Abstract
Introduction
Diclofenac has increased cardiovascular risks, but its risk profile compared with other COX-2 inhibitors remains unknown.
Aims
The aim of this study was to compare the cardiovascular risks of diclofenac versus other older and newer COX-2 inhibitors (coxibs).
Methods
Using Danish nationwide health registries (1999–2020), we conducted a series of emulated trials (n = 264). Eligible adults had no recent NSAID prescriptions, contraindications or conditions with low adherence. We included initiators of diclofenac (n = 1,600,202), meloxicam (n = 10,903), etodolac (n = 238,538), celecoxib (n = 77,591), and etoricoxib (n = 12,122). We computed the adjusted intention-to-treat incidence rate ratio (aIRR) with 95% confidence interval (CI) of major adverse cardiovascular events (MACE) within 30 days of initiation (5562 events).
Results
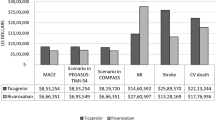
MACE was 20% increased among initiators of diclofenac compared with other older COX-2 inhibitors (aIRR 1.19, 95% CI 1.10–1.28), driven by cardiac death (aIRR 1.57, 95% CI 1.21–2.03). The effect appeared strongest for women (aIRR 1.28, 95% CI 1.15–1.43), individuals with high baseline cardiovascular risk (aIRR 1.32, 95% CI 1.05–1.66), and when comparing high-dose diclofenac with low doses of the other older COX-2 inhibitors (aIRR 1.31, 95% CI 1.13–1.52). The results reflected increased rates compared with both meloxicam (aIRR 1.46, 95% CI 0.94–2.26) and etodolac (aIRR 1.18, 95% CI 1.09–1.28). Diclofenac initiators had similar increased rates of MACE compared with coxibs (aIRR 0.96, 95% CI 0.85–1.08), consistent for celecoxib (aIRR 1.02, 95% CI 0.88–1.19) and etoricoxib (aIRR 0.85, 95% CI 0.66–1.10).
Conclusions
The increased cardiovascular risks associated with diclofenac initiation were higher than for other older COX-2 inhibitors (meloxicam/etodolac) and comparable to coxibs (celecoxib/etoricoxib).




Similar content being viewed by others
References
Schmidt M, Lamberts M, Olsen AM, Fosbøll E, Niessner A, Tamargo J, et al. Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J. 2016;37:1015–23.
European Medicines Agency (European Network of Centres for Pharmacoepidemiology and Pharmacovigilance). Call for information on effectiveness of risk minimisation on diclofenac (Referral EMEA/H/A-31/1344). 2017.
Bonnesen K, Schmidt M. Recategorization of non-aspirin nonsteroidal anti-inflammatory drugs according to clinical relevance: abandoning the traditional NSAID terminology. Can J Cardiol. 2021;37:1705–07.
McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med. 2013;10: e1001388.
Schmidt M, Sørensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018;362:k3426-10.
Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–91.
Schmidt M, Hallas J, Friis S. Potential of prescription registries to capture individual-level use of aspirin and other nonsteroidal anti-inflammatory drugs in Denmark: trends in utilization 1999–2012. Clin Epidemiol. 2014;6:155–68.
Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–9.
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90.
Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f.
Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–9.
Danaei G, Rodriguez LAG, Cantero OF, Logan R, Hernan MA. Observational data for comparative effectiveness research: An emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22:70–96.
Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available: table 1. Am J Epidemiol. 2016;183:758–64.
Schneeweiss S, Patrick AR, Til S, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45:S131–42.
Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6: e012832.
Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Collaboration, Coxib and traditional NSAID Trialists’ (CNT), et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–79.
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102.
Grosser T, Yu Y, FitzGerald GA. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33.
Arias LHM, González AM, Fadrique RS, Vazquez ES. Cardiovascular risk of nonsteroidal anti-inflammatory drugs and classical and selective cyclooxygenase-2 inhibitors: a meta-analysis of observational studies. J Clin Pharmacol. 2019;59:55–73.
McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8: e1001098.
Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial Celecoxib Long-term. Arthritis Safety Study. JAMA. 2000;284:1247–55.
Chan FKL, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376:173–9.
MacDonald TM, Hawkey CJ, Ford I, McMurray JJV, Scheiman JM, Hallas J, et al. Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT). Eur Heart J. 2017;38:1843–50.
Cannon CP, Curtis SP, FitzGerald GA, Krum H, Kaur A, Bolognese JA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2006;368:1771–81.
FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–42.
Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–7.
Aw T-J, Haas SJ, Liew D, Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:490–6.
Scott PA, Kingsley GH, Scott DL. Nonsteroidal antiinflammatory drugs and cardiac failure: metaanalyses of observational studies and randomised controlled trials. Eur J Heart Fail. 2008;10:1102–7.
Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sørensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450–d3450.
Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15.
Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357:j1909.
Gaster N, Hallas J, Pottegård A, Friis S, Schmidt M. The validity of danish prescription data to measure use of aspirin and other non-steroidal anti-inflammatory drugs and quantification of bias due to non-prescription drug use. Clin Epidemiol. 2021;13:569–79.
Adelborg K, Sundbøll J, Munch T, Frøslev T, Sørensen HT, Bøtker HE, et al. Positive predictive value of cardiac examination, procedure and surgery codes in the Danish National Patient Registry: a population-based validation study. BMJ Open. 2016;6: e012817.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by the Novo Nordisk Foundation (Grant NNF19OC0054908). The funding sources had no role in the design, conduct, analysis, or reporting of the study.
Conflicts of interest
All authors report no conflicts of interest in this work.
Ethics approval
No ethics committee approval was needed.
Consent to participate
No patient involvement.
Consent for publication
The study has been reported to the Danish Data Protection Board by Aarhus University (No. 1689).
Availability of data and material
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. Data sharing is not allowed.
Code availability
Not applicable.
Authors' contribution
MS conceived the study idea and designed the study with LAP. LAP collected the data and carried out the analyses. MS organized the writing and wrote the initial draft. All authors participated in the discussion and interpretation of the results and critically revised the manuscript for intellectual content and approved the final version before submission. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MS is the guarantor.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, M., Sørensen, H.T. & Pedersen, L. Cardiovascular Risks of Diclofenac Versus Other Older COX-2 Inhibitors (Meloxicam and Etodolac) and Newer COX-2 Inhibitors (Celecoxib and Etoricoxib): A Series of Nationwide Emulated Trials. Drug Saf 45, 983–994 (2022). https://doi.org/10.1007/s40264-022-01211-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01211-1