Abstract
Introduction
Post-marketing surveillance activities are particularly important for safety issues in children, the elderly, and patients with severe comorbidities since these populations are usually excluded from clinical trials. In addition, using electronic databases for monitoring of safety of marketed products has been of considerable interest.
Objectives
This study aimed to clarify the advantages and difficulties of the self-controlled case series method relative to cohort studies in pharmacoepidemiological studies in children, using an administrative database, and to explore the impact on results of handling the period eligible for analysis and recurrent events in different ways.
Methods
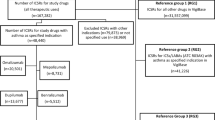
Datasets of only individuals who had the outcome of interest were derived from an anonymized hospital administrative database in Japan from April 2003 through August 2011. We calculated incidence rate ratios (IRRs) and their 95 % confidence intervals (CIs) for the risks of diarrhea, bronchitis, and eczema related to palivizumab treatment in young children. The analysis included ‘first diagnosed’ events or ‘multiple’ events during an eligible period. An eligible period was defined in two ways: first-time inpatient periods of more than 3 continuous days (EPA); and a continuous period in cases where the interval between visits was below the 75th percentile of the interval between visits for patients with the same diagnosis (EPB).
Results
We extracted data for 70,771 patients and identified 641 who were exposed to palivizumab. The age-adjusted IRRs for diarrhea, bronchitis, and eczema were 3.0 (95 % CI 1.7–5.4), 10.3 (95 % CI 8.0–13.2), and 16.9 (95 % CI 12–23), respectively, in multiple events and the EPB eligible period. The IRRs varied greatly between the two eligible periods.
Conclusions
This method could be a useful tool in pharmacoepidemiological studies in children. Careful consideration in the handling of inpatient and outpatient periods, including sensitivity analyses, is necessary because this method is a within-individual comparison.



Similar content being viewed by others
References
Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR project. Pharmacoepidemiol Drug Saf. 2011;20(1):1–11.
Linder JA, Haas JS, Iyer A, et al. Secondary use of electronic health record data: spontaneous triggered adverse drug event reporting. Pharmacoepidemiol Drug Saf. 2010;19(12):1211–5.
Bello A, Hemmelgarn B, Manns B, Tonelli M, for Alberta Kidney Disease Network. Use of administrative databases for health-care planning in CKD. Nephrol Dial Transplant. 2012; doi:10.1093/ndt/gfs163.
Smoyer Tomic KE, Amato AA, Fernandes AW. Incidence and prevalence of idiopathic inflammatory myopathies among commercially insured, medicare supplemental insured, and medicaid enrolled populations: an administrative claims analysis. BMC Musculoskelet Disord. 2012;13(1):103.
Smith EG, Zhao S, Rosen AK. Using the patient safety indicators to detect potential safety events among US veterans with psychotic disorders: clinical and research implications. Int J Qual Health Care. 2012;24(4):321–9.
Schoonen WM, Thomas SL, Somers EC, et al. Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol. 2010;70(4):588–96.
Gibson JE, Hubbard RB, Smith CJ, Tata LJ, Britton JR, Fogarty AW. Use of self-controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol. 2009;169(6):761–8.
Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25(4):1019–27.
Ministry of Health, Labour and Welfare (MHLW). National database guideline in Japan (in Japanese). Available: http://www.mhlw.go.jp/stf/shingi/2r98520000016v8d-att/2r98520000016vcn.pdf. Accessed Aug 29 2013.
Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20(5):413–9.
Akazawa M, Imai H, Igarashi A, Tsutani K. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010;8(2):146–60.
Hashikata H, Harada KH, Kagimura T, Nakamura M, Koizumi A. Usefulness of a large automated health records database in pharmacoepidemiology. Environ Health Prev Med. 2011;16(5):313–9.
Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008;337:a1227.
Pratt NL, Roughead EE, Ramsay E, Salter A, Ryan P. Risk of hospitalization for stroke associated with antipsychotic use in the elderly: a self-controlled case series. Drugs Aging. 2010;27(11):885–93.
Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–97.
The IMpact-RSV study group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(3 Pt 1):531–7.
Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–40.
Maclure M, Fireman B, Nelson JC, et al. When should case-only designs be used for safety monitoring of medical products? Pharmacoepidemiol Drug Saf. 2012;21(S1):50–61.
Conflicts of interest
This work was supported by a 2011 Research Grant from Pfizer Health Research Foundation (10-8-043) http://www.pfizer-zaidan.jp/. This report was co-authored by academic researchers and Medical Data Vision Co., Ltd (MDV). HU, SH, and KK have no conflicts of interest regarding MDV. Hanae Ueyama, Shiro Hinotsu, Shiro Tanaka, Hisashi Urushihara, Masaki Nakamura, Yuji Nakamura and Koji Kawakami have no conflicts of interest with any other organization related to the subject of this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueyama, H., Hinotsu, S., Tanaka, S. et al. Application of a Self-Controlled Case Series Study to a Database Study in Children. Drug Saf 37, 259–268 (2014). https://doi.org/10.1007/s40264-014-0148-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0148-9