Abstract
Background
Large-vessel ischemic stroke represents about 25–40% of all ischemic strokes. Few clinical trials compared ticagrelor versus clopidogrel in ischemic stroke patients; all these studies included only patients with a transient ischemic attack or minor stroke; moreover, none of these studies included patients from North Africa.
Objectives
We aimed to compare ticagrelor versus clopidogrel in the first-ever large-vessel occlusion (LVO) acute ischemic stroke in Egypt.
Methods
Our trial involved 580 first-ever LVO ischemic stroke patients who were randomly assigned to administer loading and maintenance doses of ticagrelor or clopidogrel. Screening, randomization, and start of treatment occurred during the first 24 hours of the stroke.
Results
580 patients were included in the intention-to-treat analysis. Thirty patients in the ticagrelor group and 49 patients in the clopidogrel group experienced a new ischemic or hemorrhagic stroke at 90 days (hazard ratio [HR] 0.61; 95% confidence interval [CI] 0.38–0.98; p-value = 0.04), 36 patients in the ticagrelor group, and 57 in the clopidogrel group experienced composite of a new stroke, myocardial infarction, or death due to vascular insults (HR 0.56; 95% CI 0.37–0.87; p = 0.009). Patients who received ticagrelor had better clinical outcomes regarding National Institutes of Health Stroke Scale (NIHSS) reduction and a favorable modified Rankin scale (mRS) score. There were no differences between ticagrelor and clopidogrel regarding hemorrhagic and non-hemorrhagic complications.
Conclusion
Patients with acute large-vessel ischemic stroke who received ticagrelor within the first 24 hours after ischemic stroke had better clinical outcomes based on recurrent stroke rates, NIHSS reduction, and favorable mRS rates compared with those who received clopidogrel. There were no differences between ticagrelor and clopidogrel regarding hemorrhagic and non-hemorrhagic complications.
Trial Registration
Clinical trials.gov (NCT06120725).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Large-vessel occlusion (LVO) ischemic stroke represents about 25–40% of all ischemic strokes, and it raises the mortality risk by more than double when compared with non-LVO stroke [1, 2]. The term LVO is used when there is a blockage of the intracranial part of the internal carotid artery, proximal middle cerebral arteries, intracranial vertebral artery (VA), and basilar artery (BA) [3].
Clopidogrel works by irreversibly blocking the binding of adenosine diphosphate to its platelet P2Y12 receptor and activating the glycoprotein IIb/IIIa complex, preventing platelets from sticking together in acute ischemic events [4]. Ticagrelor is a reversible P2Y12 receptor antagonist, metabolized primarily via the cytochrome P-450 (CYP3A4) enzyme, that inhibits platelet activation and aggregation irrespective of CYP2C19 genotypes [5, 6]. Subgroup analysis of many trials such as the CHANCE, CHARISMA and MATCH trials did not support combination therapy over single antiplatelet therapy in large-vessel stroke [7,8,9].
Few clinical trials compared ticagrelor versus clopidogrel in ischemic stroke patients; all these studies included only patients with a transient ischemic attack or minor stroke; moreover, none of these studies included patients from North Africa [10, 11]. We aimed in our study to compare ticagrelor versus clopidogrel in a first-ever trial for LVO moderate or moderate-to-severe ischemic stroke in Egypt.
2 Methods
2.1 Trial Design
After receiving approval from the ethics council of the Faculty of Medicine at Kafr el-Sheikh University, we executed our single-blinded, randomized controlled study and screened all patients presenting with their first-ever LVO ischemic stroke who sought medical advice in Kafr-Elsheikh Hospital and Nasr City Insurance Hospital during the period from July 1, 2022 to November 1, 2023. The last patient was enrolled in our study on July 15, 2023; we followed up patients for 90 days after stroke onset. In a one-to-one ratio, 580 first-ever LVO stroke participants were randomly assigned to receive loading and maintenance doses of ticagrelor or clopidogrel.
2.2 Participants
We had two parallel groups. The (A) group included 290 patients who received a 180-mg loading dose of ticagrelor during the first 24 hours following stroke onset, followed by 90 mg twice daily from day 2 to day 90. The (B) group included 290 patients who received a 300-mg loading dose of clopidogrel followed by 75 mg once daily from the Day 2 to Day 90).
2.3 Eligibility Criteria
We enrolled males and females aged 18–75 years with their first-ever large-vessel arterial ischemic stroke. Large-vessel stroke was defined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification when patients had clinical and brain imaging findings of >50% stenosis or occlusion of at least one of the following arterial segments on computed tomography angiography (CTA) or magnetic resonance angiography (MRA) if CTA was contraindicated: intracranial portion of internal carotid arteries, middle cerebral arteries (M1/M2), intracranial portion of vertebral arteries, and basilar artery, and patients had cortical or cerebellar lesions and brain stem or subcortical hemispheric infarcts > 1.5 cm in diameter on CT or MRI, and there were no potential sources of cardiogenic embolism [12].
We did not exclude patients with previous transient ischemic attack (TIA). All patients were ineligible for recombinant tissue plasminogen activator (rt-PA) therapy or thrombectomy to avoid clouding safety assessment [13].
We excluded patients with allergies to any of the studied drugs or who suffered from clinical seizures as a part of their stroke, those with major organ failure, malignancies, or myocardial infarction during the past 6 weeks, and patients who took regular antiplatelets or anticoagulants in the previous week to avoid clouding our drug safety assessment [13].
We excluded patients with a minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤ 3) or severe stroke (NIHSS ≥ 25), patients who had spontaneous resolution of symptoms before imaging, and patients with history of a CNS disorder (e.g., multiple sclerosis, epilepsy, meningioma). Patients were also not eligible if carotid, cerebrovascular, or coronary revascularization was planned, requiring halting study treatment within 7 days after randomization.
Patients who experienced a cardioembolic stroke either prior to or post-treatment were not included in our study. Cardio-embolic strokes were diagnosed when the patient exhibited a potential cardiac source of embolus such as mechanical cardiac valves, atrial fibrillation (AF), mitral valve prolapse, aortic valve stenosis or calcification, and patent foramen ovale [14]. Patients were diagnosed with clinical AF based on the presence of a conventional 12-lead electrocardiography (ECG) recording that exhibited a minimum of 30 seconds of cardiac rhythm, showing the absence of identifiable recurring P waves and irregular RR intervals (when atrioventricular conduction is not impaired) [15].
We excluded patients with a source of gastrointestinal bleeding such as peptic ulcers, patients with recurrent stroke based on appropriate clinical history, examination, and/or MRI brain findings, and those who had a blood glucose level < 50 or > 400 mg/dL or platelet count < 100,000 or international normalized ratio > 1.4 or prothrombin time > 18.
We excluded patients who were regular users of drugs that affected clopidogrel metabolism, such as proton pump inhibitors, ketoconazole, dihydropyridine calcium channel blockers, and rifampin [16]. We also excluded pregnant or lactating females, patients with venous infarction, and ischemic infarction secondary to hypo-perfusion.
2.4 Interventions
We evaluated all patients with their first-ever large-vessel ischemic stroke who presented to Kafr-Elsheikh Hospital and Nasr City Insurance Hospital in the period from September 1, 2021 to September 1, 2023, recording detailed personal history, risk factors assessment, and the onset of stroke. Every patient screened for eligibility had a 12-lead routine ECG and transthoracic echocardiography (TTE) to detect atrial fibrillation and valvular heart diseases before enrolment in the study.
Baseline laboratory examinations (including lipid profiles, liver functions, coagulation profiles, complete blood counts, and fasting and postprandial blood glucose levels and HbA1C), carotid duplex imaging, and continuous cardiac rhythm monitoring for 1 day were performed for all patients after enrolment. Blood pressure was also assessed, and we diagnosed hypertension when systolic blood pressure was > 130 mmHg and/or diastolic blood pressure was > 85 mm/Hg on at least three different occasions [17].
We diagnosed diabetes mellitus when the fasting plasma glucose level was > 126 mg/dL and/or casual plasma glucose was > 200 mg/dL, and/or HbA1C was > 6.5 [17], and we diagnosed admission hyperglycemia when the admission blood glucose value was > 140 mg/dL [18]. Regarding managing hyperglycemia, we aimed to maintain blood glucose levels below 140 mg/dL and 180 mg/dL. We withheld all usual antidiabetic treatments and used periodic subcutaneous regular insulin injections, adjusted according to blood glucose levels. Patients received rapid-acting insulins immediately after meals based on the amount of carbohydrates consumed. Four units of rapid-acting analog insulin were used for standard meals containing 60 g of carbohydrates, and the blood glucose level was followed up every 3 hours. If the blood glucose concentration did not reach the target at 24 and 48 hours, the subcutaneous insulin dose, including long-acting basal insulin, was increased [19].
We diagnosed hyperlipidemia when blood cholesterol was >200 mg/dL, triglycerides were >150 mg/dL, low-density lipoprotein cholesterol (LDL-C) was >100 mg/dL and/or high-density lipoprotein cholesterol (HDL-C) was <40 mg/dL [20]. Regarding the management of hyperlipidemia, patients received rosuvastatin 20 mg daily and ezetimibe 10 mg to achieve LDL-C <70 mg/dL. We chose rosuvastatin as, among the statins, simvastatin, lovastatin, and atorvastatin are metabolized by cytochrome P450 3A4 (CYP3A4), fluvastatin is metabolized by CYP2C9, while rosuvastatin undergoes little metabolism and has limited drug–drug interaction with clopidogrel [21]. After 4–12 weeks of starting anti-hyperlipidemia agents, we performed a follow-up fasting lipid profile [22].
Every patient underwent brain CT and brain MRI using the following stroke protocol: T1W, T2W, fluid-attenuated inversion recovery (FLAIR), diffusion weighted imaging (DWI), T2 gradient-echo, CTA, or MRA (if CTA was contraindicated), from the aortic arch through the circle of Willis. Two neuroradiologists blinded to treatment reviewed CT and MRI source images. Cerebrovascular vessels were divided into segments: supra-clinoid internal carotid artery, first-division middle cerebral artery (M1), second-division middle cerebral artery (M2), basilar artery (BA), and intracranial vertebral artery (VA). A neuroradiologist determined whether any of these vascular segments were occluded. If there was no vascular occlusion, the patient was documented as having no large vessel occlusion. If one or more vascular segments were occluded and the patient was ineligible for thrombectomy or arterial stenting, the case history and NIHSS score were reviewed; if the vascular occlusion was in the appropriate territory to account for the clinical findings, the case was judged as having a large-vessel occlusion.
Our study follow-up was via telephone calls twice per week and a face-to-face interview in the outpatient clinic once per month, and continued for 3 months. If any of our patients complained of symptoms suggesting recurrent stroke in the follow-up calls, they were advised to go to the hospital.
2.5 Outcomes
2.5.1 Primary Outcomes
The primary efficacy outcome was a new stroke event (ischemic or hemorrhagic) at 90 days in each group [7], while the primary safety outcome was the rate of hemorrhagic complications evaluated using the PLATO bleeding definition, which classified hemorrhagic complications into three types: major, minor, and minimal bleeding [23].
Hemorrhagic infarction was detected using a follow-up CT brain scan after 2 days and after 1 week or discharge; additionally, the European Cooperative Acute Stroke Study (ECASS) classification [24] was used to detect the type of hemorrhagic infarction.
2.5.2 Secondary Outcomes
The secondary efficacy outcomes were the rates of patients who achieved a significant reduction in NIHSS (decrease of four points or more) [25] on the day 7 or discharge, the rates of favorable outcome with modified Rankin scale (mRS) of 0–2 [26] after 1 week and 90 days, and rates of a composite of new stroke, myocardial infarction and death due to vascular events during the follow-up period. The secondary safety outcomes were the rate of treatment-related adverse effects assessed by a follow-up questionnaire and the rate of death due to vascular or non-vascular causes in each group.
2.6 Sample Size
After using Power Analysis & Sample Size System (PASS, V12, NCSS), we determined that a total of 524 large-vessel ischemic stroke patients would provide 80% power to detect a relative risk reduction of 35% in new ischemic stroke (primary outcome) in the ticagrelor group as compared with the clopidogrel group, with a final two-sided significance level of 95% and alpha error of 5%, assuming an incidence of new ischemic stroke of 13.6% [27] in the clopidogrel group and an overall dropout rate of 5%. The final size of our trial was 580 patients, 290 patients in each group.
2.7 Randomization and Blinding
Before assigning patients to a group, we collected written informed consent from them or their next of kin.
Our study was single-blinded to the investigators; an independent statistician generated a computer-generated randomization chart with a block size of four in a one-to-one ratio, and participants were randomly assigned to receive either ticagrelor or clopidogrel by a specially trained and qualified nurse. None of the investigators included in the study knew the patients’ assignments. We prepared 580 labels denoting either Drug A or Drug B. According to the randomization chart, these were placed into sequentially numbered opaque sealed envelopes numbered 1–580. Patients were given enrolment numbers starting from 1. Envelopes carrying the same number as the patient enrolment number were attached to the corresponding file. Once opened, the patients were assigned to receive Drug A or Drug B. Drug A comprised ticagrelor pills and Drug B comprised clopidogrel pills. The statistical analysis was performed by an independent statistician who did not know which treatment protocol was assigned for groups A and B. The follow-up calls were made by a specially trained and qualified nurse who was in contact with a neurology consultant; a consultant neurologist and a specially trained nurse conducted the follow-up interviews. All patients were asked not to tell the physician about their treatment. We did not use a placebo in our trial due to a lack of funding and financial support.
2.8 Statistical Analysis of the Data
We used the IBM SPSS software package, version 20.0 (IBM Corp., Armonk, NY, USA), to analyze our data and based all efficacy and safety analyses on the intention-to-treat principle. Both the primary and secondary outcomes underwent separate statistical analyses. Depending on their distribution, as determined by the Shapiro–Wilk test, we described numerical data as mean and standard deviation (SD) or median and interquartile range (IQR). We also reported categorical data using numbers and percentages. The Mann–Whitney U test was used to compare the irregularly distributed numerical data, while Pearson’s Chi-square was utilized to correlate categorical data. In our study, all the data were included. All statistical analyses were two-sided, and differences with a p value of <0.05 were considered statistically significant. To avoid type 1 statistical errors in the analysis of secondary efficacy outcomes, we used correction for multiple comparisons, and secondary efficacy outcomes differences with an adjusted p value of < 0.0125 were considered statistically significant. Survival analysis was performed using the Kaplan Meier test and log-rank method to study the effect of the antiplatelet on the incidence of outcomes during the 3-month follow-up period. The Cox regression method obtained the hazard ratio (HR) at a 95% confidence interval (CI). An HR is considered significant when it does not fall between the lower and upper CI.
3 Results
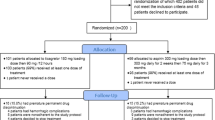
We evaluated 950 patients to assess their eligibility to be included in our trial; 370 patients were excluded from the randomization, of which 66 patients were regularly using oral anticoagulants, 28 patients had received endovascular thrombectomy, 21 patients had renal failure, 57 patients had platelet count < 100,000, 73 patients had NIHSS ≤ 3, 49 patients had NIHSS ≥ 25, 28 patients did not have large vessel occlusion on CTA, and 48 patients declined to participate. Five hundred and eighty patients (352 males, 228 females) were randomly assigned in a one-to-one ratio to receive either ticagrelor or clopidogrel; 553 patients completed the 3-month study duration (Fig. 1).
We found no statistically significant differences between the characteristics of the two groups (Table 1).
Thirty patients (10.3%) in the ticagrelor arm and 49 patients (16.9%) in the clopidogrel group experienced a new ischemic or hemorrhagic stroke at 90 days (HR 0.61; 95% CI 0.38–0.98; p = 0.04) (Table 2, Fig. 2). Eighty-nine (30.7%) patients in the ticagrelor group and 61 (21%) patients in the clopidogrel group showed a significant reduction in NIHSS after 1 week (HR 0.59; 95% CI 0.41–0.86; p = 0.008); 86 (29.7%) patients in the ticagrelor group and 58 (20%) patients in the clopidogrel group experienced favorable mRS scores after 1 week (HR 0.60; 95% CI 0.42–0.87; p = 0.007); 99 (34.1%) patients in the ticagrelor group and 70 (24.1%) in the clopidogrel group experienced favorable mRS scores after 3 months (HR 0.65; 95% CI 0.47–0.90; p = 0.008); and 36 (12.4%) patients in the ticagrelor group and 57 (19.7%) patients in the clopidogrel group experienced composite of recurrent ischemic or hemorrhagic stroke, MI, or death due to vascular insults (HR 0.56; 95% CI 0.37–0.87; p = 0.009) (Table 2).
In the ticagrelor arm, 22 (7.6%) patients had drug-related hemorrhagic complications, of which nine patients had minimal bleeding, three patients had minor bleeding, and ten patients (3.4%) experienced major bleeding; on the other hand, in the clopidogrel group, 15 (5.2%) patients had drug-related hemorrhagic complications, of which four patients had minimal bleeding, two patients had minor bleeding, and nine patients (3.1%) experienced major bleeding (HR 0.80; 95% CI 0.41–1.55; p = 0.50) (Table 3, Fig. 3).
Ten patients (3.4%) in the ticagrelor group and nine patients (3.1%) in the clopidogrel group had hemorrhagic infarction (HR 0.77; 95% CI 0.31–1.90; p = 0.57); 35 (12.1%) in the ticagrelor group and 37 (12.8%) patients in the clopidogrel group had drug-related non-hemorrhagic side effects (HR 0.78; 95% CI 0.49–1.25; p = 0.31); and four patients in the ticagrelor group and three patients in the clopidogrel group died due to vascular and non-vascular complications (HR 0.50; 95% CI 0.10–2.60; p = 0.41) (Table 3).
In our study, 404 patients had an anterior circulation stroke, of whom 207 were assigned to the ticagrelor group and 197 were assigned to the clopidogrel group, and 176 had posterior circulation stroke, of whom 83 were assigned to the ticagrelor group and 93 were assigned to the clopidogrel group.
When we performed subgroup analysis of the efficacy and safety outcomes according to the circulation affected by ischemic stroke, we found the following results: in anterior circulation stroke, 20 patients (10.3%) in the ticagrelor arm and 33 patients (16.9%) in the clopidogrel group experienced a recurrent ischemic or hemorrhagic stroke at 90 days (HR 0.47; 95% CI 0.27–0.84; p = 0.01) (Table 4, Fig. 4). Also, we found that 62 (30%) patients in the ticagrelor group and 41 (20.8%) patients in the clopidogrel group showed a significant reduction in NIHSS after 1 week (HR 0.51; 95% CI 0.32–0.82; p = 0.005), 60 (29%) patients in the ticagrelor group and 38 (19.3%) patients in the clopidogrel group experienced favorable mRS scores after 1 week (HR 0.57; 95% CI 0.3–0.87; p = 0.010), 70 (33.8%) patients in the ticagrelor group and 47 (23.9%) in the clopidogrel group experienced favorable mRS scores after 3 months (HR 0.60; 95% CI 0.40–0.88; p = 0.009), and 24 (11.6%) patients in the ticagrelor group and 40 (20.3%) patients in the clopidogrel group experienced composite of recurrent ischemic or hemorrhagic stroke, MI, or death due to vascular insults (HR 0.48; 95% CI 0.28–0.82; p = 0.007) (Table 4).
In anterior circulation stroke, 14 (6.8%) patients in the ticagrelor arm, and 9 (4.6%) patients in the clopidogrel group had drug-related hemorrhagic complications (HR 1.94; 95% CI 0.81–4.66; p = 0.14), 7 patients (3.4%) in the ticagrelor group and 6 patients (3%) in the clopidogrel group had hemorrhagic infarction (HR 2.3; 95% CI 0.66–8.04; p = 0.20), 24 (12.1%) in the ticagrelor group and 25 (12.8%) patients in the clopidogrel group had drug-related non-hemorrhagic side effects (HR 1.32; 95% CI 0.75–2.36; p = 0.32), and 2 patients in each group died due to vascular and non-vascular complications (HR 1.47; 95% CI 0.20–10.56; p = 0.70) (Table 5).
In posterior circulation stroke, ten patients (12%) in the ticagrelor arm and 16 patients (17.2%) in the clopidogrel group experienced a recurrent ischemic or hemorrhagic stroke at 90 days (HR 2.45; 95% CI 1.19–5.04; p = 0.02) (Table 6). Also, we found that 27 (32.5%) patients in the ticagrelor group and 20 (21.5%) patients in the clopidogrel group showed a significant reduction in NIHSS after 1 week (HR 2.41; 95% CI 1.23–4.71; p = 0.010), 26 (31.3%) patients in the ticagrelor group and 20 (21.5%) patients in the clopidogrel group experienced favorable mRS scores after 1 week (HR 2.61; 95% CI 1.33–5.11; p = 0.005), 29 (34.9%) patients in the ticagrelor group and 23 (24.7%) in the clopidogrel group experienced favorable mRS scores after 3 months (HR 2.23; 95% CI 1.25–3.97; p = 0.007), and 12 (14.5%) patients in the ticagrelor group and 17 (18.3%) patients in the clopidogrel group experienced composite of recurrent ischemic or hemorrhagic stroke, MI, or death due to vascular insults (HR 3.34; 95% CI 1.36–8.24; p = 0.009) (Table 6).
In posterior circulation stroke, 8 (9.6%) patients in the ticagrelor arm and 6 (6.5%) patients in the clopidogrel group had drug-related hemorrhagic complications (HR 1.58; 95% CI 0.53–4.76; p = 0.42), 3 patients (3.4%) in each group had hemorrhagic infarction (HR 0.51; 95% CI 0.08–3.15; p = 0.47), 11 (13.3%) in the ticagrelor group and 12 (12.9%) patients in the clopidogrel group had drug-related non-hemorrhagic side effects (HR 0.91; 95% CI 0.39–2.10; p = 0.82), and 2 patients in the ticagrelor group and 1 patient in the clopidogrel group died due to vascular and non-vascular complications (HR 0.58; 95% CI 0.05–6.5; p = 0.66) (Table 7).
4 Discussion
Several trials examined the effectiveness of ticagrelor in preventing ischemic stroke, including the SOCRATES and THALES trials. The SOCRATES trial concluded that ticagrelor was not more effective than aspirin in reducing the occurrence of stroke, heart attack, or death within 90 days, specifically in cases of minor ischemic stroke or TIA associated with ipsilateral atherosclerotic stenosis [28, 29], while the THALES trial identified that in patients with minor-to-moderate non-cardioembolic ischemic stroke (NIHSS score ≤ 5) or TIA, ticagrelor and aspirin decreased the risk of stroke or death within 30 days as opposed to aspirin alone. However, there was no significant difference in the occurrence of disability between the two groups, and the rate of hemorrhagic complications was higher among the ticagrelor group [30].
Other trials compared ticagrelor versus clopidogrel in ischemic stroke; for example, the CHANCE-2 trial identified that in Chinese patients with minor ischemic stroke or TIA who harbored CYP2C19 loss-of-function alleles, the occurrence of stroke within 90 days was decreased with ticagrelor as opposed to clopidogrel, and there was no statistically significant difference in the risk of severe or moderate hemorrhagic complications between the two groups. The CHANCE-2 subgroup analysis identified that patients without intra-cranial arterial stenosis (ICAS) had better clinical outcomes when treated with ticagrelor and aspirin as opposed to clopidogrel and aspirin after a minor ischemic stroke or TIA [10, 31], while Wang et al. identified that Chinese patients with minor stroke or TIA who received ticagrelor and aspirin have a lower proportion of high platelet reactivity than those who are treated with clopidogrel plus aspirin [11].
All the trials that compared ticagrelor with clopidogrel in ischemic stroke included only patients with a transient ischemic attack or minor stroke and did not include patients from Africa. Our trial differed from the previously held studies as it is the first study worldwide that compared ticagrelor and clopidogrel in moderate and moderate-to-severe LVO ischemic stroke Egyptian patients.
All of our patients received single antiplatelets as all of our patients had large-vessel moderate or moderate-to-severe disabling ischemic stroke. To the best of our knowledge, there were no such trials that compared dual versus single antiplatelet in moderate and moderate-to-severe ischemic stroke. Although the SAMMPRIS trial concluded that, in TIA and non-disabling ischemic stroke patients with intracranial arterial stenosis, dual antiplatelet therapy with clopidogrel and aspirin was superior to percutaneous transluminal angioplasty and stenting (PTAS) in reducing the risk of early stroke [32], many other trials, such as subgroup analysis of CHANCE, CHARISMA and MATCH trials [7,8,9], did not support combination therapy over single antiplatelet in large-vessel stroke; in addition, the median baseline NIHSS of all of our patients was 9–10, and this could cause a different response to dual antiplatelet therapy than the response observed in those with TIA or minor stroke, as our patients had a more severe neurological impairment and larger areas of ischemic injury, which may have a greater risk of a brain hemorrhage [33].
We found no difference between the two groups concerning hemorrhagic and non-hemorrhagic adverse effects. This result agrees with the finding of Wang et al. (2019), Yang et al. (2020), and Wang et al. [10, 11, 34].
In our trial, the ticagrelor group had fewer recurrent strokes and better clinical outcomes regarding reduction in NIHSS and favorable mRS than the clopidogrel group. Our findings agree with Wang et al. [10, 11, 31].
When we performed subgroup analysis according to the circulation affected, we found that in both anterior and posterior circulation strokes, the ticagrelor group had better results regarding the rates of any stroke at 90 days, the rates of patients who achieved favorable mRS at 1 week and 90 days, and there was no significant difference between the two groups concerning hemorrhagic and non-hemorrhagic adverse effects.
The fewer recurrent strokes and better clinical outcomes noted in the ticagrelor group in comparison with the clopidogrel group could be because ticagrelor is an active drug that is metabolized primarily via the CYP3A4 enzyme, unlike clopidogrel which is a prodrug, and the conversion of clopidogrel to its active metabolite involved two sequential oxidative steps; the first one leads to formation of 2-oxo-clopidogrel, followed by the conversion of 2-oxoclopidogrel to the active metabolite. CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4/5 are implicated as cytochrome P450 enzymes in the metabolism of clopidogrel [35]; drug–drug interactions of clopidogrel were reported with atorvastatin, the calcium-channel antagonist verapamil, and the proton-pump inhibitor omeprazole [35]. As a result, ticagrelor had a much more rapid onset of action and a more powerful effect in reducing platelet reactivity. The PRINCE trial stated that patients with minor stroke or transient ischemic attack who are treated with ticagrelor plus aspirin have a lower proportion of high platelet reactivity than those who are treated with clopidogrel plus aspirin. The reduction in platelet reactivity is associated with a reduction in recurrent vascular events [11].
In addition, inflammatory high sensitivity C-reactive protein (hs-CRP) is associated with recurrent vascular events [31], especially among patients with symptomatic intra-cranial arterial stenosis (ICAS) in whom hs-CRP reflects the intracranial atherosclerotic burden [31]. This could indicate that the inflammatory burden plays an interactive role in the effectiveness of antiplatelet therapy. Clopidogrel had weak anti-inflammatory effect and the analysis of dual antiplatelet therapy in patients with ICAS and hs-CRP in the CHANCE trial found that clopidogrel plus aspirin therapy was effective only in patients with ICAS with non-elevated hs-CRP (< 3 mg/L), not in patients with ICAS with elevated hs-CRP (≥ 3 mg/L) [31]. On the other hand, ticagrelor had a powerful anti-inflammatory effect by inhibiting neurotoxic and thrombogenic eicosanoids, including thromboxane B2 [36], and it reduced interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels in diabetic patients with non-ST segment elevation acute coronary syndrome [37]. Also, studies showed that ticagrelor combined with rosuvastatin decreased myocardial mRNA levels of IL-1β, IL-6, and NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) in diabetic rats with ischemia-reperfusion injury [38].
The metabolism of clopidogrel is affected in patients with CYP2C19 loss-of-function alleles. However, there is no such study that evaluated the prevalence of CYP2C19 loss-of-function alleles in Egypt. Nevertheless, among African and Caucasian patients, the prevalence of CYP2C19 loss-of-function alleles is about 5–10% [39], unlike the prevalence of 50–60% in the Asian population and 25–28% in the European population [40], but a genetic sub-study of the Platelet Inhibition and Patient Outcomes (PLATO) trial indicated that ticagrelor is more efficacious than clopidogrel for acute coronary syndromes, regardless of the CYP2C19 genotype [7].
Our trial had some advantages as it was the first blinded RCT worldwide that compared ticagrelor versus clopidogrel in moderate and moderate to severe LVO ischemic stroke in Africa, but it had some limitations: (i) the relatively small sample; (ii) our trial was single-blinded, where only the investigators and the statistician were blinded to the treatment groups—this issue might lead to the placebo effect [41], which might leave room for bias in adverse events assessment and drug continuation; and (iii) all the patients were Egyptian, so we need to pursue a double-blinded larger scale randomized trial powered for both safety and efficacy to establish the validity and generalizability of the results.
5 Conclusion
Patients with acute large-vessel occlusion stroke who received ticagrelor within the first 24 hours after ischemic stroke had better clinical outcomes based on recurrent stroke rates, NIHSS reduction, and favorable mRS rates compared with those who received clopidogrel. There were no differences between ticagrelor and clopidogrel regarding hemorrhagic and non-hemorrhagic complications.
References
Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8(Nov):1–5.
Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and tia. Stroke. 2009;40(12):3834–40.
Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, Olson SE, Pannell JS, et al. Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Clin Neurosurg. 2019;85(1):S4-8.
Meyer DM, Albright KC, Allison TA, Grotta JC. LOAD: a pilot study of the safety of loading of aspirin and clopidogrel in acute ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis. 2008;17(1):26–9.
Tantry US, Bliden KP, Wei C, Storey RF, Armstrong M, Butler K, et al. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3(6):556–66.
Aref HM, El-Khawas H, Elbassiouny A, Shokri HM, Zeinhom MG, Roushdy TM. A randomized pilot study of the efficacy and safety of loading ticagrelor in acute ischemic stroke. Neurol Sci. 2023;44(2):765–71. https://doi.org/10.1007/s10072-022-06525-7.
Liu L, Wong KSL, Leng X, Pu Y, Wang Y, Jing J, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology. 2015;85(13):1154–62.
Bhatt DL, Fox KAA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17.
Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet (Lond, Engl). 2004;364(9431):331–7.
Wang Y, Meng X, Wang A, Xie X, Pan Y, Johnston SC, et al. Ticagrelor versus clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. N Engl J Med. 2021;385(27):2520–30.
Wang Y, Chen W, Lin Y, Meng X, Chen G, Wang Z, et al. Ticagrelor plus aspirin versus clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: open label, blinded endpoint, randomised controlled phase II trial. BMJ. 2019;365:1–11.
Adams HPJ, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in acute stroke treatment (TOAST). Neurology. 1999;53(1):126–31.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke A. Stroke. 2019;50:344–418.
Ntaios G, Hart RG. Embolic stroke. Circulation. 2017;136(25):2403–5.
Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GA, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498.
Bates ER, Lau WC, Angiolillo DJ. Clopidogrel-drug interactions. J Am Coll Cardiol. 2011;57(11):1251–63.
Ahmed SR, Mohamed AAM, Salem HH, Helmy S, Moustafa RR, Borham SMF. Association of white matter hyperintensities with migraine phenotypes and response to treatment. Acta Neurol Belg. 2022. https://doi.org/10.1007/s13760-022-02015-x.
Alvarez-Sabín J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke. 2003;34(5):1235–41.
Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE Randomized Clinical Trial. JAMA. 2019;322(4):326–35. https://doi.org/10.1001/jama.2019.9346.
Yao YS, Di Li T, Zeng ZH. Mechanisms underlying direct actions of hyperlipidemia on myocardium: an updated review. Lipids Health Dis. 2020;19(1):23. https://doi.org/10.1186/s12944-019-1171-8.
Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. Can Med Assoc J. 2005;172(10):1307–12.
Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Vol. 52. Stroke. 2021;2021:364–467.
Schulman S, Anger SU, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202–4.
Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, et al. N Engl J. 2011;1317–29.
Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–82.
Furlan AJ. Acute stroke trials: Strengthening the underpowered. Stroke. 2002;33(6):1450–1.
Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003.
Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375(1):35–43.
Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16(4):301–10. https://doi.org/10.1016/S1474-4422(17)30038-8.
Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020;383(3):207–17.
Wang C, Jia W, Jing J, Meng X, Wang A, Xu Q, et al. Ticagrelor versus clopidogrel in minor stroke or transient ischemic attack with intracranial artery stenosis: a post hoc analysis of CHANCE-2. J Am Heart Assoc. 2023;12(21):1–12.
Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis for the SAMMPRIS Trial Investigators *. N Engl J Med. 2013;365(11):993–1003.
Wang Y, Pan Y, Li H, Amarenco P, Denison H, Evans SR, et al. Efficacy and safety of ticagrelor and aspirin in patients with moderate ischemic stroke: an exploratory analysis of the THALES randomized clinical trial. JAMA Neurol. 2021;78(9):1091–8. https://doi.org/10.1001/jamaneurol.2021.2440.
Yang Y, Chen W, Pan Y, Yan H, Meng X, Liu L, et al. Effect of ticagrelor versus clopidogrel on platelet reactivity measured by thrombelastography in patients with minor stroke or TIA. Aging (Albany NY). 2020;12(20):20085–94.
Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genom. 2010;20(7):463–5.
Van KF, Ciabattoni G, Patrono C, Schmitz PIM, Van GJ, Koudstaal PJ. Evidence for episodic platelet activation in acute ischemic stroke. Stroke. 1994;25(2):278–81.
Jeong HS, Hong SJ, Cho SA, Kim JH, Cho JY, Lee SH, et al. Comparison of ticagrelor versus prasugrel for inflammation vascular function, and circulating endothelial progenitor cells in diabetic patients with non-ST-segment elevation acute coronary syndrome requiring coronary stenting: a prospective, randomized. JACC Cardiovasc Interv. 2017;10(16):1646–58.
Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc drugs Ther. 2016;30(6):539–50.
Dehbozorgi M, Kamalidehghan B, Hosseini I, Dehghanfard Z, Sangtarash MH, Firoozi M, et al. Prevalence of the CYP2C19*2 (681 G>A), *3 (636 G>A) and *17 (-806 C>T) alleles among an Iranian population of different ethnicities. Mol Med Rep. 2018;17(3):4195–202.
Minderhoud C, Otten LS, Hilkens PHE, van den Broek MPH, Harmsze AM. Increased frequency of CYP2C19 loss-of-function alleles in clopidogrel-treated patients with recurrent cerebral ischemia. Br J Clin Pharmacol. 2022;88(7):3335–40. https://doi.org/10.1111/bcp.15282.
Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension p. Lancet. 2017;389(10088):2473–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Mohamed G. Zeinhom, Ahmed Elbassiouny, Ahmed Mahmoud Mohamed, and Sherihan Rezk Ahmed declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Author Contributions
Dr Zeinhom and Dr Ahmed were the principal investigators, Dr Zeinhom and Dr Elbassiouny were the main supervisors of the study; Dr Mohamed shared in the study plan revision and supervision, while Dr Zeinhom shared in manuscript writing. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Ethical Approval
Our study had the approval of the ethical committee of Kafr El-Sheikh University, and the ethical reference number is (MKSU:50-9-6).
Consent to Participate
Written informed consent was obtained from all subjects before the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from Mohamed G. Zeinhom on reasonable request.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zeinhom, M.G., Elbassiouny, A., Mohamed, A.M. et al. Ticagrelor Versus Clopidogrel in Acute Large-Vessel Ischemic Stroke: A Randomized Controlled Single-Blinded Trial. CNS Drugs 38, 387–398 (2024). https://doi.org/10.1007/s40263-024-01080-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-024-01080-5