Abstract
Background
The association between antidepressant exposure and type 2 diabetes mellitus is still debated. Moreover, the pharmacological mechanisms remain unknown.
Objective
The objective of this study was to investigate this putative relationship with the role of antidepressant pharmacological targets using the ‘pharmacoepidemiological–pharmacodynamic’ method.
Methods
First, we performed case/non-case analyses in VigiBase® (the World Health Organization international database of suspected adverse drug reactions) to examine a signal of increased type 2 diabetes reporting (expressed as the reporting odds ratio and its 95% confidence interval) for antidepressants in general; examine and rank type 2 diabetes signals between the different pharmacological classes of antidepressants and the different antidepressants (58 in total). Second, we performed linear regression analyses to explore the association between the type 2 diabetes signal ranked between antidepressants and their binding affinities for nine targets (serotonin, norepinephrine, dopamine transporters, 5-HT2C serotonin, D2 dopamine, α1, α2 adrenergic, M3 muscarinic and H1 histamine receptors).
Results
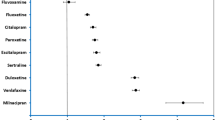
A significant type 2 diabetes signal was found for antidepressants in general, three classes of antidepressants (tricyclic antidepressants, serotonin reuptake inhibitors and “other” antidepressants) and 15 individual antidepressants in particular. Among the antidepressants, three serotonin reuptake inhibitors [escitalopram (adjusted reporting odds ratio 1.15 [1.07–1.25]), paroxetine (1.15 [1.07–1.23]), sertraline (1.23 [1.17–1.31])] and three “other” antidepressants [duloxetine (1.15 [1.07–1.23]), trazodone (1.20 [1.09–1.32]), venlafaxine (1.15 [1.08–1.23])] were the antidepressants most frequently reported with type 2 diabetes. We found a significant correlation between the type 2 diabetes signal and serotonin transporter affinity (slope = 0.14 [0.06–0.23], p = 0.003, R2 = 0.43) but not the other targets.
Conclusion
The present study suggests a potential role for serotonin transporter in antidepressant-induced type 2 diabetes.


Similar content being viewed by others
References
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–30.
Reid S, Barbui C. Long term treatment of depression with selective serotonin reuptake inhibitors and newer antidepressants. BMJ. 2010;340:c1468.
Frisard C, Gu X, Whitcomb B, Ma Y, Pekow P, Zorn M, et al. Marginal structural models for the estimation of the risk of diabetes mellitus in the presence of elevated depressive symptoms and antidepressant medication use in the Women’s Health Initiative observational and clinical trial cohorts. BMC Endocr Disord. 2015;15:56.
Pan A, Sun Q, Okereke OI, Rexrode KM, Rubin RR, Lucas M, et al. Use of antidepressant medication and risk of type 2 diabetes: results from three cohorts of US adults. Diabetologia. 2012;55:63–72.
Pérez-Piñar M, Mathur R, Foguet Q, Ayis S, Robson J, Ayerbe L. Cardiovascular risk factors among patients with schizophrenia, bipolar, depressive, anxiety, and personality disorders. Eur Psychiatry. 2016;35:8–15.
Wu C-S, Gau SS-F, Lai M-S. Long-term antidepressant use and the risk of type 2 diabetes mellitus: a population-based, nested case–control study in Taiwan. J Clin Psychiatry. 2014;75:31–8 (quiz 38).
Bhattacharya R, Ajmera M, Bhattacharjee S, Sambamoorthi U. Use of antidepressants and statins and short-term risk of new-onset diabetes among high risk adults. Diabetes Res Clin Pract. 2014;105:251–60.
Knol MJ, Geerlings MI, Egberts ACG, Gorter KJ, Grobbee DE, Heerdink ER. No increased incidence of diabetes in antidepressant users. Int Clin Psychopharmacol. 2007;22:382–6.
Sambamoorthi U, Ma Y, Findley PA, Rust G. Antidepressant use, depression, and new-onset diabetes among elderly Medicare beneficiaries. J Diabetes. 2013;5:327–35.
McIntyre RS, Soczynska JK, Konarski JZ, Kennedy SH. The effect of antidepressants on glucose homeostasis and insulin sensitivity: synthesis and mechanisms. Expert Opin Drug Saf. 2006;5:157–68.
Stahl SM, Grady MM. Stahl’s essential psychopharmacology: the prescriber’s guide. 4th ed. Cambridge: Cambridge University Press; 2011.
Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–26.
Montastruc F, Palmaro A, Bagheri H, Schmitt L, Montastruc J-L, Lapeyre-Mestre M. Role of serotonin 5-HT2C and histamine H1 receptors in antipsychotic-induced diabetes: a pharmacoepidemiological-pharmacodynamic study in VigiBase. Eur Neuropsychopharmacol. 2015;25:1556–65.
Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, et al. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature. 1995;374:542–6.
Nakajima K, Jain S, Ruiz de Azua I, McMillin SM, Rossi M, Wess J. Minireview: novel aspects of M3 muscarinic receptor signaling in pancreatic β-cells. Mol Endocrinol. 2013;27:1208–16.
Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman and Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill; 2011.
Nguyen TTH, Pariente A, Montastruc J-L, Lapeyre-Mestre M, Rousseau V, Rascol O, et al. An original pharmacoepidemiological-pharmacodynamic method: application to antipsychotic-induced movement disorders. Br J Clin Pharmacol. 2017;83:612–22.
Bate A, Lindquist M, Edwards IR. The application of knowledge discovery in databases to post-marketing drug safety: example of the WHO database. Fundam Clin Pharmacol. 2008;22:127–40.
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409–19.
Uppsala Monitoring Centre. https://signin.who-umc.org. Accessed 1 June 2018.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index. https://www.whocc.no/atc_ddd_index. Accessed 1 June 2018.
International Federation of Pharmaceutical Manufacturers and Associations. Introductory guide for standardised MedDRA queries (SMQs). https://www.meddra.org/how-to-use/support-documentation. Accessed 1 June 2018.
Faillie J-L. Case–non case studies: principles, methods, bias and interpretation (in French). Therapie. 2018;73(3):247–55.
Montastruc J-L, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72:905–8.
Hahn M, Chintoh A, Giacca A, Xu L, Lam L, Mann S, et al. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr Res. 2011;131:90–5.
Sim Y-B, Park S-H, Kang Y-J, Kim S-M, Lee J-K, Jung J-S, et al. The regulation of blood glucose level in physical and emotional stress models: possible involvement of adrenergic and glucocorticoid systems. Arch Pharm Res. 2010;33:1679–83.
British Pharmacological Society (BPS) and International Union of Basic Clinical Pharmacology (IUPHAR). The IUPHAR/BPS guide to pharmacology. https://www.guidetopharmacology.org. Accessed 1 June 2018.
Psychoactive Drug Screening Program Ki Database. https://pdsp.unc.edu/databases/kidb.php. Accessed 1 June 2018.
The Metabolomics Innovation Centre. DrugBank. https://www.drugbank.ca. Accessed 1 June 2018.
Rehman A, Setter SM, Vue MH. Drug-induced glucose alterations part 2: drug-induced hyperglycemia. Diabetes Spectr. 2011;24:234–8.
Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Investig. 2017;40:1165–74.
Bhattacharjee S, Bhattacharya R, Kelley GA, Sambamoorthi U. Antidepressant use and new-onset diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2013;29:273–84.
Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74:31–7.
Yoon JM, Cho E-G, Lee H-K, Park SM. Antidepressant use and diabetes mellitus risk: a meta-analysis. Korean J Fam Med. 2013;34:228–40.
Salvi V, Grua I, Cerveri G, Mencacci C, Barone-Adesi F. The risk of new-onset diabetes in antidepressant users: a systematic review and meta-analysis. PLoS One. 2017;12(7):e0182088.
Derijks HJ, Meyboom RHB, Heerdink ER, De Koning FHP, Janknegt R, Lindquist M, et al. The association between antidepressant use and disturbances in glucose homeostasis: evidence from spontaneous reports. Eur J Clin Pharmacol. 2008;64:531–8.
Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166:591–8.
Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–72.
Tran Y-H, Schuiling-Veninga CCM, Bergman JEH, Groen H, Wilffert B. Impact of muscarinic M3 receptor antagonism on the risk of type 2 diabetes in antidepressant-treated patients: a case-controlled study. CNS Drugs. 2017;31:483–93.
Sugimoto Y, Inoue K, Yamada J. Involvement of serotonin in zimelidine-induced hyperglycemia in mice. Biol Pharm Bull. 1999;22:1240–1.
Salvi V, Mencacci C, Barone-Adesi F. H1-histamine receptor affinity predicts weight gain with antidepressants. Eur Neuropsychopharmacol. 2016;26:1673–7.
Bascuñan LRC, Lyons C, Bennet H, Artner I, Fex M. Serotonergic regulation of insulin secretion. Acta Physiol (Oxf). 2018;23:e13101.
Patras de Campaigno E, Bondon-Guitton E, Laurent G, Montastruc F, Montastruc JL, Lapeyre-Mestre M, et al. Identification of cellular targets involved in cardiac failure caused by PKI in oncology: an approach combining pharmacovigilance and pharmacodynamics. Br J Clin Pharmacol. 2017;83:1544–55.
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29:385–96.
Bezin J, Bosco-Levy P, Pariente A. False-positive results in pharmacoepidemiology and pharmacovigilance. Therapie. 2017;72:415–20.
Acknowledgements
The authors thank the Uppsala Monitoring Centre in general, and in particular Ms. Camilla Westerberg, research pharmacist, and the VigiBase® custom search services team for providing and giving permission to use the VigiBase® data. Despite the use of the World Health Organization database, the present study results and conclusions are those of the authors and not necessarily those of the Uppsala Monitoring Centre, National Centres or the World Health Organization.
Author information
Authors and Affiliations
Contributions
Thi Thu Ha Nguyen, Anne Roussin, Vanessa Rousseau, Jean-Louis Montastruc and François Montastruc designed the study and wrote the protocol. Thi Thu Ha Nguyen performed the experiments. Thi Thu Ha Nguyen and Vanessa Rousseau analysed the data. Thi Thu Ha Nguyen, Anne Roussin, Vanessa Rousseau, Jean-Louis Montastruc and François Montastruc contributed to the interpretation of the data. Thi Thu Ha Nguyen wrote the first draft of the manuscript and Anne Roussin, Vanessa Rousseau, Jean-Louis Montastruc and François Montastruc critically revised the manuscript for important intellectual content. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Funding
Thi Thu Ha Nguyen was supported by a research grant for her PhD (of which this study is a part) from the Pierre Fabre Foundation, France and the French Embassy in Hanoi, Vietnam.
Conflict of interest
Thi Thu Ha Nguyen, Anne Roussin, Vanessa Rousseau, Jean-Louis Montastruc and François Montastruc have no conflicts of interest that are directly relevant to the content of this study.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, T.T.H., Roussin, A., Rousseau, V. et al. Role of Serotonin Transporter in Antidepressant-Induced Diabetes Mellitus: A Pharmacoepidemiological–Pharmacodynamic Study in VigiBase®. Drug Saf 41, 1087–1096 (2018). https://doi.org/10.1007/s40264-018-0693-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0693-8