Abstract
Introduction
Hypersensitivity reactions (HSRs) are among the known adverse events of intravenous (i.v.) iron products. Of these, particularly severe HSRs such as anaphylaxis are of great clinical concern due to their life-threatening potential.
Methods
This was a retrospective pharmacoepidemiological study with a case-population design evaluating the number of reported severe HSRs following administration of the two i.v. iron products—ferric carboxymaltose and iron (III) isomaltoside 1000—in relation to exposure in European countries from January 2014 to December 2017. Exposure to both products was estimated using IQVIA MIDAS sales data in European countries. Information on spontaneously reported severe HSRs was obtained from and analysed separately for the two established safety surveillance databases EudraVigilance and VigiBase™ using the MedDRA® Preferred Terms anaphylactic reaction, anaphylactic shock, anaphylactoid reaction and anaphylactoid shock associated with administration of either product.
Results
Between 2014 and 2017, the reporting rate of severe HSRs per 100,000 defined daily doses (100 mg dose equivalents of iron) varied from 0.3 to 0.5 for ferric carboxymaltose and from 2.4 to 5.0 for iron (III) isomaltoside 1000. The reporting rate ratio for iron (III) isomaltoside 1000 versus ferric carboxymaltose was between 5.6 (95% CI 3.5–9.0) and 16.2 (95% CI 9.4–27.8).
Conclusions
Findings suggest that iron (III) isomaltoside 1000 is associated with a higher reporting rate of severe HSRs related to estimated exposure than ferric carboxymaltose in European countries. Future research investigating the occurrence of severe HSRs associated with i.v. ferric carboxymaltose and iron (III) isomaltoside 1000 is needed to broaden the evidence for benefit-risk assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this pharmacoepidemiological study, data were sourced from two pharmacovigilance reporting databases to evaluate the rate of severe HSRs associated with ferric carboxymaltose and iron (III) isomaltoside 1000. |
The results suggest that iron (III) isomaltoside 1000 is associated with a higher reporting rate of severe HSRs than ferric carboxymaltose. |
The findings support the need to further evaluate differences in the safety profile of these two intravenous iron-containing products. |
1 Introduction
Globally, anaemia affects 2.36 billion individuals (approximately 34% of the global population), making anaemia one of the most common medical impairments in the world [1]. Prevalence is highest in preschool-age children (47.4%) and lowest in men (12.7%). The population most affected are women, and there is a particularly high prevalence among pregnant women (41.8%) [2]. The most common cause of anaemia is iron deficiency, which accounts for up to 50% of anaemia cases [3]. Iron-deficiency anaemia (IDA) is one of the five leading causes of years lived with disability (YLDs) in the world [4]. IDA can be caused by a variety of conditions, such as insufficient iron absorption, inadequate dietary iron, blood loss or an increased physiological requirement for iron. Adequate supplementation of iron counteracts these adverse conditions. Intravenous (i.v.) iron rather than oral formulations is usually indicated in patients with intolerance for or poor response to oral iron treatment due to gastrointestinal side effects resulting in lack of efficacy, pre-existing diseases with systemic inflammation, ongoing bleeding or non-adherence [5].
Anaemia is twice as prevalent among US patients with chronic kidney disease (CKD) compared with the general population (15.4% vs. 7.6%) [6]. Anaemia develops during the course of the disease and worsens as the underlying CKD progresses [7]. Traditionally, most i.v. administrations of iron were given in CKD populations as they require optimal management of iron homeostasis [8,9,10]. However, based on the experience with i.v. iron use in dialysis patients, the newer iron preparations, which are generally better tolerated and easier to use than the older preparations, are also widely used in other populations, including patients with inflammatory bowel disease (IBD), heavy menstrual bleeding, oncological disorders and heart failure.
Although administration of i.v. iron is generally considered safe, iron infusions can cause hypersensitivity reactions (HSRs) [11, 12]. As these reactions may range from mild to fatal, HSRs are of great clinical concern, especially anaphylaxis [13, 14]. Anaphylaxis is the umbrella term for an acute reaction defined as follows: a severe, life-threatening generalized or systemic hypersensitivity reaction. The diagnosis should be based on clinical symptoms independent of the pathomechanisms involved, and should distinguish between allergic anaphylaxis and non-immune anaphylaxis (previously classified as anaphylactoid reaction) [15].
In 2013, all i.v. iron medicinal products registered in the European Union were evaluated by the European Medicines Agency (EMA) as part of a referral procedure, wherein the risk of serious HSRs with the use of these products was investigated [11]. The conclusion was that the overall benefit/risk ratio for i.v. iron products on the European Market is positive, but no distinction was made between the individual substance classes (iron sucrose, iron dextran, iron gluconate, ferric carboxymaltose, iron (III) isomaltoside 1000). As an outcome of this referral, all labels were harmonized in all relevant sections dealing with the risk of HSRs, and it was added to the labels of all products that i.v. irons are subject to additional monitoring. The market authorization holders for i.v. iron products were requested to conduct a post-authorization safety study (PASS) to further evaluate the safety concerns related to severe HSRs. The results of this PASS are expected in 2020 [16].
As severe hypersensitivity events are rare, large sample sizes are needed to investigate differences. In 2011, Bailie and colleagues used information from the WHO global database of individual case safety reports (ICSRs), the world’s largest database of this kind, to evaluate differences in spontaneously reported severe HSRs between different i.v. preparations in Europe and North America in the period from January 2003 to June 2009 [17]. A total number of 192 anaphylaxis events were identified and the rates of anaphylaxis were 6- to 11-fold higher for iron dextran (8- to 30-fold for low molecular weight iron dextran) than for non-dextran i.v. irons. This study did not include information on ferric carboxymaltose or iron (III) isomaltoside 1000 because only substances that were on either market for more than 2 years were included. In Europe, ferric carboxymaltose received its first market authorization approval in June 2007, and iron (III) isomaltoside 1000 in December 2009; in the USA ferric carboxymaltose was approved in July 2013, and iron (III) isomaltoside 1000 is not yet on the US market. A later study based on a sample size of 688,183 patients using Medicare claims data in the USA indicated differences in the risk for hypersensitivity and related severe adverse events (AE) for i.v. iron products on the US market at that time [18]. They found that the risk for anaphylaxis at first exposure to an i.v. iron was 68 per 100,000 persons for iron dextran and 24 per 100,000 persons for all non-dextran i.v. iron products combined. This study was conducted over the period January 2003 to December 2013 and did not include ferric carboxymaltose and iron (III) isomaltoside 1000 for the above-mentioned reasons [18].
Therefore, we conducted a pharmacoepidemiological database analysis that aimed to assess the number of reported severe HSRs following administration of the two high-dose, fast-infusion i.v. iron products, ferric carboxymaltose and iron (III) isomaltoside 1000, in relation to their exposure in European countries, using information from safety databases of the EMA and the WHO (EudraVigilance and VigiBase™, respectively).
2 Methods
This was a retrospective pharmacoepidemiological study with a case-population design [19]. The 4-year study period ranged from 1 January 2014 to 31 December 2017, which is the most recent period after the conclusion of and actions taken after the EMA referral on i.v. iron-containing medicinal products (presented in September 2013) [11].
Exposure to ferric carboxymaltose or iron (III) isomaltoside 1000 was estimated using sales data for these products in European countries collected via the IQVIA MIDAS platform (Table 1). For the majority of countries, sales are captured for both hospital as well as retail settings. The coverage varies by country and setting. Both substances are mainly used as high-dose formulations; however, for both substances low-dose formulations exist. As the safety surveillance database reports cannot distinguish between low- and high-dose use, the respective low-dose formulations are included in the analysis. For our analysis we assume that sales data accurately reflect the usage and exposure to each substance (i.e. inventory levels do not vary across the 4-year period as stocks in hospitals are usually kept low). Accuracy of sales data is validated regularly in a number of studies each year and a global precision index was published with a value of 94.6% in 2016 [20]. Sales data were available for 25 countries of the European Economic Area (EEA) plus Switzerland (Table 1). Sales were normalized to 100 mg dose equivalents (DEq; 100 mg DEq of iron = 1 defined daily dose, DDD) of iron, which serves as a proxy variable for the number of administrations, as the most commonly given dose per treatment is 10 DDDs. This is in accordance with the previously published method of standardization [21].
Information on the number of spontaneously reported severe HSRs in the countries of the EEA plus Switzerland were obtained from two established safety surveillance databases—EudraVigilance [22] and VigiBase™ [23]. EudraVigilance is a centralized European database of suspected adverse reactions to medicines that are authorized or being studied in clinical trials in the EEA. Patient-specific data are only available for age and gender, and information on co-morbidities cannot be retrieved. VigiBase™ is the WHO global database of individual case safety reports (ICSRs) run by the Uppsala Monitoring Center. Reports are submitted by member countries of the WHO Programme for International Drug Monitoring, and since November 2017 it also includes EudraVigilance data reported directly to WHO. In addition, stakeholders have reporting obligations for both sources, therefore most reports can be found in both sources. In order to provide a comprehensive overview, both data sources were considered in the analysis. For this study, spontaneously reported severe HSRs were identified in both databases by using the MedDRA® Preferred Terms (PTs) anaphylactic reaction, anaphylactic shock, anaphylactoid reaction and anaphylactoid shock associated with an administration of ferric carboxymaltose or iron (III) isomaltoside 1000.
The EudraVigilance EEA-based data do not include information from Switzerland, and only ferric carboxymaltose but not iron (III) isomaltoside 1000 is on the Swiss market. Therefore, in order to make event reports from the databases comparable and to avoid bias in favour of ferric carboxymaltose with regard to sales and reported HSRs, event reports for Switzerland were directly provided by Vifor Pharma, the manufacturer of ferric carboxymaltose. Numbers of event reports from Vifor Pharma are presented separately to provide transparency on the figures available from Eudravigilance for EEA and from Vifor Pharma for Switzerland (see Table 2). For VigiBase™, the data included in the analysis comprise the EEA and Switzerland.
2.1 Reporting Rates Calculation
We conducted a descriptive analysis to evaluate reported rates of severe HSRs (i.e. anaphylactic reaction, anaphylactic shock, anaphylactoid reaction and anaphylactoid shock) in the period from 2014 to 2017 in European countries. We analysed case reports from EudraVigilance and VigiBase™ separately. Data were aggregated over all countries. As mentioned above, estimated exposure to the two substances was calculated using sales data from the IQVIA MIDAS platform. Reporting rates by substance were calculated by dividing reported severe HSRs by exposure (100,000 DDDs) in the EEA plus Switzerland. Reporting rate ratios were determined by comparing the reporting rates of both products. Homogeneity across years was tested with the Breslow-Day test. A common estimate of the reporting rate ratio was determined using the Cochran–Mantel–Haenszel method. Statistical analysis was carried out using SAS® software V9.3 (SAS Institute, Cary, NC, USA) on Windows™-based computer platforms.
3 Results
3.1 Exposure to Ferric Carboxymaltose and Iron (III) Isomaltoside 1000 Based on Sales Data
In the EEA, annual total exposure to ferric carboxymaltose steadily increased from 6.4 million DDDs in 2014 to approximately 12.4 million DDDs in 2017. Annual total exposure to iron (III) isomaltoside 1000 for the same period and region increased from 0.6 million DDDs to 1.4 million DDDs (Fig. 1).
In total, more than 80% of overall exposure to ferric carboxymaltose was recorded in Germany, Switzerland, France, UK, Spain, Italy and Belgium. Countries with the highest exposure to iron (III) isomaltoside 1000 were the UK, Poland, Netherlands, Sweden, Denmark and Germany.
3.2 Reported Number of Severe HSRs in VigiBase™ and EudraVigilance Databases
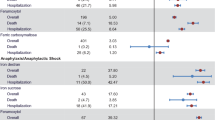
In VigiBase™ from 2014 to 2017, 143 events of severe HSRs were reported for ferric carboxymaltose and 126 for iron (III) isomaltoside 1000. In EudraVigilance, 76 events were reported for ferric carboxymaltose. Adding 45 event reports for Switzerland from Vifor Pharma, this results in 121 total reported events for ferric carboxymaltose. For iron (III) isomaltoside 1000, 137 events were reported in EudraVigilance in the EEA. The absolute numbers of anaphylactic/anaphylactoid reactions and anaphylactic/anaphylactoid shocks were comparable for both substances in both databases after adding the Swiss event reports from Vifor Pharma (Table 2). Anaphylactic/anaphylactoid reactions constituted approximately 80% of events, while anaphylactic/anaphylactoid shocks accounted for approximately 20% of HSRs.
In EudraVigilance, one fatal case each was reported for patients receiving ferric carboxymaltose and iron (III) isomaltoside 1000 as an outcome of an anaphylactoid reaction and an anaphylactic reaction, respectively.
From 2014 to 2017, the number of events per year remained in the same range for both ferric carboxymaltose (EudraVigilance: min/max 21–38 events per year, median: 31; VigiBase™: min/max 21–45 events per year, median: 38.5) and iron (III) isomaltoside 1000 (EudraVigilance: min/max 24–46 events per year, median: 33.5; VigiBase™: min/max 27–37 events per year, median: 31).
3.3 Characteristics of Patients with Reported Severe Hypersensitivity Reactions in EudraVigilance
In EudraVigilance, data on age group and gender are available for most patients with a severe HSR. For ferric carboxymaltose, almost 90% (N = 67 of 75) of reported severe HSR events occurred in female patients, and for iron (III) isomaltoside 1000 approximately two-thirds (N = 88 of 136, 64.7%) of the reported severe HSRs occurred in female patients.
The majority of patients with reported severe HSRs in EudraVigilance were below 64 years of age (ferric carboxymaltose: N = 54 of 63; 85.7%; iron (III) isomaltoside 1000: N = 93 of 130; 71.5%).
About half of the reports (N = 112; 52.6%) included information on dosing. Of these, the 45 patients receiving ferric carboxymaltose received a median dosage of 1000 mg (Q25–Q75: 500–1000 mg) and for 67 patients receiving iron (III) isomaltoside 1000, a median dose of 1000 mg (Q25–Q75: 500–1000 mg) was given.
3.4 Reporting Rates for Severe Hypersensitivity Reactions
Between 2014 and 2017, the number of reported severe HSRs per 100,000 DDDs (‘reporting rate’) varied from 0.3 to 0.5 for ferric carboxymaltose (EudraVigilance: 0.3–0.4, VigiBase™: 0.3–0.5) and from 2.4 to 5.0 for iron (III) isomaltoside 1000 (EudraVigilance: 2.4–5.0, VigiBase™: 2.5–4.6) (see Fig. 2).
Between 2014 and 2017, the reporting rate ratio of severe HSRs associated with iron (III) isomaltoside 1000 versus ferric carboxymaltose ranged from 6.4 (95% CI 3.9–10.5) to 14.8 (95% CI 9.1–24.0) based on EudraVigilance data and from 5.6 (95% CI 3.5–9.0) to 16.2 (95% CI 9.4–27.8) based on VigiBase™ data (see Table 3).
For EudraVigilance, Breslow-Day was found not to be significant (p = 0.0965), indicating no significant heterogeneity in the reporting rate between the years. The year-adjusted reporting rate for iron (III) isomaltoside 1000 was 10.7 times the reporting rate of ferric carboxymaltose between 2014 and 2017 (95% CI 8.4–13.7) (see Table 3).
For VigiBase™, there was significant heterogeneity (p = 0.0297) of the reporting rate among the years. However the value shows the same trend as the value for EudraVigilance data (see Table 3).
4 Discussion
Severe hypersensitivity reactions to i.v. iron products occur infrequently but can have serious adverse outcomes for patients. There are several i.v. iron formulations available in Europe, and therefore it is important to understand both the frequency and the severity of hypersensitivity-mediated reactions to support clinical decision making when using these products. In the absence of head-to-head comparative clinical trial data, real-world data are an established source to provide insights on the safety profile of drugs in clinical practice.
In this pharmacoepidemiological study, data from two established safety databases—VigiBase™ and EudraVigilance—complemented with data for Switzerland from Vifor Pharma, were used to evaluate the reported rate of severe HSRs (i.e. anaphylactic/anaphylactoid reactions and shocks) associated with ferric carboxymaltose and iron (III) isomaltoside 1000 in relation to the overall exposure of these iron products in European countries from 2014 to 2017. Overall, the number of reported severe HSRs associated with these iron-containing products is in the same dimension in both databases, but the exposure to ferric carboxymaltose is considerably higher in European countries than to iron (III) isomaltoside 1000. Of note, EudraVigilance and VigiBase™ are not completely independent sources of information, thus similar results do not reflect a reproduction of results in two different data sources, but the use of both substantiates the comprehensiveness of information captured. For both products, ferric carboxymaltose and iron (III) isomaltoside 1000, most severe HSRs were reported for female patients. This reflects the higher usage of i.v. iron in females as prevalence of anaemia is higher in women than in men [2]. Taking the exposure of both products into account and acknowledging that dosage was comparable for both products, the results suggest that iron (III) isomaltoside 1000 is associated with a considerably higher reporting rate per 100,000 DDDs for severe HSRs than ferric carboxymaltose.
Reporting of AEs does not necessarily reflect the occurrence of events in clinical practice, and therefore the presented results do not allow a conclusion to be drawn about the absolute and relative risk for severe HSRs associated with ferric carboxymaltose and iron (III) isomaltoside 1000. Although AE reporting can be used to estimate the relative rates of events with individual products, head-to-head data remain the gold standard because they capture exposure and outcome in a standardized manner and circumvent effects of differential prescribing and reporting. So far, no comparative safety study on severe HSRs associated with ferric carboxymaltose and iron (III) isomaltoside 1000 has been published.
However, differences in the safety profile of these iron-containing products have been observed both in a systemic review of clinical trials [24] and in a retrospective analysis of clinical data [25]. The recently published systematic network meta-analysis based on five randomised, controlled trials has compared the tolerability of different i.v. iron formulations for the treatment of IDA in patients with inflammatory bowel disease, and found differences in the safety characteristics of iron formulations. Pooled data from 1,746 patients revealed AE rates of 12.0%, 15.3%, 12.0% and 17.0% for ferric carboxymaltose, iron sucrose, iron dextran and iron isomaltoside 1000, respectively [24]. Differences in the safety profile between ferric carboxymaltose and iron (III) isomaltoside 1000 are also supported by results of a 2017 retrospective single-centre study in an unselected outpatient population of 231 patients. This study describes the occurrence of HSRs and hypophosphataemia after administration of ferric carboxymaltose and iron (III) isomaltoside 1000. The results showed a lower risk for mild HSRs and a higher risk for hypophosphataemia for patients treated with ferric carboxymaltose compared to patients treated with iron (III) isomaltoside 1000 [25]. A single-centre cohort study was recently published with similar results. The crude risk for a hypersensitivity reaction was 75% lower after ferric carboxymaltose treatment when compared to treatment with iron (III) isomaltoside 1000 (RR = 0.248, 95% CI 0.145–0.426, p < 0.0001) [27].
The results of this pharmacoepidemiological study analysing spontaneous reports have known limitations. In general, for spontaneously reported AE data from safety databases the following points need to be taken into account: use of different preferred MedDRA® terms for AEs, changes in AEs coding over time, inconsistent descriptions of AEs by clinicians, no confirmation of event term or causal relationship for AEs reported by patients, and different reports in national and international surveillance databases. As these general issues apply for both substances, the impact on a comparison of both substances is considered to be negligible. Beyond this, AE reporting rates depend on many aspects of medical practice. Under-reporting of adverse events to spontaneous reporting systems is well known [27]. However, our analysis is focused on severe events where under-reporting is less likely.
An investigation of time trends in reporting of adverse drug reactions (ADRs) indicates that physicians know the main ADRs after the first years of use and tend to report only serious ADRs [28]. The reporting of AEs may also be higher directly after launch compared to when products are well established on the market or may be influenced by a public discussion on safety concerns. In order to overcome this limitation, the 4-year period from 2014 to 2017 after the referral for iron-containing products in 2013 was chosen [11]. In this period both products had already been on the European market for several years, ferric carboxymaltose since June 2007 and iron (III) isomaltoside 1000 since December 2009. This period also allows changes in reporting rates to be captured directly after a referral when higher rates are expected than in the subsequent years. Furthermore, levels of AE reporting may also vary in different healthcare systems.
In this database analysis all available data on severe HSRs captured by the MedDRA® Preferred Terms anaphylactic/anaphylactoid reaction and shock in the EEA countries plus Switzerland were considered. For the majority of countries, both products are available for treatment of iron deficiency. However, in nine out of the 27 European countries only one product (either ferric carboxymaltose in France, Czech Republic, Belgium, Hungary, Italy, Portugal and Switzerland or iron (III) isomaltoside 1000 in Estonia and Latvia) is on the market. Therefore, an impact of differential marketing on the level of the reporting rate cannot be ruled out, but is considered to be minor.
With respect to the estimated exposure based on sales data, the following considerations should also be addressed. The MIDAS database does not capture all sales data such as direct sales to clinics and private offices within each country. In some countries, not all distribution channels (hospital/retail) are captured in the MIDAS database. For example, hospital data are not available for Estonia, Greece and Latvia. Although exposure in some European countries may be underestimated, which could lead to an overestimation of the rate of severe HSRs with respect to exposure, this does apply for both iron products. For the majority of the major markets for each product both channels are in fact covered in the sales data, therefore the impact on the results can be neglected.
Among the limitations of comparative studies sourced from pharmacovigilance data are the challenges related to confounding. As in this study controlling for confounding could not be done, the direct comparison providing a reporting rate ratio does not give definitive insight on differences between the safety profile of both iron products. While we could not control for confounding in this study, the magnitude of difference in frequency of reported severe HSRs offers reassurance in the results of this study.
Despite the limitations of basing this analysis on AE reports and using sales data as a proxy for exposure, a notable difference in the reporting rate for the two i.v. iron preparations was found. One reason that might add to the difference between the two products is that reporting tends to be less frequent for more widely used products. However, this potential difference in safety characteristics is in line with other published results. This raises the question whether this is related to inherent differences in the substances themselves.
Both compounds contain a core of iron-hydroxide encased in a carbohydrate shell or complex that serves to stabilize the core and regulate the speed of iron release. It has been observed that core size and carbohydrate chemistry influence pharmacological and biological differences between iron formulations [29]. The unique composition of iron-carbohydrate complexes impacts their stability, size, shape and surface charge. The metabolic pathway of those complexes is determined by the carbohydrate shells, resulting in potential changes in pharmacokinetics and pharmacodynamics, further leading to a potential interaction with the innate immune system [30]. Intravenous iron formulations differ in terms of the size of the core, their composition and density of the surrounding carbohydrate shell resulting in potential variations regarding efficacy and tolerability [31,32,33]. They can be classified as non-dextran-derived and dextran-derived complexes depending on their carbohydrate shell. In non-dextran-derived complexes, such as ferric carboxymaltose, a higher molecular weight leads to a more stable complex and lower labile iron release [29, 34]. In dextran-derived complexes, however, there is no correlation between complex stability and molecular weight [29, 34, 35]. As intravenous dextran was known to cause severe anaphylaxis, new intravenous iron preparations were developed consisting of dextran-free carbohydrate shells [36, 37]. Although newer intravenous iron formulations such as ferric carboxymaltose and iron (III) isomaltoside 1000 are considered to be much safer than earlier generations of iron products [14], it may be that the molecular structure, carbohydrate complexes of i.v. iron-containing products as well as the dextran heritage of iron (III) isomaltoside 1000 [38] impacts immunogenicity, resulting in differing occurrences of hypersensitivity reactions.
5 Conclusion
The findings of this pharmacoepidemiological study suggest that iron (III) isomaltoside 1000 is associated with a higher reporting rate of severe HSRs related to estimated exposure than ferric carboxymaltose in European countries. Future research investigating the occurrence of severe HSRs associated with these i.v. iron products is needed to broaden the evidence for benefit-risk assessment.
References
Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602.
Benoist Bd. Worldwide prevalence of anaemia 1993–2005 of: WHO Global Database of anaemia. Geneva: World Health Organization; 2008.
World Health Organization. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. WHO/NHD/01.3: World Health Organization. https://www.facebook.com/WHO; 2001 [cited 2018 Sep 28]. Available from: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/.
Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59.
Ford DC, Dahl NV, Strauss WE, Barish CF, Hetzel DJ, Bernard K, et al. Ferumoxytol versus placebo in iron deficiency anemia: efficacy, safety, and quality of life in patients with gastrointestinal disorders. Clin Exp Gastroenterol. 2016;9(k.A.):151–62.
Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943.
Bahrainwala J, Berns JS. Diagnosis of iron-deficiency anemia in chronic kidney disease. Semin Nephrol. 2016;36(2):94–8.
van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68(6):2846–56.
Sunder-Plassmann G, Hörl WH. Importance of iron supply for erythropoietin therapy. Nephrol Dial Transplant. 1995;10(11):2070–6.
Ahsan N. Intravenous infusion of total dose iron is superior to oral iron in treatment of anemia in peritoneal dialysis patients: a single center comparative study. J Am Soc Nephrol. 1998;9(4):664–8.
EMA CHMP. Assessment report for: Iron containing intravenous (IV) medicinal products EMA/549569/2013; 2013 [cited 2018 Aug 8]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500150771.pdf.
Hussain I, Bhoyroo J, Butcher A, Koch TA, He A, Bregman DB. Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia. 2013;2013:169107.
Girelli D, Ugolini S, Busti F, Marchi G, Castagna A. Modern iron replacement therapy: clinical and pathophysiological insights. Int J Hematol. 2018;107(1):16–30.
Auerbach M, Macdougall I. The available intravenous iron formulations: history, efficacy, and toxicology. Hemodial Int. 2017;21(Suppl 1):S83–92.
Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–6.
encepp.eu. Intravenous Iron Postauthorisation Safety Study (PASS): Evaluation of the Risk of Severe Hypersensitivity Reactions; 2018 [cited 2018 Oct 1]. Available from: http://www.encepp.eu/encepp/viewResource.htm?id=24010.
Bailie GR, Horl WH, Verhoef J-J. Differences in spontaneously reported hypersensitivity and serious adverse events for intravenous iron preparations: comparison of Europe and North America. Arzneimittelforschung. 2011;61(5):267–75.
Wang C, Graham DJ, Kane RC, Xie D, Wernecke M, Levenson M, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314(19):2062–8.
Theophile H, Laporte J-R, Moore N, Martin K-L, Begaud B. The case-population study design: an analysis of its application in pharmacovigilance. Drug Saf. 2011;34(10):861–8.
IQVIA Commercial GmbH & Co. OHG. ACTS 31st Edition: IQVIA Quality Assurance; 2018 [cited 2018 Nov 27]. Available from: https://www.iqvia.com/library/publications/acts-2017-31st-edition.
Bailie GR, Clark JA, Lane CE, Lane PL. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20(7):1443–9.
European Medicines Agency. EudraVigilance system overview [cited 2018 Nov 27]. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview.
Uppsala Monitoring Centre. Vigibase FAQs [cited 2018 Nov 27]. Available from: https://www.who-umc.org/vigibase/vigibase/know-more-about-vigibase/.
Aksan A, Isik H, Radeke HH, Dignass A, Stein J. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1303–18.
Bager P, Hvas CL, Dahlerup JF. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br J Clin Pharmacol. 2017;83(5):1118–25.
Mulder MB, van den Hoek HL, Birnie E, van Tilburg AJP, Westerman EM. Comparison of hypersensitivity reactions of intravenous iron: iron isomaltoside-1000 (Monofer®) versus ferric carboxy-maltose (Ferinject®). A single center, cohort study. Br J Clin Pharmacol. 2018. https://doi.org/10.1111/bcp.13805.
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Moulis G, Sailler L, Sommet A, Lapeyre-Mestre M, Montastruc J-L. Exposure to inhibitors of the renin-angiotensin system is a major independent risk factor for acute renal failure induced by sucrose-containing intravenous immunoglobulins: a case-control study. Pharmacoepidemiol Drug Saf. 2012;21(3):314–9.
Jahn MR, Andreasen HB, Fütterer S, Nawroth T, Schünemann V, Kolb U, et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm. 2011;78(3):480–91.
Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–94.
Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141(3):846–853.e1–2.
Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J Am Soc Nephrol. 2004;15(Suppl 2):S93–8.
Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am J Hematol. 2008;83(7):580–8.
Neiser S, Rentsch D, Dippon U, Kappler A, Weidler PG, Göttlicher J, et al. Physico-chemical properties of the new generation IV iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. Biometals. 2015;28(4):615–35.
Anderson GJ, Wang F. Essential but toxic: controlling the flux of iron in the body. Clin Exp Pharmacol Physiol. 2012;39(8):719–24.
Richter AW, Hedin HI. Dextran hypersensitivity. Immunol Today. 1982;3(5):132–8.
Funk F, Ryle P, Canclini C, Neiser S, Geisser P. The new generation of intravenous iron: chemistry, pharmacology, and toxicology of ferric carboxymaltose. Arzneimittelforschung. 2010;60(6a):345–53.
Heads of Medicines Agencies. Public Assessment Report Scientific discussion Monofer 100 mg/ml solution for injection/infusion (iron(III) isomaltoside 1000): SE/H/734/01/DC; 2009 [cited 2018 Oct 11]. Available from: https://docetp.mpa.se/LMF/Monofer%20solution%20for%20injection%20or%20infusion%20ENG%20PAR_09001be68045522a.pdf.
Acknowledgements
The authors thank Prof. Aryeh Shander and Prof. Andreas Bircher for reviewing the manuscript and providing input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Vifor Pharma International AG.
Conflict of interest
Stefan Wohlfeil is an employee of Vifor Pharma Management Ltd. Lennart Nathell is a former employee of Vifor Pharma, and during the planning, execution and publishing of the study was working for a company on a contract with Vifor Pharma Management Ltd. Vifor Pharma is the manufacturer for Ferinject® (ferric carboxymaltose). Birgit Ehlken, Annegret Gohlke, Derya Bocuk and Massoud Toussi are employees of IQVIA which received funding from Vifor Pharma International AG for the conduct of the study.
Additional information
Data derived from VigiBase™: The World Health Organization (WHO) indicates that as the information comes from a variety of sources, the likelihood that the suspected adverse reaction is drug-related is not the same in all cases, and that the information does not represent the opinion of the WHO.
Data derived from EudraVigilance: The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ehlken, B., Nathell, L., Gohlke, A. et al. Evaluation of the Reported Rates of Severe Hypersensitivity Reactions Associated with Ferric Carboxymaltose and Iron (III) Isomaltoside 1000 in Europe Based on Data from EudraVigilance and VigiBase™ between 2014 and 2017. Drug Saf 42, 463–471 (2019). https://doi.org/10.1007/s40264-018-0769-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0769-5