Abstract
Background
The management of uncontrolled severe asthma has greatly improved since the advent of novel biologic therapies. Up to August 2022, five biologics have been approved for the type 2 asthma phenotype: anti-IgE (omalizumab), anti-IL5 (mepolizumab, reslizumab, benralizumab), and anti-IL4 (dupilumab) monoclonal antibodies. These drugs are usually well tolerated, although long-term safety information is limited, and some adverse events have not yet been fully characterized. Spontaneous reporting systems represent the cornerstone for the detection of potential signals and evaluation of the real-world safety of all marketed drugs.
Objective
The aim of this study was to provide an overview of safety data of biologics for severe asthma using VigiBase, the World Health Organization global pharmacovigilance database.
Methods
We selected all de-duplicated individual case safety reports (ICSRs) attributed to five approved biologics for severe asthma in VigiBase, up to 31st August 2022 (omalizumab, mepolizumab, reslizumab, benralizumab and dupilumab). Descriptive frequency analyses of ICSRs were carried out both as a whole class and as individual products. Reporting odds ratios (ROR) with 95% confidence intervals (CIs) were used as the measure of disproportionality for suspected adverse drug reactions (ADRs) associated with the study drugs compared with either all other suspected drugs (Reference Group 1, RG1) or inhaled corticosteroids plus long-acting β-agonists (ICSs/LABAs) (Reference Group 2, RG2) or with oral corticosteroids (OCSs) (Reference Group 3, RG3).
Results
Overall, 31,724,381 ICSRs were identified in VigiBase and 167,282 (0.5%) were related to study drugs; the remaining reports were considered as RG1. Stratifying all biologic-related ICSRs by therapeutic indication, around 29.4% (n = 48,440) concerned asthma use; omalizumab was mainly indicated as the suspected drug (n = 20,501), followed by dupilumab, mepolizumab, benralizumab and reslizumab. Most asthma ICSRs concerned adults (57%) and women (64.1%). Asthma biologics showed a higher frequency of serious suspected ADR reporting than RG1 (41.3% vs 32.3%). The most reported suspected ADRs included asthma, dyspnea, product use issue, drug ineffective, cough, headache, fatigue and wheezing. Asthma biologics were disproportionally associated with several unknown or less documented adverse events, such as malignancies, pulmonary embolism and deep vein thrombosis with omalizumab; alopecia and lichen planus with dupilumab; alopecia and herpes infections with mepolizumab; alopecia, herpes zoster and eosinophilic granulomatosis with polyangiitis related to benralizumab; and alopecia with reslizumab.
Conclusions
The most frequently reported suspected ADRs of asthma biologics in VigiBase confirmed the presence of well-known adverse effects such as general disorders, injection-site reactions, nasopharyngitis, headache and hypersensitivity, while some others (e.g. asthma reactivation or therapeutic failure) could be ascribed to the indication of use. Moreover, the analysis of signals of disproportionate reporting suggests the presence of malignancies, effects on the cardiovascular system, alopecia and autoimmune conditions, requiring further assessment and investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most reported suspected adverse reactions related to biologics in asthma patients are known side effects, while some others could derive from the underlying indication of use (asthma reactivation or therapeutic failure). |
A confounding effect is exerted by corticosteroids that are often used concomitantly or immediately before starting biologic treatment. |
Several potential safety signals (e.g. malignancies, rhythm disorders, pulmonary embolism, alopecia, etc.) have been identified, requiring further investigation. Autoimmune conditions may be triggered in patients that are already affected by autoimmune diseases (e.g. eosinophilic granulomatosis with polyangiitis, sarcoidosis). |
1 Introduction
Biologics for severe asthma, targeting specific steps of T-helper cell 2 (Th2) immune inflammation, represent a revolutionary treatment option for asthma management. When asthma diagnosis is confirmed and comorbidities have been addressed (e.g. rhinitis, rhinosinusitis, nasal polyps, gastroesophageal reflux, obstructive sleep apnea), severe asthma is defined as “asthma which requires treatment with high dose inhaled corticosteroids (ICSs) plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy.” Uncontrolled asthma is associated with high rates of exacerbations and glucocorticoid dependence [1]. The evaluation of severe asthma prevalence is still debated; in literature, the estimated frequency ranges from 1.8 to 38.9% among all asthma patients [2, 3]. These values are affected by different factors [2]. One aspect to evaluate in the management of severe asthma, especially for uncontrolled asthma, is the burden of the disease due to asthma exacerbations, asthma-related emergency room admissions and hospitalizations [3].
Currently available biologics act on Th2/eosinophilic phenotype molecules, which improve the quality of life of patients with severe asthma, achieving disease control and reducing/stopping oral steroid dependence [4, 5]. Type 2 biologics for severe asthma target key points of the type 2 inflammation including immunoglobulin E (IgE) (omalizumab), interleukin 5 (IL-5) (mepolizumab, reslizumab) or its receptor (benralizumab), thymic stromal lymphopoietin (TSLP) (tezepelumab) and interleukin 4 receptor alpha subunit (IL4rα) (dupilumab), which blocks signaling cascades induced by both IL-4 and IL-13 [6, 7]. Tezepelumab is a human monoclonal antibody (mAb) to thymic stromal lymphopoietin that was approved for severe uncontrolled asthma in patients aged 12 years and older at the end of 2022 [7]. Omalizumab, mepolizumab, benralizumab, dupilumab and tezepelumab are currently approved by the US Food and Drug Administration for use in pediatric asthma. Omalizumab, mepolizumab and dupilumab are currently approved by both the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) for use in children (over 6 years of age) and/or adolescents, while benralizumab is only approved by the FDA for adolescents [6, 7].
Some biologics are authorized for therapeutic indications other than asthma, including atopic dermatitis (dupilumab), chronic spontaneous urticaria (omalizumab), chronic rhinosinusitis with nasal polyps (dupilumab, omalizumab, mepolizumab), eosinophilic esophagitis (dupilumab) and eosinophilic granulomatosis with polyangiitis [EGPA], or hypereosinophilic syndrome (mepolizumab).
Despite extensive clinical experience on the use of these biologics in asthma patients, some fundamental aspects must still be defined. These include the optimal duration of therapy, for which presently there are no precise indications in literature, as well as long-term effects even after discontinuation.
The use of biologics in asthma patients is overall safe. The most commonly reported adverse events (e.g. asthma worsening, nasopharyngitis and headache) in pivotal clinical trials were mild and well tolerated, despite those studies being based on limited sample sizes and short follow-up periods (Table 1) [8,9,10,11,12,13,14,15,16].
In recent years, a few safety alerts on possible risks associated with asthma biologics (ABs) have been issued by regulatory agencies, such as the FDA warning and the Medicines and Healthcare products Regulatory Agency (MHRA) safety alert regarding a slightly higher risk of heart and brain adverse events with omalizumab [17, 18]. A potential safety signal concerning malignancies related to omalizumab, detected in a previous analysis of the World Health Organization (WHO) pharmacovigilance database, is still being debated in the scientific community [19]. In addition, the identified risk of anaphylaxis with omalizumab and reslizumab still needs to be further characterized for other ABs also [20].
Considering that biologics are increasingly used in clinical practice, it is extremely important to better explore the safety of these drugs in real-world settings, especially concerning long-term use and rare adverse events. The aim of this study was to evaluate the post-marketing safety profile of biologics when used in patients with severe asthma using VigiBase, the WHO global pharmacovigilance database.
2 Methods
2.1 Data Source
VigiBase is the WHO global pharmacovigilance database of individual case safety reports (ICSRs), managed by the Uppsala Monitoring Centre (UMC) in Sweden. It holds over 30 million reports of suspected adverse events of medicines (August 2022) submitted, since 1968, by member countries of the WHO Programme for International Drug Monitoring (WHO PIDM). For data extraction, we used VigiLyze, a data warehousing system provided by UMC. We used the de-duplicated dataset automatically calculated by vigiMatch, a probabilistic record matching method [21]. Drugs are encoded with the WHODrug Global dictionary for medicinal information. Suspected adverse drug reactions (ADRs) are coded with the Medical Dictionary for Regulatory Activities (MedDRA, version 25.0) terminology.
2.2 Study Drugs and Data Analysis
Omalizumab, mepolizumab, reslizumab, benralizumab and dupilumab were the study drugs. We selected all de-duplicated ICSRs attributed to study drugs in VigiBase, from its inception date up to 31st August 2022. As such, the new biological drug tezepelumab was not considered as it was introduced into the European market only after the extraction date. Biologic-related ICSRs were stratified based on the different therapeutic indications. Focusing on asthma use, we selected suspected ADR reports in which biologics had a specific asthma-related therapeutic indication, including the following selected PT terms: 'Asthma', 'Asthma late onset', 'Asthma prophylaxis', 'Asthmatic crisis', 'Childhood asthma', 'Status asthmaticus'.
Descriptive frequency analyses of ICSRs in which selected biologics were reported as suspected drugs were carried out for asthma-related therapeutic indications. In particular, age and sex distribution of patients affected by biologic-related adverse events, frequency of seriousness, temporal trend in reporting, different types of reporter and outcomes were all examined. Descriptive comparisons of some ICSR characteristics for asthma use versus all other indications were carried out.
A Chi-square test was performed to compare categorical variables as appropriate. A p-value < 0.05 denoted the statistical significance.
Serious suspected ADRs were defined as adverse events leading to death or persistent/significant disability or incapacity, life-threatening, requiring in-patient hospitalization or prolongation of hospital stay, or congenital malformation/birth defects or other important medical events based on clinical judgment or Important Medical Event (IME) list [22, 23].
A suspected ADR, whose nature, severity, specificity or outcome is not consistent with the term or description used in the FDA labels [24] and in the European Public Assessment Reports (EPARs) and Risk Management Plans (RMPs) [25], has been defined as ‘unexpected’ [22].
Reporting odds ratio (ROR) was used as a measure of suspected ADR reporting disproportionality, with a statistical threshold that was defined as 95% confidence interval lower bound > 1 present in three or more reports [26,27,28,29]. These thresholds are the most frequently used, although the minimum number of cases could be modified depending on different factors, including the database and the drug/event under investigation [27].
When both these criteria were satisfied for a given drug–event combination, it was called a signal of disproportionate reporting (SDR) [30, 31]. The calculation and interpretation of disproportionality findings were performed in accordance with available regulatory guidances [31,32,33].
Specifically, disproportionality analysis was carried out for suspected ADRs reported in ICSRs with a specified asthma-related therapeutic indication at MedDRA PT level for each single study drug. When feasible on the basis of available information, case-by-case assessment for unexpected adverse events was carried out examining ICSR line listings related to the selected SDRs.
RORs were calculated by using ICSRs related to all other drugs collected in VigiBase, vaccines included, (Reference Group 1, RG1) as the primary comparison group. As restricting the comparator background to drugs for common therapeutic areas may be useful to mitigate potential confounders and to evaluate study finding robustness when implemented as a sensitivity analysis [34, 35], we also used as an additional comparison group (Reference Group 2, RG2) ICSRs including ICSs plus long-acting β-agonists (LABAs) (ICSs/LABAs) (Anatomical Therapeutic Chemical class: R03AK) as suspected drugs for asthma-related therapeutic indication.
Considering the large amount of COVID-19 vaccine-related reports received in the last 3 years, to explore the potential masking effect of COVID-19 vaccines on disproportionality analysis of biologics, we conducted a sensitivity analysis removing all vaccine-related reports (almost all of them were COVID-19 vaccine related).
In severe uncontrolled asthma, patients often start on oral corticosteroid (OCS) treatment; whenever needed thereafter, biologics are initiated to replace or reduce OCSs. For this reason, to better explore the adverse events attributable to biologic drugs, irrespective of the concomitant/previous use of OCSs, we conducted an additional sensitivity analysis selecting as comparator ICSRs in which OCSs typically used in asthmatic patients (i.e. betamethasone, deflazacort, methylprednisolone and prednisone) were reported as suspected drugs for asthma-related conditions (RG3). Furthermore, a sensitivity analysis was carried out, after excluding reports in which ABs were co-reported with one of these four OCSs as suspected drugs, to evaluate confounding in RORs derived from primary disproportionality analysis.
3 Results
3.1 General Analysis
Up to 31st August 2022, 31,724,381 de-duplicated ICSRs were collected in VigiBase; among these, 167,282 (0.5%) were related to biologics under study, while the remaining reports were considered as Reference Group 1 (RG1) (n = 31,557,099). Dupilumab was indicated as a suspected drug in 101,297 (60.5%) reports, followed by omalizumab (n = 44,043; 26.3%), mepolizumab (n = 13,909; 8.3%), benralizumab (n = 7853; 4.7%) and reslizumab (n = 475; 0.3%). In 274 reports, two or more ABs were co-reported as suspected/interacting drugs.
Stratifying all biologic-related ICSRs (n = 167,282) by therapeutic indication, around 34% of reports concerned use in atopic dermatitis (n = 56,682), 29.4% (n = 48,440) in asthma, 7% in urticaria (n = 11,495) and 3% in chronic rhinosinusitis with nasal polyps (ChRNP) (n = 4,673). The remaining reports concerned various other indications (n = 7023; 4.2%) or non-specified indications (n = 38,969; 23.3%). An ICSR could have multiple indications reported, but for disproportionality analysis the reports were counted only once (either in the asthma group or in the reference group).
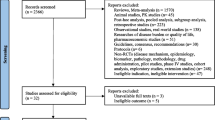
Focusing specifically on asthma-related ICSRs (n = 48,440), omalizumab was mainly reported as the suspected drug (n = 20,501; 42.3%), followed by dupilumab (n = 13,677; 28.2%), mepolizumab (n = 8731; 18.0%), benralizumab (n = 5512; 11.4%) and reslizumab (n = 219; 0.5%) (see flow chart in Fig. 1). Two or more ABs were co-reported as suspected or interacting drugs in 183 reports.
Flow chart for individual case safety report selection process in VigiBase. The sum of the reports by single asthma drug is higher than the total number of reports, since a single report could contain two or more biologic drugs as suspected. ICSRs individual case safety reports, ICSs/LABAs inhaled corticosteroids plus long-acting β-agonists, OCSs oral corticosteroids
Table 2 shows the main characteristics of biologic-related ICSRs in asthma patients as compared with those related to the same drugs for other therapeutic indications, as well as versus all other drugs in VigiBase (RG1). The female/male ratio of biologic-related asthma ICSRs was higher than that related to other therapeutic uses (2.3 vs 1.7) or all other drugs in the database (1.6).
The percentage of serious suspected ADRs with biologic drugs was much higher in asthma use (41.3%) as compared with other indications (15.7%) as well as versus RG1 (32.3%) (p < 0.001).
Table 3 describes the characteristics of biologic-related ICSRs in asthma use by a single biologic agent. Female/male ratio was comparable across all asthma biologics. The age distribution of reports concerning all biologics exhibits slight variations; dupilumab and omalizumab were associated with higher rates of ICSRs in adolescents (aged 12–17 years). Higher frequencies of serious suspected ADRs were documented for omalizumab (57.5%), mepolizumab (47.3%) and reslizumab (44.3%).
The most reported suspected ADRs for all five biologics specifically used in asthma are shown in Table 4. Asthma, dyspnea, product use issue, drug ineffective, cough, headache, fatigue and wheezing were the most frequent suspected adverse reactions included in ICSRs.
In Fig. 2, RORs of biologic-related ICSRs in asthma patients versus other indications of use (Part A) and versus all other drugs in the database (Part B) by System Organ Class (SOC) level are reported. Considering Part A, biologic-related ICSRs in asthma patients were mainly involved in the following SOCs: ‘respiratory disorders’ (Resp), ‘social circumstances’ (SocCi) and ‘cardiac disorders’ (Card). Instead, considering Part B, the SOCs mainly involved in reports of asthma study drugs were ‘respiratory disorders’ (Resp), ‘infection and infestations’ (Infec) and ‘immune system disorders’ (Immun).
RORs of System Organ Classes comparing biologics used in asthma with biologics in all other indications (Part A) and with all other drugs in VigiBase (Part B). Blood blood and lymphatic system disorders, Card cardiac disorders, Cong congenital, familial and genetic disorders, Ear ear and labyrinth disorders, Endo endocrine disorders, Eye eye disorders, Gastr gastrointestinal disorders, Genrl general disorders and administration-site conditions, Hepat hepatobiliary disorders, Immun immune system disorders, Infec infections and infestations, Inj&P injury, poisoning and procedural complications, Inv investigations, Metab metabolism and nutrition disorders, Musc musculoskeletal and connective tissue disorders, Neopl neoplasms benign, malignant and unspecified (incl cysts and polyps), Nerv nervous system disorders, Preg pregnancy, puerperium and perinatal conditions, Prod product issues, Psych psychiatric disorders, Renal renal and urinary disorders, RG1 Reference Group 1, Repro reproductive system and breast disorders, Resp respiratory, thoracic and mediastinal disorders, RORs reporting odds ratios, Skin skin and subcutaneous tissue disorders, SocCi social circumstances, Surg surgical and medical procedures, Vasc vascular disorders
Analyzing the proportion of reports by SOC for each single biologic in asthmatic patients (Fig. 3), dupilumab was mainly related to ‘social circumstances’, ‘injury, poisoning and procedural complications’ and ‘eye disorders’; omalizumab resulted in higher proportions of ‘pregnancy, puerperium and perinatal conditions’, ‘neoplasms benign, malignant and unspecified (incl cysts and polyps)’ and ‘immune disorders’; mepolizumab was mainly associated to ‘product issues’, ‘infections and infestations’ and ‘surgical and medical procedures’; benralizumab with ‘nervous system disorders’, ‘respiratory, thoracic and mediastinal disorders’ and ‘general disorders and administration site conditions’; reslizumab with ‘endocrine disorders’, ‘pregnancy, puerperium and perinatal conditions’ and ‘musculoskeletal and connective tissue disorders’.
Reports related to each single biologic used in asthma stratified by System Organ Classes (%). Blood blood and lymphatic system disorders, Card cardiac disorders, Cong congenital, familial and genetic disorders, Ear ear and labyrinth disorders, Endo endocrine disorders, Eye eye disorders, Gastr gastrointestinal disorders, Genrl general disorders and administration-site conditions, Hepat hepatobiliary disorders, Immun immune system disorders, Infec infections and infestations, Inj&P injury, poisoning and procedural complications, Inv investigations, Metab metabolism and nutrition disorders, Musc musculoskeletal and connective tissue disorders, Neopl neoplasms benign, malignant and unspecified (incl cysts and polyps), Nerv nervous system disorders, Preg pregnancy, puerperium and perinatal conditions, Prod product issues, Psych psychiatric disorders, Renal renal and urinary disorders, Repro reproductive system and breast disorders, Resp respiratory, thoracic and mediastinal disorders, Skin skin and subcutaneous tissue disorders, SocCi social circumstances, Surg surgical and medical procedures, Vasc vascular disorders
3.2 Disproportionality Analysis
Disproportionality analysis was carried out on ICSRs related to biologics with a specified asthma indication by using all other drugs in the databases (RG1) as the primary comparator and, in order to limit confounding by indication, ICS/LABA-related ICSRs as the second reference group (RG2). Many SDRs, significant in both comparator groups (vs RG1 and RG2), concerned already known side effects (reported in package inserts of asthma biologics), such as hypersensitivity conditions including anaphylaxis, headache, fatigue and injection-site reactions (see Table 4). Specifically, anaphylactic reactions were observed for omalizumab (N = 808, 3.9%) as well as for mepolizumab (N = 82, 0.9%), benralizumab (N = 79, 1.4%) and reslizumab (N = 6, 2.7%), resulting in SDRs (data not shown).
Several suspected ADRs resulting in positive SDRs (vs RG1), likely related to an underlying therapeutic indication (e.g. dyspnea, cough, etc.), did not reach statistically significance anymore when using other anti-asthma agents as comparator (RG2) (see Table 4).
We also observed several positive SDRs concerning adverse events commonly linked to corticosteroids (e.g. adrenal insufficiency, Cushing’s syndrome, diabetes mellitus, hyperglycemia, overweight, etc.). In order to mitigate the possible impact of corticosteroid use as an important confounding factor and to increase the specificity of results, an additional disproportionality analysis by each single agent was carried out using some selected OCSs (RG3) as comparator group. As a result, comparing the ROR values resulting from RG1 and RG3, the above cited adverse events lose their significance (see Table 5).
Reporting of asthma biologics was also disproportionally associated with several unexpected (on the basis of the Summaries of Product Characteristics) or less documented adverse events, including malignancies, pulmonary embolism and deep vein thrombosis, sarcoidosis, blood pressure increased, herpes zoster and erythema nodosum with omalizumab; alopecia and lichen planus with dupilumab; alopecia, polymyalgia rheumatica and herpes infections with mepolizumab; alopecia, herpes zoster and EGPA related to benralizumab; and alopecia with reslizumab (see Table 6). Most cases of biologic-induced alopecia involved females and adult patients.
In the sensitivity analysis in which all vaccine-related reports were removed, all these potential signals were confirmed, except for basal cell carcinoma with mepolizumab (OR 1.81, 95% CI 0.81–4.03), alopecia with dupilumab (OR 1.04, 95% CI 0.87–1.25) and alopecia with benralizumab (OR 1.11, 95% CI 0.84–1.46).
To examine possible differences by therapeutic indication, RORs for the most relevant adverse events related to ABs by therapeutic indication (asthma use vs all other uses) are reported in Fig. 4. Cardiac arrhythmias, solid neoplasms, anaphylactic reaction, cardio-cerebral ischemic disease, arthritis and musculoskeletal disorders were more commonly reported in ICSRs specific for asthma use, while skin and ocular disorders were more likely occur for other therapeutic uses.
RORs of some selected suspected ADRs comparing biologics used for asthma versus all other indications. Each category of adverse events was evaluated aggregating different PT terms, as follows: ‘Anaphylactic reactions’: Anaphylactic reaction; Anaphylactic shock; Anaphylactoid reaction. ‘Arthritis’: Arthritis; Arthritis infective; Osteoarthritis; Periarthritis; Polyarthritis; Rheumatoid arthritis; Spinal osteoarthritis. ‘Cardiac arrhythmias’: Arrhythmia; Arrhythmia supraventricular; Atrial fibrillation; Atrial tachycardia; Sinus tachycardia; Supraventricular tachycardia; Ventricular tachycardia; Atrioventricular block; Atrioventricular block complete. ‘Cardio-cerebral ischemic diseases’: Acute myocardial infarction; Cerebral infarction; Cerebral ischemia; Cerebral thrombosis; Cerebrovascular accident; Cerebrovascular disorder; Infarction; Ischemic stroke; Myocardial infarction; Myocardial ischemia; Thrombotic stroke. ‘Eye disorders’: Conjunctivitis; Keratitis; Ulcerative keratitis; Blepharitis; Eye pruritus; Dry eye; Eye swelling; Eye pain; Eye irritation; Eye disorder. ‘Hematological malignancies’: B-cell lymphoma; Cutaneous T-cell lymphoma; Lymphoma; Non-Hodgkin's lymphoma. ‘Muscular disorders’: Myalgia; Myopathy; Rhabdomyolysis. ‘Solid neoplasms’: Bladder cancer; Bone cancer; Brain neoplasm; Breast cancer; Colon cancer; Gastric cancer; Hepatic cancer; Lung neoplasm malignant; Malignant melanoma; Ocular neoplasm; Pancreatic carcinoma; Prostate cancer; Renal cancer; Skin cancer; Thyroid cancer; Uterine cancer. ‘Skin disorders’: Eczema; Pain of skin; Skin disorder; Skin discoloration; Skin hemorrhage; Skin plaque. ADR adverse drug reaction, PT preferred term, ROR reporting odds ratio, S suspected
In the sensitivity analysis, RORs were calculated using all other reports in VigiBase (RG1) as a comparison group, after excluding reports in which ABs were co-reported with one of the four OCSs included as suspected drugs in RG3 (n = 453). No substantial variation was observed in ROR values derived from primary analysis (data not shown).
4 Discussion
To our knowledge, this is the first extensive study that explored the safety profiles of biologics approved for severe asthma treatment using the WHO global pharmacovigilance database. Previously published ICSR analyses from VigiBase were restricted only to single compounds or to specific safety issues [19, 36,37,38,39,40].
In our analysis, ICSRs mainly involved females and adult patients, especially in asthmatic patients. This finding is expected considering the higher prevalence of female adult patients with asthma disease [41]. Among biologic-related ICSRs in asthma patients, about 40% included omalizumab as suspected drug, differently from ICSRs related to other therapeutic uses in which dupilumab was the most implicated drug.
Our findings corroborated well-known safety issues related to ABs, already described in pivotal clinical trials as well as observational studies, including general disorders (e.g. malaise, fatigue), injection-site reactions, nasopharyngitis, headache and hypersensitivity.
In our study, the majority of cases concerned adverse events that most likely result from the underlying condition (e.g. asthma re-exacerbation or therapeutic failure, cough, dyspnea, etc.). In line with this hypothesis, when using ICSs/LABAs as reference group, most of these disproportionate signals no longer reached statistical significance.
Biologics, considered as OCS-sparing agents, are used for severe asthma to control asthma exacerbations [42, 43]. In this context, it is also important to understand the relevance of phenotyping severe asthma patients through biomarkers and/or clinical features, such as comorbidities, in clinical practice [44,45,46,47,48]. Unfortunately, some severe asthma patients do not respond to biologic therapy, thus presenting asthma exacerbations or deterioration. The differences in treatment response may be multifactorial, and related to various drug and/or patient-related factors, such as the mechanisms of action, the target, dose and interval of the biological drug or the heterogeneity of asthma phenotypes and underlying endotypes [49, 50]. Persistent suboptimal responders require a re-evaluation of asthma phenotype biomarkers, and the suspected immunological pathways involved in the asthma inflammation [51].
As expected, a significant number of spontaneous reports including anaphylactic reactions was reported with greater frequencies for omalizumab and reslizumab in relation to reports of each biologic agent. In line with a recently published FAERS analysis [39], all biologic drugs showed positive signals of disproportionate reporting for anaphylactic reactions, except for dupilumab, which is the only fully human monoclonal antibody among the five biologic agents. The risk of hypersensitivity/allergic reactions and anaphylaxis could be related to the immunogenic properties of the protein component of monoclonal antibodies (mAbs). The role of excipients, such as polysorbates, has also been investigated in literature [20]. Although the incidence of anaphylaxis related to mAbs for severe asthma is low, asthma patients in general appear to have a higher risk of severe allergic reactions, including anaphylaxis, compared with patients that use mAbs for other indications, such as chronic urticaria [39]. In addition, the FDA included a black box warning on both omalizumab and reslizumab labels for the risk of anaphylaxis [24].
Potential differences among single biologic safety profiles also emerged from our findings as reported in the results section. Most of these findings were in line with available evidence for each biologic agent, including the corresponding FDA labels [24] and the EPARs and RMPs [25].
Due to the potential interference with the immune system, one of the major concerns with ABs was the risk of provoking or unmasking malignancies, although none of the investigated drugs exerts an immunosuppressive effect under a mechanistic perspective. Analogously to a previous disproportionality analysis of VigiBase [19], our study reported signals of disproportionate reporting for omalizumab and leukemia, melanoma, breast, lung, prostate, colon and thyroid cancers. Omalizumab is an anti Ig-E drug and a possible relationship between absent or very low serum Immunoglobulin E levels and cancer risk has been already suggested in literature [52]. A numeric imbalance in malignancy rates in patients with allergic asthma was observed in pivotal trials, leading the US FDA to require a post-marketing 5-year safety study (EXCELS) to assess the long-term safety of omalizumab in an observational setting, primarily the risk of malignancy [53]. In line with a pooled analysis of clinical trials, the results of the EXCELS study did not confirm a significant association between omalizumab use and risk of cancer [54,55,56,57], despite the limitation of this observational study preventing a definitive conclusion to be drawn regarding this risk [58]. Although the number of cases reported in the post-marketing setting has increased during the last few years, the difficulty of establishing a causal association in spontaneous cancer-related ICSRs prevents us from assessing this risk. A few cases of malignancy, including basal cell carcinoma and melanoma, were also reported for mepolizumab, but there is no supporting evidence from literature.
Based on the findings of the above-mentioned EXCELS study [53], an FDA safety alert regarding a potential association of omalizumab and arterial thrombotic events (ATEs) was issued in 2014 [17] and information about ATEs has been added to the drug label. In addition, results from EXCELS showed a rate of pulmonary embolism or venous thrombosis corresponding to 3.2 per 1000 patient-years with omalizumab (N = 5007) versus 1.5 per 1000 patient-years with non-omalizumab treatment (N = 2829) [59]. Accordingly, in our analysis several spontaneous cases of ATEs (i.e. myocardial infarction, transient ischemic attack and stroke) were reported with omalizumab. We also observed cases of venous thromboembolic disorders, such as pulmonary embolism and deep vein thrombosis. Some case reports describing the association of omalizumab with pulmonary vein thrombosis were also published [60, 61]. However, the effects of omalizumab and other ABs on the cardiovascular system remain controversial.
Statistically significant signals of disproportionate reporting of alopecia were identified for all ABs under study. Hair loss is listed among the side effects in the omalizumab package insert, but unlisted for the other study drugs. Several published case reports and a previous analysis based on VigiBase considered the potential correlation between the onset of hair disorders and dupilumab therapy [62,63,64]. Nevertheless, a paradoxical effect of dupilumab as a beneficial treatment for alopecia areata has been reported [65]. In addition, a case report of mepolizumab-associated alopecia has been published [66]. In this case, the authors suggested that autoimmune mechanisms could be unmasked by the suppression of eosinophils following treatment with mepolizumab.
Several AB-related ICSRs, describing adverse events in which autoimmunity could play a role (e.g. sarcoidosis, lichen planus, myasthenia gravis, polymyalgia rheumatica, EGPA), have been detected in VigiBase. Most cases of autoimmune conditions attributed to ABs were also described in literature [36, 67,68,69,70,71,72,73,74]. It is not clear if the biologic agent could act as a trigger in patients with genetic predispositions or if it causes directly similar, but not true, autoimmune conditions. Furthermore, some reported cases could reflect a delay in the diagnosis of the autoimmune disease, more than a true side effect. In the case of EGPA, the natural history of the disease, almost invariantly characterized by severe asthma as a prodromal stage, might account for its onset in patients undergoing anti Th2 mAbs for severe asthma. In that light, EGPA occurrence could be related to the inability of the biologic therapy to prevent the disease evolution more then to a side effect triggered by the drug.
In our study, a significant disproportionality related to spontaneous abortion following omalizumab use was highlighted, but this finding could be confounded by the underlying disease. Actually, numerous studies showed a link between uncontrolled asthma during pregnancy and the increased risk of perinatal mortality, congenital anomalies, prematurity, low birth weight and adverse maternal outcomes [75,76,77].
Some studies have investigated the potential link between asthma and the development of herpes zoster infection caused by the re-activation of latent varicella zoster virus [78,79,80]. Many risk factors could play a role in this re-activation, such as stress, immunosuppression condition, age or some debilitating diseases that compromise the immune system, like asthma [79]. For this reason, the herpes zoster immunization is primarily suggested for asthmatic adults aged 50 years or more [78, 80]. Nevertheless, a recent review focusing on the risks of asthma biologic therapy underlined that a small percentage of patients receiving dupilumab, mepolizumab and benralizumab developed a herpes zoster infection and this risk is already labelled for mepolizumab [81].
A significant confounder in our analysis is represented by concomitant or recent use of OCSs that are typically administered to patients with severe asthma, according to the European Respiratory Society (ERS)/American Thoracic Society (ATS) guidelines [1]. In general, after starting biologics, OCS sparing up to withdrawal may be considered a primary outcome in the management of severe asthma patients to minimize side effects [82].
Frequent or continuous use of corticosteroids (OCSs or long-term high-dose ICSs) could be associated with an array of potential adverse events, such as fluid retention, bone damage, elevated blood sugars and psychiatric problems, which could be clinically relevant, especially when high doses are required for a prolonged period of time. Furthermore, the reduction or suppression of OCSs during therapy with biologics for asthma may unmask some comorbidity symptoms or patients’ underlying diseases (e.g. psoriasis, multiple inflammatory diseases, etc.), known as glucocorticoid deprivation syndrome. For instance, the potential signals identified in our study of EGPA related to benralizumab or sarcoidosis with omalizumab offer a possible alternative explanation in the unmasking effect of biologics after interruption of OCS therapy, as already discussed in some published case reports [73, 83, 84]. Furthermore, several OCS-related long-term effects may emerge during therapy with biologics and be erroneously attributed to them. In our analysis, numerous ICSRs related to adrenal insufficiency, hyperglycemia, fractures, Cushing's syndrome, weight increase and other typical steroid adverse events were reported in association with asthma biologics. However, when using OCS as comparator, most of those signals no longer reached statistical significance, thus supporting our hypothesis.
Some variations in reporting trends by therapeutic use were also detected. In detail, we observed that several types of malignancies, cardiovascular adverse events, pneumonia and musculoskeletal events are more likely to be reported for asthma treatment than other therapeutic uses, whereas cutaneous and ocular events are more often reported for other indications (e.g. atopic dermatitis). These results need to be interpreted, in view of the different reporting trends of single biologic agents by therapeutic indication. While omalizumab is the most represented drug in asthma-specific reports, for other therapeutic uses, mainly atopic dermatitis, a higher frequency of reports is observed for dupilumab that is commonly related to ocular and cutaneous adverse events, especially in patients with atopic dermatitis, as already shown in previous WHO pharmacovigilance analyses [37, 40]. Another FAERS study [85] found that patients in treatment with dupilumab for atopic dermatitis have more ocular complications than asthmatic patients, suggesting that a potential drug–disease interaction could enhance the risk of ocular complications following dupilumab administration. Furthermore, the safety profiles of biologics used in such different populations, who have different background rates of adverse events, are sometimes reflections of the characteristics of the patients being treated rather than effects of the drug under study.
4.1 Strengths and Limitations
Our data were extracted from VigiBase and the major strength is the fact that VigiBase is a global spontaneous reporting database with more than 30 million reports coming from different countries. Analysis of this worldwide spontaneous reporting system enables better identification of rare and long-term adverse events and broader generalization of study results.
Our analysis aimed to assess the overall safety profiles of all the biologics approved for asthma treatment stratifying by therapeutic indication. Even if the safety profile of reports with a missing indication was similar to our study reports, we preferred to exclude these reports from our disproportionality analyses in order to precisely explore safety data of biologics by therapeutic use. We provided additional evidence concerning several safety issues, some of which have been previously discussed in scientific literature or by regulatory agencies. Case-by-case assessment related to unexpected suspected ADRs was carried out and referred to available data contained in line listings.
However, there are some limitations to acknowledge. In general, spontaneous reporting data are subject to several biases, including under-reporting, selective reporting and the lack of a denominator (total number of drug users), all of which prevent measuring absolute risk of suspected ADRs [86]. Disproportionality findings require cautious interpretation, evaluation of the risk of bias and consideration for alternative explanations other than causal association between the drug and the adverse event. Furthermore, potential limitations in sensitivity and precision of these methods should be taken into account [27, 28]. Indeed, clinical assessment (qualitative analysis) remains essential before drawing any causal inference from disproportionality measures. Therefore, we cannot exclude that some signals of disproportionate reporting could be confounded by the underlying conditions, baseline risk and comorbidities in asthmatic patients who are treated with biologics, even if we conducted sensitivity analyses. Moreover, ROR values may also be influenced by several biases due to specific reporting trends in the spontaneous reporting system and to different marketing authorization dates of studied biologics.
As known, few ICSRs in VigiBase provide the desired quality level of information [87]. In our study, we excluded reports with missing indication even if they could be related to patients with asthma. Reports of suspected ADRs were often incomplete for causality assessment due to unavailability of clinical details in line listings and incomplete reporting of age, drug dosing, time to onset, comorbid conditions and concomitant drugs, thus limiting case-by-case assessment.
5 Conclusions
Overall, this study found good safety profiles of biologic drugs used in patients with severe asthma. Our findings confirmed well-known side effects related to asthma biologics already described such as general disorders, injection-site reactions, nasopharyngitis, headache and hypersensitivity, while some others (e.g. asthma reactivation or therapeutic failure) could be ascribed to the indication of use. Regarding anaphylactic reactions, all study biologics, except dupilumab, showed positive signals of disproportionate reporting; this risk is probably related to the immunogenic properties of the protein component of mAbs. Confounding effect by previous or concomitant use of corticosteroids that are used often concomitantly or immediately before starting biologic treatment has also been managed by performing an additional sensitivity analysis. Several potential safety signals (e.g. malignancies, rhythm disorders, pulmonary embolism, alopecia, etc.) have been identified. Further additional studies are required to assess and validate these potential safety signals.
References
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. https://doi.org/10.1183/09031936.00202013. (Erratum in: Eur Respir J. 2014 Apr;43(4):1216. Dosage error in article text. Erratum in: Eur Respir J. 2018 Jul 27;52(1): Erratum in: Eur Respir J. 2022 Jun 9;59(6)).
Vianello A, Caminati M, Andretta M, Menti AM, Tognella S, Senna G, Degli EL. Prevalence of severe asthma according to the drug regulatory agency perspective: An Italian experience. World Allergy Organ J. 2019;12(4): 100032. https://doi.org/10.1016/j.waojou.2019.100032.
Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–88. https://doi.org/10.1080/03007995.2018.1505352.
Mavissakalian M, Brady S. The current state of biologic therapies for treatment of refractory asthma. Clin Rev Allergy Immunol. 2020;59(2):195–207. https://doi.org/10.1007/s12016-020-08776-8.
Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, Gaga M, Kellermeyer L, Khurana S, Knight S, McDonald VM, Morgan RL, Ortega VE, Rigau D, Subbarao P, Tonia T, Adcock IM, Bleecker ER, Brightling C, Boulet LP, Cabana M, Castro M, Chanez P, Custovic A, Djukanovic R, Frey U, Frankemölle B, Gibson P, Hamerlijnck D, Jarjour N, Konno S, Shen H, Vitary C, Bush A. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1): 1900588. https://doi.org/10.1183/13993003.00588-2019.
Russo D, Di Filippo P, Attanasi M, Lizzi M, Di Pillo S, Chiarelli F. Biologic therapy and severe asthma in children. Biomedicines. 2021;9(7):760. https://doi.org/10.3390/biomedicines9070760.
Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218: 107414. https://doi.org/10.1016/j.rmed.2023.107414.
Lommatzsch M, Brusselle GG, Canonica GW, Jackson DJ, Nair P, Buhl R, Virchow JC. Disease-modifying anti-asthmatic drugs. Lancet. 2022;399(10335):1664–8. https://doi.org/10.1016/S0140-6736(22)00331-2.
Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–98. https://doi.org/10.1016/j.chest.2016.03.032.
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, Murphy K, Maspero JF, O’Brien C, Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66. https://doi.org/10.1016/S2213-2600(15)00042-9. (Erratum in: Lancet Respir Med. 2015 Apr;3(4):e15. Erratum in: Lancet Respir Med. 2016 Oct;4(10 ):e50).
Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, Rosen KE, Eisner MD, Wong DA, Busse W. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–82. https://doi.org/10.7326/0003-4819-154-9-201105030-00002. (Erratum in: Ann Intern Med. 2019 Oct 1;171(7):528).
Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–90. https://doi.org/10.1067/mai.2001.117880.
Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59(7):701–8. https://doi.org/10.1111/j.1398-9995.2004.00533.x.
Ferguson GT, FitzGerald JM, Bleecker ER, Laviolette M, Bernstein D, LaForce C, Mansfield L, Barker P, Wu Y, Jison M, Goldman M; BISE Study Investigators. Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2017;5(7):568–76. https://doi.org/10.1016/S2213-2600(17)30190-X.
Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, Yancey SW, Ortega HG. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–20701. https://doi.org/10.1016/j.clinthera.2016.07.010.
Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, Katelaris C, Tohda Y, Zhang B, Staudinger H, Pirozzi G, Amin N, Ruddy M, Akinlade B, Khan A, Chao J, Martincova R, Graham NMH, Hamilton JD, Swanson BN, Stahl N, Yancopoulos GD, Teper A. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–96. https://doi.org/10.1056/NEJMoa1804092.
Food and Drug Administration Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-asthma-drug-xolair-omalizumab-including. Accessed June 13, 2023.
Medicines and Healthcare products Regulatory Agency (MHRA). Omalizumab: potential risk of arterial thrombotic events. https://www.gov.uk/drug-safety-update/omalizumab-potential-risk-of-arterial-thrombotic-events. Accessed June 13, 2023.
Mota D, Rama TA, Severo M, Moreira A. Potential cancer risk with omalizumab? A disproportionality analysis of the WHO’s VigiBase pharmacovigilance database. Allergy. 2021;76(10):3209–11. https://doi.org/10.1111/all.15008.
Baddini-Martinez J, Leitão Filho FS, Caetano LSB. Anaphylactic risks associated with immunobiological agents in asthma therapy. Rev Assoc Med Bras. 2023;69(3):367–9. https://doi.org/10.1590/1806-9282.20221358.
Tregunno PM, Fink DB, Fernandez-Fernandez C, Lázaro-Bengoa E, Norén GN. Performance of probabilistic method to detect duplicate individual case safety reports. Drug Saf. 2014;37(4):249–58. https://doi.org/10.1007/s40264-014-0146-y.
ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Glossary of ICH terms and definitions; 2023. https://cioms.ch/publications/product/glossary-of-ich-terms-and-definitions. Accessed July 31, 2023.
European Medicines Agency (EMA). Inclusion/exclusion criteria for the “Important Medical Events” list. https://www.ema.europa.eu/en/documents/other/inclusion-exclusion-criteria-important-medical-events-list-meddra_en.pdf Accessed July 31, 2023.
Food and Drug Administration. FDA Online Label Repository. https://labels.fda.gov/. Accessed June 21, 2023.
European Medicines Agency (EMA). Medicines EPAR. https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human/ema_group_types/ema_medicine. Accessed June 21, 2023.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–36. https://doi.org/10.1002/pds.1742.
Wisniewski AF, Bate A, Bousquet C, Brueckner A, Candore G, Juhlin K, Macia-Martinez MA, Manlik K, Quarcoo N, Seabroke S, Slattery J, Southworth H, Thakrar B, Tregunno P, Van Holle L, Kayser M, Norén GN. Good signal detection practices: evidence from IMI PROTECT. Drug Saf. 2016;39(6):469–90. https://doi.org/10.1007/s40264-016-0405-1.
Candore G, Juhlin K, Manlik K, Thakrar B, Quarcoo N, Seabroke S, Wisniewski A, Slattery J. Comparison of statistical signal detection methods within and across spontaneous reporting databases. Drug Saf. 2015;38(6):577–87. https://doi.org/10.1007/s40264-015-0289-5.
Khouri C, Revol B, Lepelley M, Mouffak A, Bernardeau C, Salvo F, Pariente A, Roustit M, Cracowski JL. A meta-epidemiological study found lack of transparency and poor reporting of disproportionality analyses for signal detection in pharmacovigilance databases. J Clin Epidemiol. 2021;139:191–8. https://doi.org/10.1016/j.jclinepi.2021.07.014.
Hauben M, Aronson JK. Defining “signal” and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32(2):99–110. https://doi.org/10.2165/00002018-200932020-00003.
European Medicines Agency (EMA, 2017). Guideline on good pharmacovigilance practices (GVP)—Module IX Addendum I– Methodological aspects of signal detection from spontaneous reports of suspected adverse reactions. EMA/209012/2015.
Council for International Organizations of Medical Sciences, editor. Practical aspects of signal detection in pharmacovigilance: report of CIOMS Working Group VIII. Geneva: CIOMS; 2010.
European Medicines Agency (EMA, 2016). EMA/849944/2016. Screening for adverse reactions in EudraVigilance. https://www.ema.europa.eu/en/documents/ Accessed July 12, 2023.
Grundmark B, Holmberg L, Garmo H, Zethelius B. Reducing the noise in signal detection of adverse drug reactions by standardizing the background: a pilot study on analyses of proportional reporting ratios-by-therapeutic area. Eur J Clin Pharmacol. 2014;70(5):627–35. https://doi.org/10.1007/s00228-014-1658-1.
Alkabbani W, Gamble JM. Active-comparator restricted disproportionality analysis for pharmacovigilance signal detection studies of chronic disease medications: an example using sodium/glucose cotransporter 2 inhibitors. Br J Clin Pharmacol. 2023;89(2):431–9. https://doi.org/10.1111/bcp.15178.
Cohen Aubart F, Lhote R, Amoura A, Valeyre D, Haroche J, Amoura Z, Lebrun-Vignes B. Drug-induced sarcoidosis: an overview of the WHO pharmacovigilance database. J Intern Med. 2020;288(3):356–62. https://doi.org/10.1111/joim.12991.
Bettuzzi T, Drucker A, Staumont-Sallé D, Bihan K, Lebrun-Vignes B, Sbidian E. Adverse events associated with dupilumab in the World Health Organization pharmacovigilance database. J Am Acad Dermatol. 2022;86(2):431–3. https://doi.org/10.1016/j.jaad.2021.09.050.
Alroobaea R, Rubaiee S, Hanbazazah AS, Jahrami H, Garbarino S, Damiani G, Wu J, Bragazzi NL. IL-4/13 Blockade and sleep-related adverse drug reactions in over 37,000 Dupilumab reports from the World Health Organization individual case safety reporting pharmacovigilance database (VigiBase™): a big data and machine learning analysis. Eur Rev Med Pharmacol Sci. 2022;26(11):4074–81. https://doi.org/10.26355/eurrev_202206_28977.
Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4): e12038. https://doi.org/10.1002/clt2.12038.
Hirai E, Haruki T, Baba T, Miyazaki D. Analyses of dupilumab-related ocular adverse drug reactions using the WHO’s VigiBase. Adv Ther. 2023. https://doi.org/10.1007/s12325-023-02573-3.
Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17(3):19. https://doi.org/10.1007/s11882-017-0686-1.
Chanez P, Contin-Bordes C, Garcia G, Verkindre C, Didier A, De Blay F, de Lara MT, Blanco P, Moreau JF, Robinson P, Bourdeix I, Trunet P, Le Gros V, Humbert M, Molimard M. Omalizumab-induced decrease of FcξRI expression in patients with severe allergic asthma. Respir Med. 2010;104(11):1608–17. https://doi.org/10.1016/j.rmed.2010.07.011.
Diamant Z, Vijverberg S, Alving K, Bakirtas A, Bjermer L, Custovic A, Dahlen SE, Gaga M, Gerth van Wijk R, Giacco SD, Hamelmann E, Heaney LG, Heffler E, Kalayci Ö, Kostikas K, Lutter R, Olin AC, Sergejeva S, Simpson A, Sterk PJ, Tufvesson E, Agache I, Seys SF. Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy. 2019;74(10):1835–51. https://doi.org/10.1111/all.13806.
Roche N, Anzueto A, Bosnic Anticevich S, Kaplan A, Miravitlles M, Ryan D, Soriano JB, Usmani O, Papadopoulos NG, Canonica GW; Respiratory Effectiveness Group Collaborators. The importance of real-life research in respiratory medicine: manifesto of the Respiratory Effectiveness Group: Endorsed by the International Primary Care Respiratory Group and the World Allergy Organization. Eur Respir J. 2019;54(3):1901511. https://doi.org/10.1183/13993003.01511-2019.
Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, Busby J, Jackson DJ, Pfeffer PE, Rhee CK, Cho YS, Canonica GW, Heffler E, Gibson PG, Hew M, Peters M, Harvey ES, Alacqua M, Zangrilli J, Bulathsinhala L, Carter VA, Chaudhry I, Eleangovan N, Hosseini N, Murray RB, Price DB. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. https://doi.org/10.1016/j.chest.2019.10.053. (Erratum in: Chest. 2021;160(5):1989).
Heffler E, Paoletti G, Giorgis V, Puggioni F, Racca F, Del Giacco S, Bagnasco D, Caruso C, Brussino L, Rolla G, Canonica GW. Real-life studies of biologics used in asthma patients: key differences and similarities to trials. Expert Rev Clin Immunol. 2019;15(9):951–8. https://doi.org/10.1080/1744666X.2019.1653758.
Eger K, Kroes JA, Ten Brinke A, Bel EH. Long-term therapy response to anti-il-5 biologics in severe asthma—a real-life evaluation. J Allergy Clin Immunol Pract. 2021;9(3):1194–200. https://doi.org/10.1016/j.jaip.2020.10.010.
Kavanagh JE, d’Ancona G, Elstad M, Green L, Fernandes M, Thomson L, Roxas C, Dhariwal J, Nanzer AM, Kent BD, Jackson DJ. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. https://doi.org/10.1016/j.chest.2020.03.042.
Kroes JA, Zielhuis SW, van Roon EN, Ten Brinke A. Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem Pharmacol. 2020;179: 113978. https://doi.org/10.1016/j.bcp.2020.113978.
Khaleva E, Rattu A, Brightling C, Bush A, Bourdin A, Bossios A, Chung KF, Chaudhuri R, Coleman C, Djukanovic R, Dahlén SE, Exley A, Fleming L, Fowler SJ, Gupta A, Hamelmann E, Koppelman GH, Melén E, Mahler V, Seddon P, Singer F, Porsbjerg C, Ramiconi V, Rusconi F, Yasinska V, Roberts G. Definitions of non-response and response to biological therapy for severe asthma: a systematic review. ERJ Open Res. 2023;9(3): 00444-2022. https://doi.org/10.1183/23120541.00444-2022.
Pepper AN, Hanania NA, Humbert M, Casale TB. How to assess effectiveness of biologics for asthma and what steps to take when there is not benefit. J Allergy Clin Immunol Pract. 2021;9(3):1081–8. https://doi.org/10.1016/j.jaip.2020.10.048.
Ferastraoaru D, Bax HJ, Bergmann C, Capron M, Castells M, Dombrowicz D, Fiebiger E, Gould HJ, Hartmann K, Jappe U, Jordakieva G, Josephs DH, Levi-Schaffer F, Mahler V, Poli A, Rosenstreich D, Roth-Walter F, Shamji M, Steveling-Klein EH, Turner MC, Untersmayr E, Karagiannis SN, Jensen-Jarolim E. AllergoOncology: ultra-low IgE, a potential novel biomarker in cancer-a Position Paper of the European Academy of Allergy and Clinical Immunology (EAACI). Clin Transl Allergy. 2020;10:32. https://doi.org/10.1186/s13601-020-00335-w.
Long AA, Fish JE, Rahmaoui A, Miller MK, Bradley MS, Taki HN, Demeo AN, Tilles SA, Szefler SJ. Baseline characteristics of patients enrolled in EXCELS: a cohort study. Ann Allergy Asthma Immunol. 2009;103(3):212–9. https://doi.org/10.1016/S1081-1206(10)60184-6.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, Canvin J. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-9.e6. https://doi.org/10.1016/j.jaci.2012.01.033.
Long A, Rahmaoui A, Rothman KJ, Guinan E, Eisner M, Bradley MS, Iribarren C, Chen H, Carrigan G, Rosén K, Szefler SJ. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4. https://doi.org/10.1016/j.jaci.2014.02.007.
Bagnasco D, Canevari RF, Del Giacco S, Ferrucci S, Pigatto P, Castelnuovo P, Marseglia GL, Yalcin AD, Pelaia G, Canonica GW. Omalizumab and cancer risk: current evidence in allergic asthma, chronic urticaria, and chronic rhinosinusitis with nasal polyps. World Allergy Organ J. 2022;15(12): 100721. https://doi.org/10.1016/j.waojou.2022.100721.
Ali Z, Egeberg A, Thyssen JP, Sørensen JA, Vestergaard C, Thomsen SF. No association between omalizumab use and risk of cancer: a nationwide registry-based cohort study. Br J Dermatol. 2022;186(4):746–8. https://doi.org/10.1111/bjd.20941.
Li J, Goulding M, Seymour S, Starke P. EXCELS study results do not rule out potential cancer risk with omalizumab. J Allergy Clin Immunol. 2015;135(1):289. https://doi.org/10.1016/j.jaci.2014.09.017.
Iribarren C, Rahmaoui A, Long AA, Szefler SJ, Bradley MS, Carrigan G, Eisner MD, Chen H, Omachi TA, Farkouh ME, Rothman KJ. Cardiovascular and cerebrovascular events among patients receiving omalizumab: Results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol. 2017;139(5):1489-1495.e5. https://doi.org/10.1016/j.jaci.2016.07.038.
Narukonda S, Vinod NR, Joshi M. A case of pulmonary vein thrombosis associated with treatment of omalizumab. J Investig Med High Impact Case Rep. 2017;5(3): 2324709617724176. https://doi.org/10.1177/2324709617724176.
Oblitas CM, Galeano-Valle F, Vela-De La Cruz L, Del Toro-Cervera J, Demelo-Rodríguez P. Omalizumab as a provoking factor for venous thromboembolism. Drug Target Insights. 2019;13:1177392819861987. https://doi.org/10.1177/1177392819861987.
Beaziz J, Bouaziz JD, Jachiet M, Fite C, Lons-Danic D. Dupilumab-induced psoriasis and alopecia areata: case report and review of the literature. Ann Dermatol Venereol. 2021;148(3):198–201. https://doi.org/10.1016/j.annder.2021.02.003.
Sachdeva M, Witol A, Mufti A, Maliyar K, Yeung J. Alopecia areata related paradoxical reactions in patients on dupilumab therapy: a systematic review. J Cutan Med Surg. 2021;25(4):451–2. https://doi.org/10.1177/1203475421995186 (Erratum in: J Cutan Med Surg. 2021;12034754211011779).
Park S, Park SH, Byun YJ, Choi SA. Short communication: Comments on hair disorders associated with dupilumab based on VigiBase. PLoS One. 2022;17(7): e0270906. https://doi.org/10.1371/journal.pone.0270906.
Darrigade AS, Legrand A, Andreu N, Jacquemin C, Boniface K, Taïeb A, Seneschal J. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol. 2018;179(2):534–6. https://doi.org/10.1111/bjd.16711.
Nixon R, Despiney R, Pfeffer P. Case of paradoxical adverse response to mepolizumab with mepolizumab-induced alopecia in severe eosinophilic asthma. BMJ Case Rep. 2020;13(2): e233161. https://doi.org/10.1136/bcr-2019-233161.
Seeborg FO, Rihal PS, Czelusta A, Sanchez R, Hanson IC. Lichen planus associated with omalizumab administration in an adult with allergic asthma. Ann Allergy Asthma Immunol. 2009;102(4):349–51. https://doi.org/10.1016/S1081-1206(10)60343-2.
Yung S, Han D, Lee JK. Cutaneous sarcoidosis in a patient with severe asthma treated with omalizumab. Can Respir J. 2015;22(6):315–6. https://doi.org/10.1155/2015/265734.
Mangin MA, Lienhart A, Gouraud A, Roux S, Hodique F, Jouen F, Balme B, Dalle S, Debarbieux S. Onset of acquired haemophilia A after omalizumab treatment in severe bullous pemphigoid—a report on two cases successfully treated with mycophenolate mofetil. Ann Dermatol Venereol. 2021;148(1):57–9. https://doi.org/10.1016/j.annder.2020.09.577.
To Y, Kono Y, Tsuzuki R, Kaneko H, To M. Rheumatoid arthritis-like polyarthralgia after the initiation of omalizumab treatment: a case series. J Allergy Clin Immunol Pract. 2021;9(9):3510–2. https://doi.org/10.1016/j.jaip.2021.05.027.
Kern L, Kleinheinrich L, Feldmann R, Sator P, Stella A, Breier F. Dupilumab-induced lichen planus: a case with oral and cutaneous eruptions. Case Rep Dermatol. 2022;14(3):356–61. https://doi.org/10.1159/000527918.
Wechsler ME, Wong DA, Miller MK, Lawrence-Miyasaki L. Churg-strauss syndrome in patients treated with omalizumab. Chest. 2009;136(2):507–18. https://doi.org/10.1378/chest.08-2990.
Caminati M, Maule M, Nalin F, Senna G, Lunardi C. Onset of eosinophilic granulomatosis with polyangiitis in a patient treated with an IL-5 pathway inhibitor for severe asthma. Rheumatology. 2021;60(2):e59–60. https://doi.org/10.1093/rheumatology/keaa572.
Caminati M, Olivieri B, Dama A, Micheletto C, Paggiaro P, Pinter P, Senna G, Schiappoli M. Dupilumab-induced hypereosinophilia: review of the literature and algorithm proposal for clinical management. Expert Rev Respir Med. 2022;16(7):713–21. https://doi.org/10.1080/17476348.2022.2090342.
Blais L, Kettani FZ, Elftouh N, Forget A. Effect of maternal asthma on the risk of specific congenital malformations: a population-based cohort study. Birth Defects Res A Clin Mol Teratol. 2010;88(4):216–22. https://doi.org/10.1002/bdra.20651.
National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol. 2005;115(1):34–46. https://doi.org/10.1016/j.jaci.2004.10.023 (Erratum in: J Allergy Clin Immunol. 2005 Mar;115(3):477).
Namazy J, Cabana MD, Scheuerle AE, Thorp JM Jr, Chen H, Carrigan G, Wang Y, Veith J, Andrews EB. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135(2):407–12. https://doi.org/10.1016/j.jaci.2014.08.025.
Kwon HJ, Bang DW, Kim EN, Wi CI, Yawn BP, Wollan PC, Lahr BD, Ryu E, Juhn YJ. Asthma as a risk factor for zoster in adults: a population-based case-control study. J Allergy Clin Immunol. 2016;137(5):1406–12. https://doi.org/10.1016/j.jaci.2015.10.032.
Shrestha AB, Umar TP, Mohammed YA, Aryal M, Shrestha S, Sapkota UH, Adhikari L, Shrestha S. Association of asthma and herpes zoster, the role of vaccination: a literature review. Immun Inflamm Dis. 2022;10(11): e718. https://doi.org/10.1002/iid3.718.
Safonova E, Yawn BP, Welte T, Wang C. Risk factors for herpes zoster: should people with asthma or COPD be vaccinated? Respir Res. 2023;24(1):35. https://doi.org/10.1186/s12931-022-02305-1.
Sitek AN, Li JT, Pongdee T. Risks and safety of biologics: a practical guide for allergists. World Allergy Organ J. 2023;16(1): 100737. https://doi.org/10.1016/j.waojou.2022.100737.
Canonica GW, Blasi F, Paggiaro P, Senna G, Passalacqua G, Spanevello A, Aliberti S, Bagnasco D, Bonavia M, Bonini M, Brussino L, Bucca C, Caiaffa MF, Calabrese C, Camiciottoli G, Caminati M, Carpagnano GE, Caruso C, Centanni S, Conte ME, Corsico AG, Cosmi L, Costantino MT, Crimi N, D'Alò S, D'Amato M, Del Giacco S, Farsi A, Favero E, Foschino Barbaro MP, Guarnieri G, Guida G, Latorre M, Lo Cicero S, Lombardi C, Macchia L, Mazza F, Menzella F, Milanese M, Montagni M, Montuschi P, Nucera E, Parente R, Patella V, Pelaia G, Pini L, Puggioni F, Ricciardi L, Ricciardolo FLM, Richeldi L, Ridolo E, Rolla G, Santus P, Scichilone N, Spadaro G, Vianello A, Viviano V, Yacoub MR, Zappa MC, Heffler E; SANI (Severe Asthma Network Italy). Oral CorticoSteroid sparing with biologics in severe asthma: a remark of the Severe Asthma Network in Italy (SANI). World Allergy Organ J. 2020;13(10):100464. https://doi.org/10.1016/j.waojou.2020.100464.
Hočevar A, Kopač P, Rotar Ž, Novljan MP, Škrgat S. Eosinophilic granulomatosis with polyangiitis evolution during severe eosinophilic asthma treatment with benralizumab. J Allergy Clin Immunol Pract. 2020;8(7):2448–9. https://doi.org/10.1016/j.jaip.2020.04.006.
Holubar J, Arnaud E, Broner J, Pers YM, Proust A, Goulabchand R. New-onset eosinophilic granulomatosis with polyangiitis in 2 patients during treatment with IL-5 pathway inhibitors. Immunol Res. 2022;70(6):721–4. https://doi.org/10.1007/s12026-022-09317-5.
Wang Y, Jorizzo JL. Retrospective analysis of adverse events with dupilumab reported to the United States Food and Drug Administration. J Am Acad Dermatol. 2021;84(4):1010–4. https://doi.org/10.1016/j.jaad.2020.11.042.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96. https://doi.org/10.2165/00002018-200629050-00003.
Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65–77. https://doi.org/10.1007/s40264-013-0131-x (Erratum in: Drug Saf. 2019 Mar 12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Conflict of interest
Gianluca Trifirò has served on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead and Amgen in the past three years; he was the scientific director of a Master’s program on pharmacovigilance, pharmacoepidemiology and real-world evidence which has received non-conditional grants from various pharmaceutical companies; he coordinated a pharmacoepidemiology team at the University of Messina until October 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also scientific coordinator of the academic spin-off ‘INSPIRE srl’ which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). None of the above-mentioned activities are related to the topic of the manuscript. The other authors have no conflict of interest to disclose.
UMC statement
VigiBase, the WHO global database of ICSRs, is the source of our information. The information comes from a variety of sources, and the probability that the suspected adverse event is drug-related is not the same in all cases; the information does not represent the opinion of the UMC or WHO.
Data availability
The datasets generated and/or analyzed in the current study are available in the VigiBase repository. The data presented in this study are obtainable on request from the corresponding author.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cutroneo, P.M., Arzenton, E., Furci, F. et al. Safety of Biological Therapies for Severe Asthma: An Analysis of Suspected Adverse Reactions Reported in the WHO Pharmacovigilance Database. BioDrugs 38, 425–448 (2024). https://doi.org/10.1007/s40259-024-00653-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-024-00653-6