Abstract
Introduction
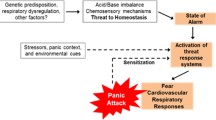
Depression, anxiety, and/or panic disorder are often comorbid and have a complex etiology mediated through the same neuronal network. Cholecystokinin-tetrapeptide (CCK-4), a synthetic analog of the endogenous neuropeptide cholecystokinin (CCK), is thought to be implicated in this network. The CCK-4 challenge model is an accepted method of investigating the pathophysiology of panic and has been shown to mediate neuronal activation via the transient receptor potential canonical (TRPC) ion channels.
Objectives
This study aimed to assess the pharmacodynamic effects of BI 1358894, a small-molecule inhibitor of TRPC ion channel members 4 and 5 (TRPC4/5), on CCK-4-induced anxiety/panic-like symptoms and evaluate circuit engagement.
Methods
Twenty healthy male CCK-4-sensitive volunteers entered a Phase I, double blind, randomized, two-way cross-over, single dose, placebo-controlled trial. Randomization was to oral BI 1358894 100 mg in the fed state followed by oral placebo in the fed state, or vice versa. Treatments were administered 5 h prior to intravenous CCK-4 50 µg. The primary endpoint was maximum change from baseline of the Panic Symptom Scale (PSS) sum intensity score after CCK-4 injection. Further endpoints included the emotional faces visual analog score (EVAS), the Spielberger State-Trait Anxiety Inventory (STAI), plasma adrenocorticotropic hormone (ACTH), and serum cortisol values. The safety and tolerability of BI 1358894 was assessed based on a number of parameters including occurrence of adverse events (AEs). All pharmacodynamic, pharmacokinetic, and safety endpoints were analyzed using descriptive statistics.
Results
Single oral doses of BI 1358894 were generally well tolerated by the healthy male volunteers included in this study. Adjusted mean maximum change from baseline in PSS sum intensity score was 24.4 % lower in volunteers treated with BI 1358894 versus placebo, while adjusted mean maximum change from baseline of EVAS was reduced by 19.2 % (BI 1358894 vs placebo). The STAI total score before CCK-4 injection was similar in both groups (placebo: 25.1; BI 1358894: 24.3). Relative to placebo, BI 1358894 reduced CCK-4-induced mean maximum plasma ACTH and serum cortisol values by 58.6 % and 27.3 %, respectively. Investigator-assessed drug-related AEs were reported for 13/20 participants (65.0 %). There were no serious or severe AEs, AEs of special interest, AEs leading to discontinuation of trial medication, or deaths.
Conclusions
Overall, BI 1358894 reduced psychological and physiological responses to CCK-4 compared with placebo, as measured by PSS, subjective EVAS and objectively measured stress biomarkers. BI 1358894 had a positive safety profile, and single oral doses were well tolerated by the healthy volunteers. This trial (NCT03904576/1402-0005) was registered on Clinicaltrials.gov on 05.04.19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Compared to placebo, BI 1358894 reduced psychological and physiological responses to cholecystokinin-tetrapeptide, as measured by the panic symptom scale, subjective emotional faces visual analog score and objectively measured stress biomarkers (adrenocorticotrophic hormone, cortisol). |
BI 1358894 was safe and well tolerated at the tested doses. |
1 Introduction
Depression, anxiety, and/or panic disorders are often comorbid; the etiology of these disorders is complex and appears to be mediated through the same neuronal network and involve several distinct regions of the brain [1,2,3,4]. Cholecystokinin-tetrapeptide (CCK-4), a synthetic analog of the endogenous neuropeptide cholecystokinin (CCK), is thought to be implicated in this network [4]. Of the different CCK receptors identified, the CCK-2 receptor exhibits a high affinity for CCK agonists (CCK-4 and the synthetic pentapeptide pentagastrin) and induces anxiety-like behavior in rats when bound to CCK-4 [5, 6]. Anxiety and short panic attacks that closely resemble spontaneous panic symptomatology have been induced by CCK-4 in patients with panic disorder [5, 7, 8]. Additionally, it has been shown that administration of CCK-4 leads to increases in regional cerebral blood flow in hypothalamic and claustrum-insular regions during peak panic symptoms [4, 9]. There is evidence for an interaction of CCK-4 with several other neurotransmitter systems; the serotonergic, noradrenergic and gamma-amino-butyric-acid (GABA) systems seem to be involved in the pathophysiology of CCK-4-induced panic [10, 11]. Based on these findings, experimental panic induction using CCK-4 was established as a challenge model to investigate the pathophysiology of panic [5]. Although the translational validity of this model of panic symptoms in healthy individuals has been questioned, a recent evaluation of CCK-4 studies suggests that CCK-4 in high doses is a useful and reliable agent to model panic attacks and assess the efficacy of new therapies for panic disorder [12, 13]. Sensitivity to CCK-4 is enhanced in patients with panic disorder (although not major depressive disorder [MDD]) relative to healthy controls [7, 14, 15]. In addition, it has been shown that CCK-4-induced neuronal activation is mediated via transient receptor potential canonical member 5 (TRPC5) ion channels [16], supporting targeting of TRPC5 ion channels as a potential treatment of panic symptoms. Several compounds, including benzodiazepines (lorazepam and alprazolam), selective serotonin reuptake inhibitors (SSRIs; escitalopram and fluvoxamine) and gamma-aminobutyric acid (GABA; tiagabine) reuptake inhibitors have been tested for their anti-panic properties using the CCK-4 challenge model [17,18,19,20,21]. Taken together, such studies corroborate the validity of the CCK-4 challenge model, and suggest that this is a suitable model to assess circuit engagement of TRPC ion channel inhibition.
Transient receptor potential canonical members 4 and 5 (TRPC4/5) are predominantly expressed in the amygdala and hippocampus [22, 23]. (TRPC)4/5 ion channels have been shown to be involved in anxiety and fear responses in rodent models [22, 24, 25], while TRPC4/5 inhibitors suppressed CCK-4-induced anxiety behaviors and antidepressant effects in preclinical studies in mice [25, 26]. When TRPC4/5 ion channels are not activated, they remain closed and block calcium from entering the axon terminal, which prevents transmission of a neuronal signal and generation of a physiological response [27, 28]. However, when an emotional stimulus is detected, these channels open, calcium enters the axon terminal, and a neuronal signal is transmitted, which generates a physiological response [27, 28]. Therefore, inhibition of these channels would prevent the influx of calcium into the neuronal axon terminal, dampening the related physiological responses. BI 1358894 is a small-molecule inhibitor of TRPC4/5 being developed for the treatment of post-traumatic stress disorder (PTSD) and MDD. Testing of this compound for potential interactions with more than 50 G protein coupled receptors, enzymes, transporters, and ion channels demonstrated no significant interactions at the expected free exposure levels with a 100 mg dose (data not published). In a Phase I clinical trial, BI 1358894 was well tolerated at a dose of ≤ 200 mg in healthy Caucasian volunteers [29]. As a result of the promising effects of TRPC4/5 inhibition on CCK-4-induced neuronal activation in vitro and anxiety in animal studies, a Phase I study (NCT03904576) exploring the effects of BI 1358894 on CCK-4-induced anxiety/panic-like symptoms in healthy humans was conducted, and results are presented here.
1.1 Objectives
The primary objective of the study was to investigate the pharmacodynamic effects of a single dose of BI 1358894 100 mg in the fed state on CCK-4-induced anxiety/panic-like symptoms using the Panic Symptom Scale (PSS) in preselected CCK-4-sensitive healthy volunteers in order to confirm central target engagement of BI 1358894. A further objective was to explore the pharmacodynamic effect of BI 1358894 on these symptoms using the emotional faces visual analog score (EVAS), Spielberger State-Trait Anxiety Inventory (STAI), and the effect on plasma adrenocorticotropic hormone (ACTH) and serum cortisol values as biomarkers/indicators of physiological stress.
2 Materials and Methods
2.1 Study Design
This was a Phase I, double blind, randomized (1:1), two-way cross-over, single dose, placebo-controlled study, in healthy male CCK-4 sensitive participants, comparing treatment with BI 1358894 to placebo (NCT03904576). The study took place at one site, PRA Health Sciences in the Netherlands. Treatments consisted of oral BI 1358894 100 mg (four 25-mg tablets) or oral matching placebo, both administered in the fed state (high fat/caloric meal 30 min before drug administration) and 5 hours (h) prior to the challenge agent, intravenous (IV) CCK-4 acetate 50 µg, which was administered at 13:00. Consistent timing of BI 1358894 and CCK-4 administration was maintained to avoid confounding factors relating to effects of circadian rhythms on data output. Treatment administration 5 h prior to the challenge agent was based on prior clinical data to ensure intended plasma concentrations of BI 1358894 were reached in > 95 % of participants. Participants were randomly allocated to one of two treatment sequences (BI 1358894 followed by placebo or placebo followed by BI 1358894), with a washout period of ≥ 17 days between treatments (Fig. 1). Randomization was performed by the sponsor using a validated system that was verified by an independent statistician. The block size was four and the randomization list was generated using a validated pseudo-random number generator and a supplied seed number such that the resulting allocation was both reproducible and non-predictable. The randomization list contained additional blocks to allow for participant replacement. Participants, investigators and persons directly involved in the conduct of the trial were blinded to trial treatments.
Study design. *Preselected CCK-4 sensitive healthy volunteers were randomized to one of two treatment sequences: oral BI 1358894 100 mg in the fed state followed by oral placebo in the fed state, or vice versa (washout period ≥17 days). Treatments were administered 5 h prior to IV CCK-4 (50 µg). CCK-4 cholecystokinin-tetrapeptide, IV intravenous, QD once daily, R randomization, S screening
The PSS and EVAS tests were performed prior to study initiation (CCK-4 screening visit); additional tests took place on Day 1, at the time of first treatment (BI 1358894 or placebo; time = 0 h), then at 4 h, 4:45 h, 5:05 h, 5:10 h, 5:20 h, and 5:30 h; Day 9 and Days 35–40 (end of study), with scores calculated for each time point. The STAI testing was carried out on Day 1 at 4:45 h posttreatment, Day 9 and Days 35–40 (end of study). Adrenocorticotropic hormone/cortisol samples were taken at screening, then on Day 1 at 4:45 h, 5:05 h, 5:10 h, 5:20 h, 5:30 h, 6:00 h, 7:00 h, 8:00 h, and 10:00 h. For each patient, the maximum concentration of the analyte (Emax) and the area under the effect–time curve of the analyte in plasma over the time interval 4:45 h to 10:00 h (AUEC4.75–10) was assessed and the mean change from baseline in plasma ACTH and cortisol was calculated for each time point.
2.2 Participants
Eligible participants were CCK-4 sensitive, healthy male volunteers, aged ≥ 18–65 years, with a body mass index (BMI) of 18.5–29.9 kg/m2. This study was limited to male participants as reproductive toxicology studies had not been performed at the time of this study. Sufficient CCK-4 sensitivity was defined as achievement of a sudden onset of anxiety and panic along with presence of at least 4 symptoms in the PSS with either a score of ≥ 2 on PSS item 15 (Anxiety and Fear for Apprehension) or achievement of a total PSS sum intensity score > 20 after IV CCK-4.
Key exclusion criteria included any finding deviating from normal in the medical examination that was considered to be clinically relevant, any evidence of a concomitant disease deemed clinically relevant by the investigator, disease of the central nervous system, being a smoker (more than 5 cigarettes or 3 pipes per day), drug abuse or a positive drug screening, inability to comply with the dietary regimen of the trial site, and any suicidal ideation of type 2–5 on the Columbia-Suicide Severity Rating Scale (C-SSRS) in the last 12 months.
2.3 Endpoints and Assessments
2.3.1 Pharmacodynamic Endpoints
The primary endpoint was maximum change from baseline of the PSS sum intensity score after CCK-4 injection. Further endpoints of interest were: time to maximum change from baseline in PSS sum intensity score, maximum PSS sum intensity score, PSS total number of symptoms, change from baseline in the PSS total number of symptoms, change from baseline in EVAS score, time to maximum change from baseline in EVAS score, maximum EVAS score, the value of each STAI for state anxiety (form Y1) before CCK-4 injection (Epre), and plasma levels of ACTH and serum levels of cortisol following CCK-4 injection (maximum concentration, AUEC of plasma/serum ACTH/cortisol concentrations over the time interval 4.45 h to 10 h [AUEC4.75–10] and time to maximum concentration of the analyte in plasma/serum [tmax]). The change from baseline in PSS and EVAS scores were defined as the score values after CCK-4 injection at each time point minus the last score value before CCK-4 injection.
The PSS is a patient-reported outcome measurement tool derived from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Revised (DSM-5-R), which is designed to assess CCK-4-induced panic symptoms in patients [14]. The PSS lists 18 symptoms to assess CCK-4-induced panic symptoms, including 4 somatic symptoms and 4 anxiety symptoms. Key symptoms are measured on a 5-point scale from 0 (not present) to 4 (severe) and a composite score is determined by summing the number and intensity rating of symptoms for each subject. These key symptoms include: dyspnea, palpitations/rapid heartbeat, sweating, faintness, unsteady feeling, dizziness, shaking/trembling, nausea, abdominal distress, choking, chest pain/discomfort, paresthesia, hot flushes/cold chills, feeling unreal/detached, anxiety/fear/apprehension [14].
The EVAS, an adaptation of the visual analog score (VAS), provides a subjective measure of current general emotional status of the participant using a 100-mm long horizontal line with verbal descriptors or word anchors at each end to express the extremes of each feeling [30]. Participants marked a position on the line that ranged between 6 facial expressions (smiling face to crying face), in accordance with their emotional status. This position was measured in mm from 0 mm (no symptoms) to 100 mm (severe symptoms).
The STAI is used to distinguish state anxiety (a temporary condition) from trait anxiety (a general tendency to perceive saturations as threatening). Participants rate themselves on a scale of 1–4 for 20 questions to evaluate their level of anxiety. Higher scores indicate greater levels of anxiety. Within this trial setting, as the STAI assessment was conducted before the CCK-4 challenge, the measure was considered to be an indicator of potential anticipatory anxiety in expectation of the response to CCK-4 [31].
2.3.2 Pharmacokinetic Endpoints
Pharmacokinetics were evaluated as further endpoints and were calculated for BI 1358894 and included maximum measured concentration of the analyte in plasma (Cmax), tmax (plasma), area under the concentration-time curve (AUC) of the analyte in plasma over the interval from 0 to the last quantifiable data point (AUC0–tz), AUC4.75–5.5 of the analyte, and the terminal half-life of the analyte in plasma (t1/2).
2.3.3 Safety and Tolerability Endpoints
The safety and tolerability of BI 1358894 was assessed based on adverse events (AEs; including clinically relevant findings from the physical and neurological examinations); safety laboratory tests; 12 lead electrocardiogram (ECG) measurements; continuous ECG monitoring; vital signs (blood pressure, pulse); and suicidality assessment (C-SSRS).
The provoked panic symptoms in response to CCK-4 injection (as documented by the PSS score) were considered pharmacodynamic parameters. As they were an expected and desired response in the context of this trial (even though they constitute an untoward medical occurrence), they were not recorded as AEs. All other observations not included on the PSS questionnaire that occurred during the AE reporting time period were documented as AEs.
2.4 Ethical Considerations
The trial was carried out in compliance with the clinical trial protocol, which was in accordance with the principles of the Declaration of Helsinki [32], the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice (ICH GCP) guidelines, applicable regulatory requirements and Boehringer Ingelheim standard operating procedures. All participants provided informed written consent in accordance with ICH GCP and local procedures. The study protocol was reviewed and approved by the local independent ethics committee (Medisch Ethische Toetsings Commissie van de Stichting Beoordeling Ethiek Bio-Medisch Onderzoek, Assen, The Netherlands) on April 10, 2019.
2.5 Statistical Analysis
All pharmacodynamic, pharmacokinetic, and safety endpoints were assessed descriptively. The adjusted mean maximum change from baseline in respective measures of PSS (sum intensity score) and EVAS (total score) per patient, and the Epre for STAI total score were assessed using a linear mixed-effects model. Covariates included effects for treatment, period, and subject, in addition to period baseline and subject baseline. Adjusted means, as well as two-sided 90 % confidence intervals (CI) were calculated. The change in plasma ACTH and serum cortisol following CCK-4 stimulation were compared to baseline levels by examining Emax and AUEC4.75–10 within each treatment group.
Sample size was determined based on the probability of observing a meaningful reduction in PSS sum intensity score after a single dose of BI 1358894 when compared with placebo. A reduction of at least 20% in PSS sum intensity score relative to placebo was considered meaningful. For these calculations, the following assumptions were made: the standard deviation (SD) of the maximum change from baseline of the treatment contrast of PSS sum intensity score relative to placebo is 20%; the maximum change from baseline for PSS sum intensity score relative to placebo is 0% after the placebo treatment. Based on these calculations, a sample size of 20, with at least 15 evaluable patients, was considered sufficient to achieve the aims of this exploratory study.
With respect to missing data, for PSS and EVAS, if the period baseline value was missing in treatment period 1, it was imputed by the corresponding value measured in treatment period 2. If the period baseline value was missing in treatment period 2, it was imputed by the corresponding value measured in treatment period 1. For the endpoint STAI, missing values were not imputed.
3 Results
3.1 Study Population and Disposition
A total of 20 healthy male participants entered the trial. Two participants were reported as protocol deviations and participated only in the first treatment period (one received BI 1358894 only and one received placebo only); meaning that 19 participants were treated with BI 1358894 and placebo, respectively, as shown in the supplemental figure (Online Resource 1). All 20 participants were included in the pharmacodynamic set and treated set, and 19 participants were included in the pharmacokinetic set. The mean (SD) age was 26.7 (9.5) years, the mean (SD) BMI was 23.0 (2.8) kg/m2, and 16/20 (80.0 %) were White. The baseline demographic characteristics were generally similar across all treatment groups as shown in the supplemental table (Online Resource 2).
3.2 Pharmacodynamics
3.2.1 PSS
The adjusted mean maximum change from baseline of PSS sum intensity score was lower with BI 1358894 treatment (22.6) than with placebo (29.9), with a decrease of 24.4 % relative to placebo and an absolute treatment difference of − 7.3 (90 % CI − 11.0, − 3.7) (Table 1; Fig. 2). Maximum change from baseline in mean PSS sum intensity score was reached 5 min after CCK-4 administration and returned almost to baseline within 10 min (Online Resource 3 and Fig. 2). Intra-individual comparisons of maximum PSS sum intensity scores during the CCK-4 challenge showed that for most subjects, maximum values were lower following administration of BI 1358894 than placebo (Fig. 2).
Mean effect-time profiles of a PSS change from baseline (± SD) and b EVAS change from baseline (± SD), and intra-individual and mean comparisons of c maximum PSS sum intensity score and d maximum EVAS scores during CCK-4 challenge administered 5 hours after a single oral administration of BI 1358894 or placebo. BI 1358894 or placebo were administered 5 h prior to CCK-4 challenge. CCK-4 cholecystokinin-tetrapeptide, Emax maximum PSS/EVAS score or change from baseline, EVAS emotional faces visual analog scale, PSS Panic Symptom Scale, SD standard deviation
The mean PSS total number of symptoms showed similar values at baseline (mean Ebase [SD]) for both BI 1358894 (0.3 [0.6]) and placebo (0.2 [0.4]); following CCK-4 treatment, mean maximum [SD] total number of symptoms was lower in the BI 1358894 group (11.0 [3.5]) versus the placebo group (12.6 [3.4]) (Online Resource 3). Maximum change from baseline in mean PSS total number of symptoms rated ≥1 was reached at 5 min after CCK-4 administration and declined within 20 min nearly to baseline (Online Resource 3). The maximum total number of symptoms was slightly lower following BI 1358894 administration than placebo (Fig. 2). Further PSS pharmacodynamic parameters are summarized in the supplemental table (Online Resource 3).
3.2.2 EVAS
The adjusted mean maximum change from baseline of EVAS score was lower with BI 1358894 treatment (53.3 mm) than with placebo (66.0 mm), with an absolute treatment difference of − 12.7 mm (90% CI − 20.0, − 5.4) and a percentage change relative to placebo of 19.2% (Table 1). The maximum change from baseline in mean EVAS score was reached at 5 min after CCK-4 administration and declined within 20 min nearly to baseline (Fig. 2 and Online Resource 3). Intra-individual comparisons of EVAS scores during the CCK-4 challenge demonstrated that for most participants, maximum values were lower following administration of BI 1358894 100 mg than following placebo (Fig. 2). Further EVAS pharmacodynamic parameters are summarized in the supplemental table (Online Resource 3).
3.2.3 Spielberger STAI
The Epre of the STAI total score was similar with placebo (25.1) and BI 1358894 treatment (24.3), revealing an absolute treatment difference of − 0.8 (90 % CI − 2.8, 1.2). Relative to placebo, BI 1358894 100 mg reduced the adjusted mean Epre of STAI total score by 3.1% (Table 1).
3.2.4 Biomarkers: ACTH and Cortisol
Maximum plasma ACTH and serum cortisol concentrations were reduced by 58.6% and 27.3%, respectively, following BI 1358894 administration compared with placebo (ACTH: mean [SD], 38.5 [22.6] pg/mL vs 92.9 [88.8] pg/mL; cortisol: 13.6 [4.4] µg/dL vs 18.7 [5.1] µg/dL, respectively). Similarly, the mean AUEC4.75–10 was lower with BI 1358894 than placebo for ACTH (98.9 [43.4] h pg/mL vs 131 [64.8] h pg/mL, respectively) and for cortisol (37.3 [9.5] h µg/dL vs 46.5 [14.0] h µg/dL). Both plasma ACTH and serum cortisol concentrations reached maximum levels by 5 min after CCK-4 administration (planned time 5 h) and declined within 1 h almost to baseline (Fig. 3). For ACTH, median tmax was similar for both treatments (5.08–5.17 h). For cortisol, median tmax was the same for both treatments (5.33 h).
Mean effect-time profiles of a ACTH and b cortisol (± SD) in blood during the CCK-4 challenge after a single administration of BI 1358894 100 mg or placebo. BI 1358894 or placebo were administered 5 h prior to CCK-4 challenge. ACTH adrenocorticotropic hormone, CCK-4 cholecystokinin-tetrapeptide, SD standard deviation
3.3 Pharmacokinetics
Plasma concentrations of BI 1358894 increased until the maximum was reached (geometric mean [gMean] Cmax: 469 nmol/L) at a median tmax of 7 h (range 1.5–12 h) post-dose, then declined in a multiphasic manner with a long terminal phase (Online Resource 4). During the CCK-4 challenge period, the gMean BI 1358894 plasma concentrations ranged between 293 and 364 nmol/L. The range of maximum concentrations was 326–669 nmol/L. The gMean t1/2 was calculated as 112 h and exposure (gMean [range]) during the CCK-4 challenge (AUC4.75–5.5) was 248 h nmol/L (91.2–420 h nmol/L). Pharmacokinetic parameters are summarized in the supplemental table (Online Resource 5).
3.4 Safety
Treatment-emergent adverse events (TEAEs) were reported for 19/20 (95.0%) participants. The number of participants with ≥ 1 TEAE was higher following BI 1358894 administration (14/19 participants, 73.7%) than following placebo (10/19 participants, 52.6%). The most frequently reported TEAEs by system order class (SOC) were nervous system disorders (BI 1358894: 12/19 [63.2%] participants in total, including headache 10/19 [52.6%] and dizziness 2/19 [10.5%] participants; placebo: 1/19 [5.3%] participants [headache]) and general disorders and administration site conditions (BI 1358894: 4/19 [21.1%] participants in total, including fatigue 3/19 [15.8%] and medical device site irritation 1/19 [5.3%]; placebo: 6/19 [31.6%] participants in total, including fatigue 1/19 [5.3%] and medical device site irritation 1/19 [5.3%]). Overall, investigator-assessed drug-related AEs (DRAEs) were reported for 13/20 participants (65.0%), with a higher frequency following BI 1358894 (11/19 participants, 57.9%) than following placebo (5/19 participants, 26.3%). The most frequently reported DRAEs by SOC were nervous system disorders (BI 1358894: 9/19 [47.4%] participants; placebo: 1/19 [5.3%]) and gastrointestinal disorders (BI 1358894: 3/19 [15.8%] participants; placebo: 3/19 [15.8%] participants). No other significant AEs (according to the European Medicines Agency ICH E3 [reference number: CPMP/ICH/137/95]), AEs leading to discontinuation of trial medication, AEs of severe intensity, AEs of special interest, deaths, or other serious AEs were reported. An overall summary of TEAEs is provided in the supplemental table (Online Resource 6).
4 Discussion
The present study investigated the effect of BI 1358894, a small-molecule inhibitor of TRPC4/5, on CCK-4-induced anxiety and panic. The primary endpoint, maximum change from baseline in PSS sum intensity score after CCK-4 injection, indicated a positive effect of BI 1358894 on CCK-4-provoked anxiety, with a lower maximum change recorded in the BI 1358894 group than the placebo group. Similarly, BI 1358894 had a positive effect on emotional status during CCK-4 challenge, as indicated by a lower maximum change from baseline in EVAS score versus placebo, and reduced levels of the stress hormones ACTH and cortisol during CCK-4 challenge compared with placebo. However, contribution of supra-pituitary sites to CCK-4 activation cannot be excluded, as CCK-4-induced stress hormone release may also be susceptible to cortisol-feedback inhibition [33]. Overall, the 3 independent subjective measures (PSS, EVAS, and ACTH/cortisol) examined during this study all indicated a positive effect of BI 1358894 on CCK-4-induced anxiety/panic-like symptoms versus placebo and confirm central target engagement of BI 1358894.
The amygdala plays a central role in emotional control as a critical component of the brain circuitry that processes emotional stimuli (both fearful and rewarding), for instance, the expression of fear and aggression, and the retrieval of emotional and fear-related memories [31, 34,35,36]. The diminished anxiety resulting from a lack of TRPC4/5 antagonism is paralleled by reduced CCK-4 responses in the amygdala in mice [22, 24, 25], suggesting a role for amygdala TRPC4/5 and CCK-4 activity in anxiety-like behaviors. Further support for the targeting of amygdala TRPC4/5 signaling is provided by a recent clinical study of BI 1358894-treated patients with MDD who exhibited attenuated amygdala hyperreactivity in response to negative faces and scenes [37].
Cholecystokinin-tetrapeptide, a synthetic analog of CCK, has been identified as a suitable mechanistic panic-response model in humans that may aid investigations into the anxiolytic effects of novel compounds; hence its use here [5, 11,12,13]. Using the CCK-4 challenge model, the efficacy of various drugs in attenuating panic symptoms has been demonstrated, including the CCK receptor antagonist CI-988, tricyclic antidepressants, SSRIs, and benzodiazepines [8, 20, 38,39,40]. CI-988, administered as a single oral dose, was moderately more effective than placebo in reducing panic frequency and induced a 14 % decrease in the sum intensity of symptoms following CCK-4 administration in healthy volunteers [39]. Whereas in patients with panic disorder, treatment with the SSRI citalopram had a more pronounced effect on the intensity of panic symptoms and decreased the frequency of panic attacks induced with CCK-4 in patients by 50%; however, this was following 8 weeks of treatment [40]. Similarly, another SSRI, fluvoxamine, administered over 8 weeks was reported to result in a statistically significant treatment effect on CCK-4-induced panic attacks when compared with placebo-treated patients using the Hamilton Anxiety Scale [20]. Although lorazepam, a benzodiazepine, did not modify CCK-4-induced cerebral activity or attenuate CCK-4-induced panic symptoms as assessed using the PSS in healthy volunteers [18], an equivalent dose of alprazolam led to a marked reduction of panic symptoms following CCK-4 challenge in healthy volunteers [8]. While direct comparison of results from this study with previous trials is difficult due to the different methods used for evaluation, dosage, and treatment, the effects of BI 1358894 on panic symptoms are similar in nature and magnitude to those of other candidate drugs also assessed as a single dose. Previous studies have shown CCK agonists (CCK-4 and pentagastrin) induce robust activation of the hypothalamic-pituitary-adrenal (HPA) axis and stimulate the release of cortisol and ACTH in healthy participants [5, 41, 42]. This is mirrored in the present study, where both ACTH and cortisol levels were seen to increase after the CCK-4 challenge. Interestingly, however, van Megan and colleagues demonstrated that HPA activation, as measured by prolactin and cortisol responses, does not accompany CCK-4 challenge in patients with panic disorder; moreover, increases in plasma 3-methoxy-4-hydroxy-phenylglycol, the principal noradrenergic metabolite, were not observed [11], and CCK-4-induced stress hormone release may be susceptible to cortisol-feedback inhibition [33].
Panic attacks are accompanied by numerous physiological symptoms including palpitations, shortness of breath, nausea or dizziness, fearful thoughts, and fainting or loss of control; patients often experience worry about future panic attacks, termed anticipatory anxiety [43, 44]. Such anticipatory anxiety has been characterized by a state of body hypervigilance and helplessness that imbues a negative affect state [43,44,45]. In a study that described the natural fluctuations in anticipatory anxiety in everyday existence, a computerized Ecological Momentary Assessment approach was used to test theoretically derived hypotheses relating to the correlates of anticipatory anxiety and its relation to panic attacks [43]. In patients with panic disorder, the presence of anticipatory anxiety and anxiety sensitivity played a role in the onset of panic disorder [43]. Furthermore, anticipatory anxiety has been identified as highest in the morning, with higher morning cortisol levels and a higher cortisol awakening response compared with controls [46]. In view of these findings, it was important to determine whether high levels of anticipatory anxiety were already present before participants received CCK-4 injection in the current study. Findings revealed that the baseline ratings of the STAI were not different between placebo and CCK-4, and were low prior to CCK-4 administration, indicating minimal or no anticipatory anxiety during the study. These findings suggest that the occurrence of increased anxiety after CCK-4 administration cannot be explained by the presence of a prior increased anticipatory anxiety.
The study had several key strengths. The cross-over study design meant that each participant served as their own control allowing for comparisons to be made within subjects rather than between subjects. This reduced variability and minimized the number of participants necessary for the study. In addition, the study was carried out in a controlled setting at one site, which limited any additional variability to the data. A limitation of this study included the modest sample size of each treatment group, and exclusively healthy male volunteers. However, considering the variable effects of CCK-4 due to hormonal changes in females [47, 48], the inclusion of males only likely reduces variability in this study. Potential interindividual variability in the pharmacokinetic and panic responses to CCK-4 administration represents a further limitation and may affect the outcome of the trial in terms of any apparent panicolytic effects. However, CCK-sensitive participants were selected in this trial, ensuring adequate CCK-4-induced effects. Furthermore, both the placebo and treatment groups were intravenously administered with CCK-4, thereby minimizing the impact of any potential variation in CCK-4 responses in this study.
This study investigated the effects of a single dose of BI 1358894 100 mg. The 100-mg dose was selected based on preclinical effects and previous clinical study results [29]. However, additional studies evaluating dose ranges as well as longer-term treatment with BI 1358894 may be warranted.
5 Conclusions and Future Research
BI 1358894, a small-molecule inhibitor of TRPC4/5, reduced psychological and physiological responses to CCK-4 compared with placebo, demonstrating a physiological response and brain circuit engagement. Consistent with the findings of two parallel studies evaluating the safety, pharmacokinetic and food effect of single and multiple rising doses of BI 1358894 (NCT03210272 and NCT03754959), single oral doses of BI 1358894 were generally well tolerated by the healthy volunteers included in this study [49]. An additional functional magnetic resonance imaging proof of clinical principal (PoCP) study for BI 1358894 to treat MDD (NCT03854578) has found that BI 1358894 significantly reduced activation in several brain regions involved in emotional processing [37], and thus has the potential to impact multiple transmitter systems.
References
Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11(1):2221.
Lueken U, Straube B, Yang Y, Hahn T, Beesdo-Baum K, Wittchen HU, et al. Separating depressive comorbidity from panic disorder: a combined functional magnetic resonance imaging and machine learning approach. J Affect Disord. 2015;184:182–92.
Wu Z, Cao L, Peng D, Mellor D, Zhang C, Li H, et al. The clinical correlates of comorbid anxiety symptoms and syndromal anxiety in patients with major depressive disorder. Psychiatry Res. 2018;269:251–7.
Zwanzger P, Domschke K, Bradwejn J. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress Anxiety. 2012;29(9):762–74.
Eser D, Schüle C, Baghai T, Floesser A, Krebs-Brown A, Enunwa M, et al. Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study. Psychopharmacol. 2007;192:479–87.
Wang H, Wong PT, Spiess J, Zhu YZ. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci Biobehav Rev. 2005;29(8):1361–73.
Bradwejn J, Koszycki D, Meterissian G. Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder. Can J Psychiatry. 1990;35(1):83–5.
Zwanzger P, Eser D, Aicher S, Schüle C, Baghai TC, Padberg F, et al. Effects of alprazolam on cholecystokinin-tetrapeptide-induced panic and hypothalamic–pituitary–adrenal-axis activity: a placebo-controlled study. Neuropsychopharmacology. 2003;28(5):979–84.
Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry. 1999;45(7):872–82.
Bradwejn J, Koszycki D, Couëtoux du Tertre A, Paradis M, Bourin M. Effects of flumazenil on cholecystokinin-tetrapeptide-induced panic symptoms in healthy volunteers. Psychopharmacology. 1994;114(2):257–61.
van Megen HJGM, Westenberg HGM, Den Boer JA, Kahn R. The panic-inducing properties of the cholecystokinin tetrapeptide CCK4 in patients with panic disorder. Euro Neuropsychopharmacol. 1996;6(3):187–94.
Kellner M. Experimental panic provocation in healthy man—a translational role in anti-panic drug development? Dialogues Clin Neurosci. 2011;13(4):485–93.
Rehfeld JF. Cholecystokinin and panic disorder: reflections on the history and some unsolved questions. Molecules. 2021;26(18):5657.
Bradwejn J, Koszycki D, Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch Gen Psychiatry. 1991;48(7):603–10.
Koszycki D, Copen J, Bradwejn J. Sensitivity to cholecystokinin-tetrapeptide in major depression. J Affect Disord. 2004;80(2–3):285–90.
Wang S, Zhang AP, Kurada L, Matsui T, Lei S. Cholecystokinin facilitates neuronal excitability in the entorhinal cortex via activation of TRPC-like channels. J Neurophysiol. 2011;106(3):1515–24.
Leicht G, Mulert C, Eser D, Sämann PG, Ertl M, Laenger A, et al. Benzodiazepines counteract rostral anterior cingulate cortex activation induced by cholecystokinin-tetrapeptide in humans. Biol Psychiatry. 2013;73(4):337–44.
Schunck T, Mathis A, Erb G, Namer IJ, Hode Y, Demazières A, et al. One milligram of lorazepam does not decrease anxiety induced by CCK-4 in healthy volunteers: investigation of neural correlates with BOLD MRI. J Psychopharmacol (Oxford, England). 2011;25(1):52–9.
Tõru I, Maron E, Raag M, Vasar V, Nutt DJ, Shlik J. The effect of 6-week treatment with escitalopram on CCK-4 challenge: a placebo-controlled study in CCK-4-sensitive healthy volunteers. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2013;23(7):645–52.
van Megen HJ, Westenberg HG, den Boer JA, Slaap B, Scheepmakers A. Effect of the selective serotonin reuptake inhibitor fluvoxamine on CCK-4 induced panic attacks. Psychopharmacology. 1997;129(4):357–64.
Zwanzger P, Eser D, Nothdurfter C, Baghai TC, Möller HJ, Padberg F, et al. Effects of the GABA-reuptake inhibitor tiagabine on panic and anxiety in patients with panic disorder. Pharmacopsychiatry. 2009;42(6):266–9.
Riccio A, Li Y, Tsvetkov E, Gapon S, Yao GL, Smith KS, et al. Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. J Neurosci Off J Soc Neurosci. 2014;34(10):3653–67.
Allen Institute for Brain Science. Allen Human Brain Atlas. 2004. https://human.brain-map.org. Accessed 31 May 2023.
Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137(4):761–72.
Yang L-P, Jiang F-J, Wu G-S, Deng K, Wen M, Zhou X, et al. Acute treatment with a novel TRPC4/C5 channel inhibitor produces antidepressant and anxiolytic-like effects in mice. PLoS One. 2015;10(8):e0136255-e.
Just S, Chenard BL, Ceci A, Strassmaier T, Chong JA, Blair NT, et al. Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS One. 2018;13(1): e0191225.
Hyman SE. Neurotransmitters. Curr Biol. 2005;15(5):R154–8.
Kawamoto EM, Vivar C, Camandola S. Physiology and pathology of calcium signaling in the brain. Front Pharmacol. 2012;3:61.
Fuertig R, Goettel M, Höfler J, Sharma VP. 450 A phase I single-rising-dose study of oral BI 1358894 in healthy male volunteers: safety, pharmacokinetics and food effect. Eur Neuropsychopharmacol. 2020;40:S256–7.
Pross N, Demazières A, Girard N, Barnouin R, Metzger D, Klein A, et al. Effects of changes in water intake on mood of high and low drinkers. PLoS One. 2014;9(4): e94754.
Young KD, Zotev V, Phillips R, Misaki M, Drevets WC, Bodurka J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: a review. Psychiatry Clin Neurosci. 2018;72(7):466–81.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Demiralay C, Jahn H, Kellner M, Yassouridis A, Wiedemann K. Differential effects to CCK-4-induced panic by dexamethasone and hydrocortisone. World J Biol Psychiatry. 2012;13(7):526–34.
Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–92.
Mandell D, Siegle GJ, Shutt L, Feldmiller J, Thase ME. Neural substrates of trait ruminations in depression. J Abnorm Psychol. 2014;123(1):35–48.
Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin N Am. 2009;32(3):549–75.
Grimm S, Kreicher C, Paret C, Niedtfeld I, Mennes M, Just S et al. BI 1358894 leads to significant attenuation of brain activity in the Cortico-Limbic system as shown by task-based BOLD-fMRI using emotional paradigms in patients with MDD; 2020.
Bradwejn J, Koszycki D. Imipramine antagonism of the panicogenic effects of cholecystokinin tetrapeptide in panic disorder patients. Am J Psychiatry. 1994;151(2):261–3.
Bradwejn J, Koszycki D, Paradis M, Reece P, Hinton J, Sedman A. Effect of CI-988 on cholecystokinin tetrapeptide-induced panic symptoms in healthy volunteers. Biol Psychiatry. 1995;38(11):742–6.
Shlik J, Aluoja A, Vasar V, Vasar E, Podar T, Bradwejn J. Effects of citalopram treatment on behavioural, cardiovascular and neuroendocrine response to cholecystokinin tetrapeptide challenge in patients with panic disorder. J Psychiatry Neurosci JPN. 1997;22(5):332–40.
Abelson JL, Liberzon I. Dose response of adrenocorticotropin and cortisol to the CCK-B agonist pentagastrin. Neuropsychopharmacology. 1999;21(4):485–94.
Eser D, di Michele F, Zwanzger P, Pasini A, Baghai TC, Schüle C, et al. Panic induction with cholecystokinin-tetrapeptide (CCK-4) increases plasma concentrations of the neuroactive steroid 3α, 5α tetrahydrodeoxycorticosterone (3α, 5α-THDOC) in healthy volunteers. Neuropsychopharmacology. 2005;30(1):192–5.
Helbig-Lang S, Lang T, Petermann F, Hoyer J. Anticipatory anxiety as a function of panic attacks and panic-related self-efficacy: an ambulatory assessment study in panic disorder. Behav Cogn Psychother. 2012;40(5):590–604.
Schmidt NB, Lerew DR, Trakowski JH. Body vigilance in panic disorder: Evaluating attention to bodily perturbations. J Consult Clin Psychol. 1997;65(2):214–20.
Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55(11):1247–63.
Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, Sondeijker FE, Ormel J, Verhulst FC. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatr Scand. 2007;116(2):137–44.
Frick G, Bremme K, Sjögren C, Lindén A, Uvnäs-Moberg K. Plasma levels of cholecystokinin and gastrin during the menstrual cycle and pregnancy. Acta Obstet Gynecol Scand. 1990;69(4):317–20.
Le Mellédo JM, Merani S, Koszycki D, Bellavance F, Palmour R, Gutkowska J, et al. Sensitivity to CCK-4 in women with and without premenstrual dysphoric disorder (PMDD) during their follicular and luteal phases. Neuropsychopharmacology. 1999;20(1):81–91.
Fuertig R, Goettel M, Herich L, Hoefler J, Wiebe ST, Sharma V. Effect of single and multiple ascending doses of BI 1358894 in healthy male volunteers on safety, tolerability and pharmacokinetics: two Phase I randomised studies (IN PRESS). CNS Drugs. 2023.
Acknowledgements
The authors would like to thank Karin Hoermann for conducting the pharmacokinetic and biomarker analyses for the study. Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing, and referencing) was provided by Lesley Blogg, PhD., of Fishawack Communications Ltd, part of Fishawack Health, and was funded by Boehringer Ingelheim International GmbH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Boehringer Ingelheim (BI study numbers 1402-0005; NCT03904576). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report.
Conflicts of interest
MG, RF, SRM, SJ and AW are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. VS is an employee of Boehringer Ingelheim International GmbH. JdB is an employee of PRA Health Sciences. All authors did not receive any direct compensation relating to the development of the manuscript.
Availability of data and material
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfil their role and obligations as authors under the ICMJE criteria. Furthermore, clinical study documents (e.g., study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/. Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Clinical Study Reports and Related Clinical Documents can also be requested via the link https://trials.boehringer-ingelheim.com/. All requests will be governed by a Document Sharing Agreement. Bona fide, qualified scientific and medical researchers may request access to de-identified, analyzable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request. Researchers should use the https://trials.boehringer-ingelheim.com/ link to request access to study data.
Ethics approval
The trial was carried out in compliance with the clinical trial protocol and approved by an independent ethics committee of the participating center. All procedures were conducted in accordance with the principles of the 1964 Declaration of Helsinki.
Consent to participate
Each volunteer signed and dated an informed consent form according to the local regulatory and legal requirements and Good Clinical Practices.
Consent for publication
Not applicable
Code availability
Not applicable
Author contributions
MG, RF, SRM, SJ, VS, AW and JdB contributed to the study concept or design, and data analyses or interpretation. All authors contributed toward the preparation of the manuscript, approved the final submitted version, and agreed to be listed as authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Goettel, M., Fuertig, R., Mack, S.R. et al. Effect of BI 1358894 on Cholecystokinin-Tetrapeptide (CCK-4)-Induced Anxiety, Panic Symptoms, and Stress Biomarkers: A Phase I Randomized Trial in Healthy Males. CNS Drugs 37, 1099–1109 (2023). https://doi.org/10.1007/s40263-023-01042-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01042-3