Abstract
Introduction
Electronic healthcare record (EHR) databases are used within pharmacoepidemiology studies to confirm or refute safety signals arising from spontaneous adverse event reports. However, there has been limited routine use of such data earlier in the signal management process, to help rapidly contextualise signals and strengthen preliminary assessment or to inform decisions regarding action including the need for further studies. This study explores the value of EHR used in this way within a regulatory environment via an automated analysis platform.
Methods
Safety signals raised at the UK Medicines and Healthcare products Regulatory Agency (MHRA) between July 2014 and June 2015 were individually reviewed by a multi-disciplinary team. They assessed the feasibility of identifying the exposure and event of interest using primary care data from the Clinical Practice Research Datalink (CPRD) within the Commonwealth Vigilance Workbench (CVW) Longitudinal Module platform, which was designed to facilitate routine descriptive analysis of signals using EHR. Three signals, where exposure and event could be well identified, were retrospectively analysed using the platform.
Results
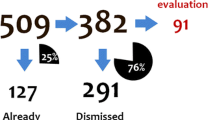
Of 69 unique new signals, 20 were for drugs prescribed predominately in secondary care or available without prescription, which would not be identified in primary care. A further 17 were brand, formulation, or dose-specific issues, were related to mortality, were relevant only to a subgroup of patients, or were drug interactions, and hence could not be reviewed using the platform given its limitations. Analyses of exposure and incidence of the adverse event could be produced using CPRD within the CMV Longitudinal Module for 32 (46%) signals. The case studies demonstrated that the data provided supporting evidence for confirming initial assessment of the signal and deciding upon the need for further action.
Conclusions
CPRD can routinely provide useful early insights into clinical context when assessing a large proportion of safety signals within a regulatory environment provided that a flexible approach is adopted within the analysis platform.




Similar content being viewed by others
References
Foy M, Barrow P, Raine JM. Spontaneous reporting: United Kingdom. In: Andrews EB, Moore N, editors. Mann’s pharmacovigilance. Oxford: Wiley; 2014.
Dal Pan GJ, Lindquist M, Gelperin K. Postmarketing spontaneous pharmacovigilance reporting systems. In: Pharmacoepidemiology. Oxford: Wiley; 2012.
Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36.
Candore G, Juhlin K, Manlik K, Thakrar B, Quarcoo N, Seabroke S, et al. Comparison of statistical signal detection methods within and across spontaneous reporting databases. Drug Saf. 2015;38:577–87.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30(10):891–8.
Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32:19–31.
Ogdie A, Langan SM, Parkinson J, Dattani H, Kostev K, Gelfrand JM. Medical record databases. In: Pharmacoepidemiology. Wiley; 2012.
Smeeth L, Cook C, Fombonne E, Heavey L, Rodrigues LC, Smith PG, Hall AJ. MMR vaccination and persuasive developmental disorders: a case-control study. Lancet. 2004;365:963–9.
Oteri A, Mazzaglia G, Pecchioli S, Molokhia M, Ulrichson SP, Pedersen L, et al. Prescribing pattern of antipsychotic drugs during the years 1996–2010: a population-based database study in Europe with a focus on torsadogenic drugs. Br J Clin Pharmacol. 2016;82:487–97.
Douglas I, Evans S, Rawlins MD, Smeeth L, Tabrizi SJ, Wexler NS. Juvenile Huntington’s disease: a population-based study using the General Practice Research Database. BMJ Open. 2013;3:e002085.
Donegan K, Beau-Lejdstrom R, King B, Seabroke S, Thomson A, Bryan P. Bivalent human papillomavirus vaccine and the risk of fatigue syndromes in girls in the UK. Vaccine. 2013;31:4961–7.
Mahaux O, Bauchau V, Van Holle L. Pharmacoepidemiological considerations in observed-to-expected analyses for vaccines. Pharmacoepidemiol Drug Saf. 2016;25:215–22.
Star K, Watson S, Sandberg L, Johansson J, Edwards IR. Longitudinal medical records as a complement to routine drug safety signal analysis. Pharmacoepidemiol Drug Saf. 2015;24:486–94.
Ball R, Robb M, Anderson SA, Dal Pan G. The FDA’s Sentinel initiative—a comprehensive approach to medical product surveillance. Clin Pharmacol Ther. 2016;99:1532–5.
Pacurariu AC, Straus SM, Trifirò G, Schuemie MJ, Gini R, Herings R, et al. Useful interplay between spontaneous ADR reports and electronic healthcare records in signal detection. Drug Saf. 2015;38:1201–10.
Guideline on good pharmacovigilance practices (GVP) Annex 1—Definitions (Rev 4). European Medicines Agency, 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143294.pdf. Accessed 8 Jan 2018.
Guideline on good pharmacovigilance practices (GVP) Module IX—Signal Management (Rev 1). European Medicines Agency, 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/10/WC500236408.pdf. Accessed 8 Jan 2018.
A guideline on summary of product characteristics (SmPC). European Commission, September 2009. Available at: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf. Accessed 8 Jan 2018.
Herret E, Gallagher AM, Bhaskeran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44:827–36.
ISAC protocol 15_217: Descriptive characteristics of the Clinical Practice Research Dataklink (CPRD) EMIS database. Available at: https://www.cprd.com/isac/Protocol_15_217.asp. Accessed 24 July 2017.
Norén N, Hopstadius J, Bate A, Star K, Edwards IR. Temporal pattern discovery in longitudinal electronic patient records. Data Min Knowl Disc. 2010;20:361–87.
Cederholm S, Hill G, Asiimwe A, Bate A, Bhayat F, Persson Brobert G, et al. Structured assessment for prospective identification of safety signals in electronic medical records: evaluation in The Health Improvement Network. Drug Saf. 2015;38:87–100.
Trifiro G, Pariente A, Coloma PM, Kors JA, Polimeni G, Miremont-Salame G, et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: which events to monitor? Pharmacoepidemiol Drug Saf. 2009;18:1176–84.
National Institute for Clinical Excellence, Social Care Institute for Excellence. Dementia: Supporting People with Dementia and Their Carers in Health and Social Care. NICE/SCIE, 2006.
Bate A, Brown EG, Goldman SA, Hauben M. Terminological challenges in safety surveillance. Drug Saf. 2012;35:79–84.
Acknowledgements
The authors would like to thank Olutoyin Agbaje, Claire Davies, Temitope Ibitoye, and Jenny Wong for the comments they provided within early stages of the pilot and on the output from the three case studies, and Arlene Gallagher for providing additional expertise on the use of CPRD data.
Author information
Authors and Affiliations
Contributions
The study was planned and led by KD, PT and RO. Preliminary screening of the DECs included was conducted by PT, RO and BB, with additional assessment support from KD, HB and AS for the second stage of the review. The three case studies were conducted by HB, BB and AS with input from KD. The paper was drafted by KD with contributions from all authors.
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Access to the CVW Longitudinal Module was provided by Commonwealth Informatics Inc. The CVW Longitudinal software is engineered by Commonwealth Informatics, Inc., and builds on research performed by the Uppsala Monitoring Centre.
Conflict of interest
At the time of the study, all authors (Katherine Donegan, Rebecca Owen, Helena Bird, Brian Burch, Alex Smith, and Phil Tregunno) were employed by the MHRA, which is an Executive Agency of the Department of Health, but have no conflicts of interest that are directly relevant to the contents of this study. The MHRA has statutory responsibility to monitor the safety of medicinal products on the UK market, and the study was undertaken independently of the Department of Health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Donegan, K., Owen, R., Bird, H. et al. Exploring the Potential Routine Use of Electronic Healthcare Record Data to Strengthen Early Signal Assessment in UK Medicines Regulation: Proof-of-Concept Study. Drug Saf 41, 899–910 (2018). https://doi.org/10.1007/s40264-018-0675-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0675-x