Abstract
Introduction
During clinical trials, mirabegron, a β3-adrenoreceptor agonist, was associated with increased vital signs vs placebo in patients with overactive bladder.
Objective
The purpose of this study was to compare incidence rates of adverse cardiovascular (CV) outcomes following mirabegron or antimuscarinic use.
Methods
We conducted an observational post-marketing safety study utilising real-world data. The study population was identified within five sources: Danish and Swedish National Registers, Clinical Practice Research Datalink (UK), Optum (USA) and Humana (USA). Episodes of time when patients were new users of mirabegron or antimuscarinics (October 2012–December 2018) were sourced from prescriptions and matched on propensity scores. Occurrences of major adverse cardiovascular events (MACE), acute myocardial infarction (AMI), stroke, CV mortality and all-cause mortality were identified. Outcome incidence rates and hazard ratios from Cox models were estimated.
Results
Overall, 152,026 mirabegron and 152,026 antimuscarinic episodes were matched. The population consisted of 63.1% women and 72.6% were ≥ 65 years old. There were no appreciable differences in the incidence rates of MACE, AMI or stroke between users of mirabegron and antimuscarinics. Incidence rates of CV mortality (hazard ratio 0.83, 95% confidence interval 0.73–0.95) and all-cause mortality (hazard ratio 0.80, 95% confidence interval 0.76–0.84) were no higher with mirabegron vs antimuscarinics. Results restricted to episodes at high risk for CV events or stratified by age (< 65 years, ≥ 65 years) or prior overactive bladder medication use were consistent with overall findings.
Conclusions
This large, multinational study found no higher risk of MACE, AMI, stroke, CV mortality or all-cause mortality among users of mirabegron relative to users of antimuscarinics.
Plain Language Summary
During clinical trials, mirabegron, which is a treatment for overactive bladder, was associated with small increases in heart rate and blood pressure. This study was conducted to compare the frequency of cardiac events following the use of mirabegron or antimuscarinics, a group of treatments also used to treat overactive bladder. We obtained the data for this study from four countries: Denmark, Sweden, the UK and the USA. We identified people who were new users of mirabegron or antimuscarinics from 2012 to 2018 using prescription or dispensing records. Occurrences of major cardiac events, heart attack, stroke, death due to cardiac events and death from any cause were evaluated. Overall, we identified 152,026 times when mirabegron or antimuscarinics were each used as new treatments. Most of the people in the study were women (63.1%) and at least 65 years old (72.6%). There were no notable differences between the treatment groups with regard to how often major cardiac events, heart attack or stroke occurred. Further, death due to cardiac events and death from any cause were no higher with mirabegron compared with antimuscarinics. We obtained similar results when the data were assessed for patients who were at high risk for cardiac events or split by age (less than 65 years or at least 65 years) or a history of overactive bladder medication use. In conclusion, this large study involving data from several countries found no higher risk of major cardiac events, heart attack, stroke or death among people prescribed mirabegron compared with antimuscarinics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study was conducted to compare the incidence of adverse cardiovascular (CV) outcomes following the use of two types of treatment for overactive bladder, mirabegron and antimuscarinics. |
In total, 152,026 occurrences of new use for each of mirabegron or antimuscarinics were matched. No higher risk of major adverse CV events, acute myocardial infarction, stroke, CV mortality or all-cause mortality was noted among current users of mirabegron relative to current users of antimuscarinics. |
Similar results to the overall findings were observed when the data were restricted to patients who were at high risk for CV events or stratified by age (< 65 years, ≥ 65 years) or prior overactive bladder medication use. |
1 Introduction
Overactive bladder (OAB) syndrome is a common condition with an estimated prevalence between 11 and 36% [1,2,3]. The syndrome is defined as the presence of urinary urgency, with or without urinary incontinence, generally with increased daytime frequency and nocturia, without any proven infection or other obvious pathology [4]. The effect of OAB on the quality of life of affected individuals is well understood, with patients reporting increased levels of anxiety and depression, as well as impairments in sexual activity and their ability to work [5, 6].
Several phase III clinical trials investigated the safety and efficacy of the β3-adrenoreceptor agonist, mirabegron, for treating patients with OAB [7,8,9]. The results from these trials informed the approval of mirabegron for the treatment of patients with OAB symptoms by the US Food and Drug Administration and the European Medicines Agency in 2012 [10, 11]. Findings obtained during the OAB clinical programme indicated that mirabegron, administered at a once daily dose of 50 mg, was associated with an increase in pulse rate of approximately 1 beat per minute and a systolic blood pressure/diastolic blood pressure increase of 0.4 to 0.6 mmHg compared with placebo [12]. An electrocardiogram study also found that a supratherapeutic dose of mirabegron 200 mg caused QT prolongation in female subjects [13]. In the context of the vital sign observations, regulatory authorities requested that a post-authorisation safety study (PASS) be conducted to address concerns about the potential cardiovascular (CV) safety of mirabegron. The current study was conducted to address this request using antimuscarinics as an active comparator group.
2 Methods
2.1 Data Sources
This study used five data sources from four countries and was a collaborative effort by partners from the University of Southern Denmark (Danish National Registers); the Centre for Pharmacoepidemiology, Karolinska Institutet (Swedish National Registers); RTI Health Solutions (Clinical Practice Research Datalink [CPRD]; UK); Optum (Optum Research Database [ORD]; USA) and Humana Healthcare Research (Humana Database; USA). The CPRD population was analysed as two groups, CPRD-linked (data from general practices that permitted hospital and mortality data linkage) and CPRD-unlinked (no linkage). The two US data sources were linked to the National Death Index.
Each research partner followed the same core protocol and statistical analysis plan, although methodological details varied at each site to account for differing data environments (see Electronic Supplementary Material [ESM]). Within each site, standard operating procedures and quality-control measures guided study conduct.
2.2 Study Design
This non-interventional, cohort study using secondary data included patients exposed to mirabegron or antimuscarinic medications from October 2012 through December 2018. Study periods within each source varied according to data availability. Exposure was based on prescription or dispensing data (hereafter prescriptions). The analysis incorporated a new user design and was conducted at the episode-of-use level as switching was expected to occur. Each mirabegron episode was matched to one antimuscarinic episode that started in the same year within each data source and according to year-specific propensity scores (PSs), with forced matching on age and sex. Incidence rates of major adverse cardiovascular events (MACE), acute myocardial infarction (AMI), stroke, CV mortality and all-cause mortality were estimated within person-time of current exposure of the matched mirabegron and antimuscarinic episodes. Hazard ratios (HRs) were obtained from Cox proportional hazard models that compared mirabegron with antimuscarinics.
2.3 Inclusion and Exclusion Criteria
Episode inclusion criteria were: a prescription for mirabegron or antimuscarinics (darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, trospium) during the study period with no previous use of that specific medication in the prior 12 months (new use). Trospium was not available for use in Sweden during the study period. Additionally, patients were required to be ≥ 18 years old and have ≥ 12 months of prior enrolment in the data source. Prescriptions of two study medications on the same day and non-tablet antimuscarinics were excluded. Patients could contribute multiple episodes if each episode met the above criteria. Additional site-specific inclusion and exclusion criteria were implemented without impacting the overall study design (ESM).
2.4 Exposure Definition
Each person-day was classified as currently exposed to medication if the person-day was within the days of supply of the prescription plus a grace period of 50% [14]. The grace period accounted for variable adherence to medications where a patient still had some medication available beyond the days of supply due to forgetting some scheduled doses or changes in dosing schedule. Methods for calculating total days of supply varied according to the information available from each data source (ESM).
Current person-time for a treatment group was terminated upon a prescription of a medication from the other treatment group or following the end of the days of supply (after applying the grace period). When days of supply overlapped between prescriptions, the first prescription was truncated on the day before the subsequent prescription. Any patient could contribute current exposure person-time for mirabegron and antimuscarinics if they switched during the study period, but not simultaneously.
2.5 Study Outcomes
The main outcome was MACE, defined as the first occurrence of AMI, stroke or CV mortality. Additional outcomes included the individual events of AMI, stroke, CV mortality and all-cause mortality. AMI outcomes included both ST elevation myocardial infarction and non-ST elevation myocardial infarction [15] and stroke outcomes included haemorrhagic and ischaemic subtypes only [16, 17]. CV mortality was defined as death due to coronary heart disease or cerebrovascular disease, and all-cause mortality was defined as death due to any cause. Outcome identification and ascertainment methods varied across the data sources (ESM). It was assumed that any CV effects due to the OAB medications would present shortly after new use and continue while patients received medication, and that the effects would decline after discontinuation.
2.6 Follow-Up Period
Follow-up for outcome occurrence started on the day after the start of the episode and ended at the earliest of: occurrence of the outcome, end of the study period, last date with validated adverse CV or death outcomes, disenrolment from the data source (e.g. emigration, death), prescription of non-tablet antimuscarinics or a prescription for multiple OAB medications on the same day. Patients were eligible to contribute additional treatment episodes until the earliest of the end of the study period, last date of data with validated adverse CV or death outcomes or disenrolment from the data source.
Once an outcome occurred, patients were no longer followed for subsequent occurrences of the same outcome. Patients could experience multiple outcome types. However, the occurrence of CV or all-cause mortality censored the treatment episode and the study follow-up. For MACE, the treatment episode and the follow-up were censored at the date of the first targeted CV event.
2.7 Statistical Methods
Data were analysed separately for the five data sources and all coding was done independently by each partner. A meta-analysis was then conducted to combine the results.
Within each population, PSs were estimated for each eligible treatment episode by modelling the probability for treatment with mirabegron or antimuscarinics, conditional on the baseline covariates. A unique calendar year-specific PS was calculated for each eligible episode using relevant baseline variables from the 12 months prior to the start of each episode. In the USA, mirabegron was approved in June 2012, thus episodes from 2012 and 2013 were combined. The PS model was built using pre-specified variables from all databases (e.g. age group, prior OAB medication use), database-specific variables (e.g. length of health plan enrolment) and database-specific empirically defined variables (e.g. most frequent International Classification of Diseases diagnosis codes). Several checks were conducted to support the variable selection process, including correlations among pre-defined covariates and empirically defined variables and review of the PS distributions.
The mirabegron episodes were matched to the antimuscarinic episodes (1:1 ratio) based on the PSs using a greedy matching algorithm [18] with a calliper of 0.009. Matches were restricted to episodes from the same calendar year and patients of the same sex and age category (< 65 years, ≥ 65 years). If a patient contributed more than one antimuscarinic episode (even if the drugs were different), these episodes were joined together to form a single period of current exposure to antimuscarinics. This only occurred if the prescription date for the second treatment episode was within the grace period for the first episode.
Absolute standardised mean differences were calculated to assess the final PS model balance [19]. Variables with a difference ≤ 0.1 were considered balanced. If a variable had a difference of > 0.1 to ≤ 0.2, further assessment was required. A difference > 0.2 was defined as unbalanced and required further PS model modifications (e.g. interaction terms), or adjustment for the variable in the outcome model.
For the matched episodes, current exposure duration and the number of prescriptions per episode of current exposure were analysed, along with the number of matched episodes and episode type (mirabegron or antimuscarinics) contributed per patient. Baseline characteristics for the mirabegron and individual antimuscarinic episodes were evaluated before and after matching (data before matching not shown). Descriptive analyses were conducted using summary measures (frequencies, proportions, medians and interquartile ranges).
For each outcome, incidence rates per 1000 person-years and 95% confidence intervals (CIs) were calculated. Cox proportional hazard models were used to estimate HRs to compare outcome incidence rates between the mirabegron and the antimuscarinic episodes.
The incidence rate of each outcome within current exposure of the mirabegron episodes was compared with the antimuscarinic episodes as a group. This analysis was also conducted by age category, OAB medication use prior to the start of the treatment episode (naïve, non-naïve) and restricting episodes to those at high risk for CV events. Non-naïve users were defined as patients who received a prescription for another OAB medication during the prior 12 months. Episodes were defined as at high risk for CV events if the patient had at least one prior diagnosis for stroke, transient ischaemic attack, coronary artery disease, angina, AMI, heart failure, cardiac arrhythmias, chronic renal insufficiency or peripheral arterial or vascular disease or at least one prior percutaneous transluminal coronary angioplasty, coronary artery bypass graft or carotid endarterectomy. Episodes from patients with three or more of the following, hyperlipidaemia, hypertension, diabetes mellitus or age ≥ 80 years, were also considered to be at high risk. Secondary analyses were conducted that separated results according to sex and a prior history of AMI or stroke; investigated the potential effect of residual confounding by alcohol/substance abuse, obesity and tobacco smoking for each data source and censored all adverse CV outcomes upon occurrence of the first individual event. For the residual confounding analysis, assessments were performed prior to pooling estimates across data sources using a spreadsheet based on previously published formula and approaches [20].
Pooled HR estimates across data sources were generated using Comprehensive Meta-Analysis software (RRID:SCR_012779) Version 3.0 [21]. Given anticipated heterogeneity in episode characteristics, prescribing patterns and the availability of covariate information across data sources, both random-effects and fixed-effects models were implemented, with point estimates representing a weighted HR of the results from the individual data sources using an inverse-variance-weighting approach [22]. Heterogeneity across data sources was assessed using the I2 test, with I2 > 50% used to indicate substantial heterogeneity. Individual study population data were also generated, and the HRs were calculated using Cox proportional hazard models and the corresponding Wald-based CIs were derived from these models.
3 Results
3.1 Treatment Episodes
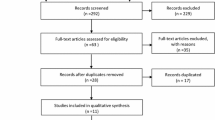
A total of 896,422 mirabegron episodes and 7,040,463 antimuscarinic episodes were identified prior to applying the inclusion and exclusion criteria (Fig. 1). Of these, 178,150 mirabegron and 691,548 antimuscarinic episodes were eligible for PS matching. A total of 152,026 mirabegron and 152,026 antimuscarinic episodes were subsequently matched one-to-one; the reasons for exclusion of the episodes are shown in the ESM. Furthermore, no episodes of oxybutynin use were identified in Denmark.
Matched cohort creation. CPRD Clinical Practice Research Datalink, OAB overactive bladder. aEach OAB medication prescription was an episode considered for inclusion in the study. bEpisodes eligible for matching were those with prescriptions of mirabegron or antimuscarinics that met all study inclusion criteria. cMatched episodes were those that met all study inclusion/exclusion criteria and were propensity score matched using a 1:1 ratio
The median duration of current exposure for the matched treatment episodes ranged from 67 days (Humana database) to 165 days (Danish data) for mirabegron, and from 45 days (CPRD-linked) to 114 days (Danish data) for antimuscarinics. Median length of episode follow-up for the matched treatment episodes ranged from 173 days (ORD) to 564 days (Danish data) for mirabegron, and from 183 days (ORD) to 401 days (Danish data) for antimuscarinics. The median number of prescriptions per episode of current exposure ranged from one (Swedish data and Humana) to two (Danish data, CPRD-linked, CPRD-unlinked and ORD) for mirabegron, and also from one (Danish data, Swedish data, CPRD-linked, CPRD-unlinked and Humana) to two (ORD) for antimuscarinics.
Within each data source, the majority of the 268,144 total patients contributed only one matched episode, ranging from 79.6% of patients from the Danish register to 92.7% of patients from the Swedish register. Of the 31,231 total patients who contributed two or more matched episodes, approximately 60% contributed both mirabegron and antimuscarinic episodes (from 44.8% of patients from the Swedish register to 69.5% of patients from the CPRD-linked database).
3.2 Baseline Characteristics
Strong balance was achieved for each data source across a range of measured baseline covariates (Table 1). Owing to sex and age group category matching, identical data were generated for these parameters in the mirabegron and antimuscarinic cohorts. Overall, 72.6% (220,798) and 63.1% (192,006) of the episodes were from patients who were ≥ 65 years old and female, respectively. Although differences in CV disease risk factors were observed across data sources, the matched mirabegron and antimuscarinic episodes within each data source were well balanced with respect to these risk factors.
3.3 CV and Mortality Outcomes
The results below are from pooled analyses unless otherwise stated. As the findings were usually similar with the fixed-effects and the random-effects models, the results shown are primarily from the fixed-effects model. When substantial heterogeneity was observed across data sources, results from both models are included.
The incidence rates per 1000 person-years during current mirabegron exposure were 21.99 (95% CI 20.97–23.05) for MACE, 5.12 (95% CI 4.63–5.64) for AMI, 12.78 (95% CI 12.00–13.59) for stroke, 5.67 (95% CI 5.17–6.22) for CV mortality and 34.95 (95% CI 33.66–36.28) for all-cause mortality (Table 2). During current antimuscarinic exposure, the incidence rates were 22.71 (95% CI 21.59–23.86) for MACE, 5.32 (95% CI 4.80–5.89) for AMI, 12.05 (95% CI 11.25–12.90) for stroke, 7.09 (95% CI 6.48–7.74) for CV mortality and 41.10 (95% CI 39.60–42.65) for all-cause mortality.
The incidence rates of MACE, AMI and stroke were similar among current users of mirabegron relative to current users of antimuscarinics, with HRs close to 1 and the 95% CIs encompassing 1 (MACE: HR 0.94, 95% CI 0.88–1.01; AMI: HR 0.94, 95% CI 0.81–1.08; stroke: HR 1.00, 95% CI 0.91–1.10). The incidence rates of CV mortality and all-cause mortality were no higher with mirabegron compared with antimuscarinics, with HRs of 0.83 (95% CI 0.73–0.95) and 0.80 (95% CI 0.76–0.84), respectively. The HRs for each outcome in the individual study populations (non-pooled data) were generally similar to those in the combined population (Fig. 2 and ESM).
Adverse CV and mortality outcomes during current use of mirabegron or antimuscarinics (matched episodes), by individual data source. AMI acute myocardial infarction, CI confidence interval, CPRD Clinical Practice Research Datalink, CV cardiovascular, HR hazard ratio, MACE major adverse cardiovascular events. HRs were calculated using Cox proportional hazard models and the corresponding Wald-based CIs were generated from the Cox models
In the analysis according to age category, the HRs for MACE, AMI and stroke ranged from 0.74 to 1.04 (< 65 years) and from 0.94 to 0.98 (≥ 65 years), and the corresponding 95% CIs all encompassed 1 (Table 3). Among those aged < 65 years, the HR for all-cause mortality was 0.76 (95% CI 0.62–0.93). In contrast to the overall results, the 95% CI was relatively large and encompassed 1 for the HR for CV mortality in this age category (HR 0.92, 95% CI 0.53–1.59). Among those aged ≥ 65 years, the HRs for CV mortality and all-cause mortality displayed the same associations as the overall results.
For the analysis by prior OAB medication use, results showed that the HRs among non-naïve OAB medication users ranged from 0.97 to 1.09 and the 95% CIs encompassed 1 (Table 4). In naïve users, the HRs for MACE, AMI and stroke ranged from 0.89 to 0.96; the 95% CIs for AMI and stroke encompassed 1, whereas the upper limit of the CI for the MACE estimate was slightly less than 1 (HR 0.90, 95% CI 0.83–0.97). The HR for all-cause mortality for naïve users was 0.73 (95% CI 0.69–0.78). Substantial heterogeneity was observed across the data sources in terms of CV mortality for naïve users (I2 = 62%). The HR for CV mortality was 0.79 (95% CI 0.68–0.92) using the fixed-effects model and 0.75 (95% CI 0.56–1.02) using the random-effects model.
Approximately 70% of the mirabegron and antimuscarinic episodes were considered at high risk for CV events (Table 5). When the data were restricted to these episodes, similar findings to the overall results were observed.
For the analysis by sex, the HRs among female patients were similar to the overall results (ESM). The results among male patients were also generally similar to the overall results. However, substantial heterogeneity among male patients was observed across the data sources for both MACE (I2 = 59%) and CV mortality (I2 = 53%). For male patients, the HRs for the fixed-effects model were 0.95 (95% CI 0.86–1.04) for MACE and 0.91 (95% CI 0.76–1.08) for CV mortality. With the random-effects model, the HRs were 0.92 (95% CI 0.76–1.12) for MACE and 0.89 (95% CI 0.65–1.21) for CV mortality.
For the analysis by prior history of AMI or stroke, similar results were observed to the overall findings, with the exception of CV mortality (ESM). For patients with a prior history of AMI or stroke, the 95% CIs for CV mortality encompassed 1 (HR 0.84, 95% CI 0.69–1.03) and for patients without a prior history, the upper confidence limit was equal to 1 (HR 0.84, 95% CI 0.71–1.00). In addition, substantial heterogeneity was observed for CV mortality in patients who had a prior history of AMI or stroke (I2 = 59%) and the HR for the random-effects model was 0.80 (95% CI 0.57–1.14). Substantial heterogeneity was also observed for MACE in patients without a prior history of AMI or stroke (I2 = 53%). The HR for MACE was 0.97 (95% CI 0.88–1.06) using the fixed-effects model and 0.96 (95% CI 0.82–1.12) using the random-effects model.
The residual confounding analysis indicated that the presence of bias was < 9% for smoking, alcohol/substance abuse and obesity in each of the data sources (ESM). When the data were censored for all CV outcomes upon occurrence of the first event, the results were nearly identical to those generated in the main analysis (ESM).
4 Discussion
This large, multinational study showed no appreciable differences in the incidences of MACE, AMI and stroke between current users of mirabegron and current users of antimuscarinics. Furthermore, the risks of CV mortality and all-cause mortality were no higher with mirabegron compared with antimuscarinics. These findings were consistently noted across the subgroup analyses.
In advance of this study, CV validation studies were conducted for each data source to confirm outcome definitions and to assess the suitability of each data source for the post-authorisation safety programme (results published for the studies conducted using the CPRD and Danish register data [24,25,26], Swedish register data were presented at the International Conference on Pharmacoepidemiology and Therapeutic Risk Management in 2016 [27], results unpublished for the ORD and Humana database studies). As mirabegron exposure results were not widely available at the time these validation studies were initiated, the investigations were conducted using data from antimuscarinic users only. Although specific associations were observed in the analyses conducted for some of the data sources [24, 27], no clear and consistent trends in the incidence rate ratios for adverse CV outcomes were observed following exposure to a range of antimuscarinics. In view of these observations, as well as the evidence that patient characteristics were similar in individuals who initiated each of the antimuscarinics, it was decided to undertake the primary analysis for the current study using a comparison cohort that comprised all new users of antimuscarinics.
The MACE findings in our study are consistent with the findings of a systematic literature review conducted to examine the CV safety of β3-adrenoreceptor agonists [28]. This review showed no increased risk of Antiplatelet Trialists’ Collaboration/MACE events among patients treated with mirabegron compared with patients treated with placebo (relative risk 0.24, 95% CI 0.02–1.69) in five 12-week trials [7,8,9, 28,29,30]. Similarly, a 1-year study included in the review found no evidence that the risk of these events was different for mirabegron vs tolterodine (relative risk 0.75, 95% CI 0.18–3.60) [28, 31]. However, this investigation lacked statistical power.
The findings for AMI and stroke are consistent with previous similar non-interventional studies using real-world data. An analysis conducted by the Food and Drug Administration from November 2012 to May 2014 (the Mini-Sentinel programme) involved investigating diagnoses of acute AMI and stroke events in matched new users of mirabegron or oxybutynin [32]. In agreement with our findings, the Mini-Sentinel programme found no evidence for differences in the incidences of AMI (HR 1.00, 95% CI 0.14–7.10) or stroke (HR 0.80, 95% CI 0.21–2.98) between the two groups, although estimates were imprecise and wide CIs were generated. The analyses from the Mini-Sentinel programme were subsequently replicated using two US administrative claims databases that did not contribute to Mini-Sentinel, IMS PharMetrics Plus and Truven (now IBM) MarketScan [33]. For both of these databases, no increased risks of AMI or stroke were found among users of mirabegron compared with users of oxybutynin (PharMetrics—AMI: HR 0.67, 95% CI 0.33–1.37; stroke: HR 0.62, 95% CI 0.34–1.13; MarketScan—AMI: HR 0.57, 95% CI 0.17–1.95; stroke: HR 0.69, 95% CI 0.30–1.62). The CV outcomes in these studies were not validated against source information; in contrast, our study adopted a more robust approach by validating potential adverse CV outcomes through direct linkage to registries or review by adjudication panels.
In the current study, the risks of CV mortality and all-cause mortality were no higher with mirabegron relative to antimuscarinics. Although our study controlled for several risk factors for CV and mortality outcomes, it is possible that unmeasured risk factors for these specific events differed between users of mirabegron and antimuscarinics and these factors may explain the findings that suggest mirabegron may be associated with reduced mortality. Examples of such factors include frailty markers or social determinants of health, which are known predictors of mortality [34,35,36].
The current study has several strengths. Our investigation comprised a large, multinational source population, which to the best of our knowledge included the highest number of mirabegron users of any study conducted to date. In addition, our study investigated CV disease risk following the administration of drugs used for treating OAB in a variety of real-world clinical practice settings. The study also investigated outcomes that have been found to be reliably recorded in electronic healthcare databases. The presence of these outcomes was confirmed through direct linkage to registries, via medical record review or physician questionnaires. Additionally, this study compiled a wide array of data on patient characteristics, health systems, drug utilisation and medical practices across populations in different countries, which enhances the generalisability of the study findings to the population of mirabegron users in routine clinical practice throughout Europe and the USA.
The current study also has limitations. Although PS methods were implemented to minimise the potential for confounding, residual confounding by unmeasured covariates cannot be ruled out. By updating the PS for each episode, time-dependent changes in key baseline covariates were used in the matching process to reduce potential confounding. In addition, it is possible that some residual confounding by lifestyle factors may have occurred as most of the data sources did not capture complete information on these risk factors. However, the secondary analyses that we conducted to assess the impact of potential confounding due to inadequately measured lifestyle factors showed minimal residual confounding of the observed effect estimates (< 9% bias). For patients contributing more than one treatment episode to the study, the analysis assumed independence of the treatment episodes. As 88% of patients contributed only one matched treatment episode, any impact of including additional episodes from the same patients would be relatively small. Patients were allowed to have different outcome types within the same episode or during subsequent episodes. As occurrences of AMI and stroke are known to affect the risk of subsequently developing other CV events [37, 38], we conducted a secondary analysis to censor all adverse CV outcomes upon occurrence of the first event. As stated above, this analysis yielded nearly identical results to the primary analysis and therefore it was assumed that the inter-related nature of the study outcomes had a minimal effect.
Additional limitations are related to the data sources and data capture on the real-world use of OAB medications. The current study included analyses from a variety of data sources, each set up for different purposes and over different time periods. For example, health outcomes that do not result in billed medical services were not identifiable from the US data. For the Danish, Swedish and CPRD data sources, data on drugs that were administered in hospitals or nursing homes, over-the-counter drugs or drugs provided as samples were not captured, although this may only constitute a small proportion of patients. Further, there was no information on the prescribed daily dosage or indication of use. We assume that the drugs were taken as prescribed, but they may have been used on an ‘as-needed’ basis. However, we believe these limitations are mitigated by the extensive exposure data that were assessed in this study and validation of the study outcomes. Assessing the severity of hypertension or whether hypertension is controlled by medication and non-pharmaceutical treatments in studies like this one is challenging. This is because the data are obtained from existing data sources, where blood pressure results are generally not available and the proxies may not be accurate. Therefore, we did not conduct analyses to assess the effect of uncontrolled hypertension on the CV outcomes in this study. Furthermore, a European study that assessed the effectiveness of a direct healthcare communication letter to decrease the proportion of patients with uncontrolled hypertension prior to mirabegron initiation found that uncontrolled hypertension was uncommon before starting treatment with mirabegron even before the dissemination of the letter [39]. While it is possible that mirabegron might have influenced hypertension control in some patients, the previously mentioned results indicate that mirabegron initiation is quite rare in patients with uncontrolled hypertension, so there would be little to be learned from this type of analysis.
5 Conclusions
This PASS found no higher risk of MACE, AMI, stroke, CV mortality or all-cause mortality among current users of mirabegron compared with current users of antimuscarinics. Given the diverse nature of the study population, these study findings may be generalisable to mirabegron users in similar healthcare systems beyond those included in this study.
References
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–8. https://doi.org/10.1111/j.1464-410X.2010.09993.x.
Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77:1081–7. https://doi.org/10.1016/j.urology.2010.08.039.
Coyne KS, Sexton CC, Bell JA, Thompson CL, Dmochowski R, Bavendam T, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn. 2013;32:230–7. https://doi.org/10.1002/nau.22295.
Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33:622–4. https://doi.org/10.1002/nau.22609.
Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80:90–6. https://doi.org/10.1016/j.urology.2012.04.004.
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101:1388–95. https://doi.org/10.1111/j.1464-410X.2008.07601.x.
Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189:1388–95. https://doi.org/10.1016/j.juro.2012.10.017.
Khullar V, Amarenco G, Angulo JC, Cambronero J, Høye K, Milsom I, et al. Efficacy and tolerability of mirabegron, a β3-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol. 2013;63:283–95. https://doi.org/10.1016/j.eururo.2012.10.016.
Herschorn S, Barkin J, Castro-Diaz D, Frankel JM, Espuna-Pons M, Gousse AE, et al. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013;82:313–20. https://doi.org/10.1016/j.urology.2013.02.077.
New class of treatment for overactive bladder approved in Europe. https://www.astellas.com/system/files/news/2018-06/131111_eg.pdf. Accessed 11 Jun 2021.
News releases. FDA approves overactive bladder treatment Myrbetriq™ (mirabegron) from Astellas. https://newsroom.astellas.us/2012-06-28-FDA-Approves-Overactive-Bladder-Treatment-Myrbetriq-mirabegron-from-Astellas. Accessed 11 Jun 2021.
Nitti VW, Chapple CR, Walters C, Blauwet MB, Herschorn S, Milsom I, et al. Safety and tolerability of the β3-adrenoceptor agonist mirabegron, for the treatment of overactive bladder: results of a prospective pooled analysis of three 12-week randomised Phase III trials and of a 1-year randomised Phase III trial. Int J Clin Pract. 2014;68:972–85. https://doi.org/10.1111/ijcp.12433.
Malik M, van Gelderen EM, Lee JH, Kowalski DL, Yen M, Goldwater R, et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: a randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin Pharmacol Ther. 2012;92:696–706. https://doi.org/10.1038/clpt.2012.181.
Gomes T, Juurlink DN, Mamdani MM. Comparative adherence to oxybutynin or tolterodine among older patients. Eur J Clin Pharmacol. 2012;68:97–9. https://doi.org/10.1007/s00228-011-1090-8.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. https://doi.org/10.1161/CIR.0b013e31826e1058.
Kirkman MA, Mahattanakul W, Gregson BA, Mendelow AD. The accuracy of hospital discharge coding for hemorrhagic stroke. Acta Neurol Belg. 2009;109:114–9.
Saver JL. Proposal for a universal definition of cerebral infarction. Stroke. 2008;39:3110–5. https://doi.org/10.1161/STROKEAHA.108.518415.
Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Presented as Paper 214-26 at the Proceedings of the 26th Annual SAS Users Group International Conference, 22–25 April 2001, Long Beach, CA, USA. https://support.sas.com/resources/papers/proceedings/proceedings/sugi26/p214-26.pdf. Accessed 11 Jun 2021.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. https://doi.org/10.1002/sim.3697.
Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. https://doi.org/10.1002/pds.1200.
Comprehensive Meta Analysis Version 3.0 manual. https://www.meta-analysis.com/downloads/Meta-Analysis%20Manual%20V3.pdf. Accessed 11 Jun 2021.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. https://doi.org/10.1002/jrsm.12.
Rothman KJ. Epidemiology: an introduction. New York: Oxford University Press; 2002.
Arana A, Margulis AV, McQuay LJ, Ziemiecki R, Bartsch JL, Rothman KJ, et al. Variation in cardiovascular risk related to individual antimuscarinic drugs used to treat overactive bladder: a UK cohort study. Pharmacotherapy. 2018;38:628–37. https://doi.org/10.1002/phar.2121.
Margulis AV, Hallas J, Pottegård A, Kristiansen NS, Atsma WJ, Franks B, et al. Comparison of cardiovascular events among treatments for overactive bladder: a Danish nationwide cohort study. Eur J Clin Pharmacol. 2018;74:193–9. https://doi.org/10.1007/s00228-017-2359-3.
Arana A, Margulis AV, Varas-Lorenzo C, Bui CL, Gilsenan A, McQuay LJ, et al. Validation of cardiovascular outcomes and risk factors in the Clinical Practice Research Datalink in the United Kingdom. Pharmacoepidemiol Drug Saf. 2021;30:237–47. https://doi.org/10.1002/pds.5150.
Linder M, Margulis AV, Anveden-Berglind I, Bahmanyar S, Bui CL, Atsma WJ, et al. Cardiovascular risk in users of antimuscarinic drugs for overactive bladder: a cohort study in the Swedish national registers. Pharmacoepidemiol Drug Saf. 2016;25(Suppl. 3):493.
Rosa GM, Ferrero S, Nitti VW, Wagg A, Saleem T, Chapple CR. Cardiovascular safety of β3-adrenoceptor agonists for the treatment of patients with overactive bladder syndrome. Eur Urol. 2016;69:311–23. https://doi.org/10.1016/j.eururo.2015.09.007.
Chapple CR, Dvorak V, Radziszewski P, Van Kerrebroeck P, Wyndaele JJ, Bosman B, et al. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J. 2013;24:1447–58. https://doi.org/10.1007/s00192-013-2042-x.
Yamaguchi O, Marui E, Kakizaki H, Homma Y, Igawa Y, Takeda M, et al. Phase III, randomised, double-blind, placebo-controlled study of the β3-adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int. 2014;113:951–60. https://doi.org/10.1111/bju.12649.
Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63:296–305. https://doi.org/10.1016/j.eururo.2012.10.048.
Leonard CE, Reichman ME, Toh D, Kulldorff M, Nelson JC, Gagne JJ, et al. Mini-Sentinel prospective surveillance plan: prospective routine observational monitoring of mirabegron (September 19, 2016). https://www.sentinelinitiative.org/sites/default/files/Drugs/Assessments/Mini-Sentinel_Modular-Program-Report_to12_prompt_mira_mpl3r_wp01_nsdp_v01_1-4.pdf. Accessed 11 Jun 2021.
Simeone JC, Nordstrom BL, Appenteng K, Huse S, D’Silva M. Replication of Mini-Sentinel study assessing mirabegron and cardiovascular risk in non-Mini-Sentinel databases. Drugs Real World Outcomes. 2018;5:25–34. https://doi.org/10.1007/s40801-017-0124-7.
Crow RS, Lohman MC, Titus AJ, Bruce ML, Mackenzie TA, Bartels SJ, et al. Mortality risk along the frailty spectrum: data from the National Health and Nutrition Examination Survey 1999 to 2004. J Am Geriatr Soc. 2018;66:496–502. https://doi.org/10.1111/jgs.15220.
Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47:193–200. https://doi.org/10.1093/ageing/afx162.
Wallace LMK, Theou O, Pena F, Rockwood K, Andrew MK. Social vulnerability as a predictor of mortality and disability: cross-country differences in the survey of health, aging, and retirement in Europe (SHARE). Aging Clin Exp Res. 2015;27:365–72. https://doi.org/10.1007/s40520-014-0271-6.
Wang Y, Li J, Zheng X, Jiang Z, Hu S, Wadhera RK, et al. Risk factors associated with major cardiovascular events 1 year after acute myocardial infarction. JAMA Netw Open. 2018;1: e181079. https://doi.org/10.1001/jamanetworkopen.2018.1079.
Ducrocq G, Amarenco P, Labreuche J, Alberts MJ, Mas J-L, Ohman EM, et al. A history of stroke/transient ischemic attack indicates high risks of cardiovascular event and hemorrhagic stroke in patients with coronary artery disease. Circulation. 2013;127:730–8. https://doi.org/10.1161/CIRCULATIONAHA.112.141572.
Heintjes EM, Bezemer ID, Prieto-Alhambra D, Smits E, Booth HP, Dedman D, et al. Evaluating the effectiveness of an additional risk minimization measure to reduce the risk of prescribing mirabegron to patients with severe uncontrolled hypertension in four European countries. Clin Epidemiol. 2020;12:423–33. https://doi.org/10.2147/CLEP.S242065.
Acknowledgements
Medical writing support was provided by Michael Parsons from Envision and funded by the study sponsor. The authors acknowledge all of the members of the mirabegron PMR-PASS study group: Cheryl Enger, John Seeger (Optum), Veena Hoffman (former Optum employee) and Kelesitse Phiri (former Optum employee); Jesper Hallas, Morten Olesen (University of Southern Denmark) and Nina Sahlertz Kristiansen (former University of Southern Denmark employee); Shahram Bahmanyar, Marie Linder, Helle Kieler (Centre for Pharmacoepidemiology, Karolinska Institutet) and Ingvild Odsbu (former Centre for Pharmacoepidemiology, Karolinska Institutet employee); Alejandro Arana, Lisa McQuay, Andrea Margulis, Susana Perez-Gutthann and Ryan Ziemiecki (RTI Health Solutions); Su Bunniran, Brandon Suehs, Claudia Uribe, Yihua Xu (Humana Healthcare Research) and Libby Horter (former Humana Healthcare Research employee); Kwame Appenteng, Stefan de Vogel, Noah Jamie Robinson, Songlin Xue, Josie Wolfram, Achim Steup, Jena Giese-Pagac, Raymond van Aarle, Neha Sheth, David Burns, Natalie Boone, Mary Beth Blauwet (Astellas Pharma), Milbhor D’Silva (former Astellas employee), Billy Franks (former Astellas employee), Willem Jan Atsma (former Astellas employee) and Tim Auton (posthumously; former Astellas employee) and Edeltraut Garbe, Anders Ekbom, Todd Lee, Noel Weiss and John Rumsfeld (Scientific Advisory Board). Additional thanks are due to Sara Yuewen Gao, Laura Karslake, Nan Liu, Katherine Reed, Bruce Turnbull, Jing Yang (Optum), Nicole Brooks (former Optum employee) and Kathleen Mortimer (former Optum employee). This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the National Health Service (NHS) as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Copyright © (2018), re-used with the permission of The Health & Social Care Information Centre. All rights reserved.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This study was funded by Astellas Pharma Inc.
Conflicts of Interest/Competing Interests
Cheryl Enger and John D. Seeger are employees of Optum. Veena Hoffman and Kelesitse Phiri were employees of Optum at the time the study was conducted. Veena Hoffman, Kelesitse Phiri, Cheryl Enger and John D. Seeger hold stock in UnitedHealth Group, Optum’s parent company. UnitedHealthcare, a UnitedHealth subsidiary, is a major purchaser of pharmaceutical products. The work was funded with a research contract between Optum and Astellas. Jesper Hallas and Morten Olesen have worked on a project commissioned by Astellas, with funding paid to their employer, the University of Southern Denmark. Nina Sahlertz Kristiansen was an employee of the University of Southern Denmark at the time the study was conducted. The contract granted the research team at the University of Southern Denmark independent publication rights. Marie Linder is an employee of the Centre for Pharmacoepidemiology, Karolinska Institutet. Ingvild Odsbu was an employee of the Centre for Pharmacoepidemiology at the time the study was conducted. The Centre receives grants from several entities (pharmaceutical companies, regulatory authorities and contract research organisations), including Astellas and Pfizer, for performance of drug safety and drug utilisation studies. Andrea V. Margulis, Alejandro Arana and Susana Perez-Gutthann are employees of RTI Health Solutions. RTI Health Solutions is a unit of RTI International, an independent non-profit organisation that conducts work for government, public and private organisations, including pharmaceutical companies. The RTI authors participated in this work in the course of employment as work for hire, pursuant to a contract to conduct an independent research study for a client (Astellas). The authors received no compensation other than their annual salary from their employer. Brandon T. Suehs and Yihua Xu are employees of Humana Healthcare Research, which received funding from Astellas in connection with the performance of this study. Libby Horter was an employee of Humana Healthcare Research at the time the study was conducted. Kwame Appenteng and Stefan de Vogel are employees of Astellas.
Ethics Approval
The New England Institutional Review Board (IRB) approved the part of the study conducted by Optum. According to Danish law, register-based studies are exempt from approval by the Danish National Committee on Biomedical Research Ethics. Neither IRBs nor internal ethics committees are used/required at the University of Southern Denmark. The regional ethical board at Karolinska Institutet approved the part of the study conducted by Karolinska Institutet. The RTI International IRB determined that RTI researchers would not be engaged in research with human subjects. With this designation, additional review, approval or exemption by the RTI International IRB was not necessary. The Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research approved the use of the CPRD database by RTI Health Solutions. Advarra (formerly known as Schulman IRB) approved the part of the study conducted by Humana Healthcare Research.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
This observational study is based on individual patient data maintained by five separate data sources. Therefore, we are not able to make these data available to the public.
Code Availability
Proprietary code was developed to perform the analyses on the data. The code applied to each data source and is maintained and archived according to the policies specific to each source. Researchers desiring access to the code would be required to obtain permission from the creator.
Authors’ Contributions
All authors provided substantial contributions to the conception or design of the study or the acquisition, analysis or interpretation of the data for the manuscript and the drafting of the manuscript or the revision of the manuscript for important intellectual content. The authors also provided final approval of the version to be submitted and agree to be accountable for all aspects of the manuscript.
Additional information
The mirabegron PMR-PASS study group members are listed in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hoffman, V., Hallas, J., Linder, M. et al. Cardiovascular Risk in Users of Mirabegron Compared with Users of Antimuscarinic Treatments for Overactive Bladder: Findings from a Non-Interventional, Multinational, Cohort Study. Drug Saf 44, 899–915 (2021). https://doi.org/10.1007/s40264-021-01095-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-021-01095-7