Abstract
Premenstrual dysphoric disorder (PMDD) is characterized by the predictable onset of mood and physical symptoms secondary to gonadal steroid fluctuation during the luteal phase of the menstrual cycle. Although menstrual-related affective dysfunction is responsible for considerable functional impairment and reduction in quality of life worldwide, currently approved treatments for PMDD are suboptimal in their effectiveness. Research over the past two decades has suggested that the interaction between allopregnanolone, a neurosteroid derivative of progesterone, and the gamma-aminobutyric acid (GABA) system represents an important relationship underlying symptom genesis in reproductive-related mood disorders, including PMDD. The objective of this narrative review is to discuss the plausible link between changes in GABAergic transmission secondary to the fluctuation of allopregnanolone during the luteal phase and mood impairment in susceptible individuals. As part of this discussion, we explore promising findings from early clinical trials of several compounds that stabilize allopregnanolone signaling during the luteal phase, including dutasteride, a 5-alpha reductase inhibitor; isoallopregnanolone, a GABA-A modulating steroid antagonist; and ulipristal acetate, a selective progesterone receptor modulator. We then reflect on the implications of these therapeutic advances, including how they may promote our knowledge of affective regulation more generally. We conclude that these and other studies of PMDD may yield critical insight into the etiopathogenesis of affective disorders, considering that (1) symptoms in PMDD have a predictable onset and offset, allowing for examination of affective state kinetics, and (2) GABAergic interventions in PMDD can be used to better understand the relationship between mood states, network regulation, and the balance between excitatory and inhibitory signaling in the brain.
Similar content being viewed by others

References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). [Internet]. 2013.
Hartlage SA, Arduino KE, Gehlert S. Premenstrual dysphoric disorder and risk for major depressive disorder: a preliminary study. J Clin Psychol. 2001;57(12):1571–8. https://doi.org/10.1002/jclp.1119.
Kuehner C, Nayman S. Premenstrual exacerbations of mood disorders: findings and knowledge gaps. Curr Psychiatry Rep. 2021;23(11):78. https://doi.org/10.1007/s11920-021-01286-0.
Rubinow DR, Roy-Byrne P, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry. 1984;141(5):684–6. https://doi.org/10.1176/ajp.141.5.684.
Muse KN, Cetel NS, Futterman LA, Yen SC. The premenstrual syndrome. Effects of “medical ovariectomy.” N Engl J Med. 1984;311(21):1345–9. https://doi.org/10.1056/NEJM198411223112104.
Hammarbäck S, Bäckström T. Induced anovulation as treatment of premenstrual tension syndrome. A double-blind cross-over study with GnRH-agonist versus placebo. Acta Obstet Gynecol Scand. 1988;67(2):159–66. https://doi.org/10.3109/00016348809004191.
Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual syndrome: effect of symptom severity and type in a controlled trial. Obstet Gynecol. 1994;84(5):779–86.
West CP, Hillier H. Ovarian suppression with the gonadotrophin-releasing hormone agonist goserelin (Zoladex) in management of the premenstrual tension syndrome. Hum Reprod. 1994;9(6):1058–63. https://doi.org/10.1093/oxfordjournals.humrep.a138633.
Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–16. https://doi.org/10.1056/nejm199801223380401.
Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017;174(10):980–9. https://doi.org/10.1176/appi.ajp.2017.16101113.
Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcón G, Schmidt PJ, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305–14. https://doi.org/10.1176/appi.ajp.2012.12030385.
Duan C, Cosgrove J, Deligiannidis KM. Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep. 2017;19(10):70. https://doi.org/10.1007/s11920-017-0824-4.
Wang X, Tao J, Li L, Zhong Z, Liu S, Jiang T, et al. Alterations in white matter fractional anisotropy in subsyndromal perimenopausal depression. BMC Psychiatry. 2014;14:367. https://doi.org/10.1186/s12888-014-0367-8.
Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44(1):111–28. https://doi.org/10.1038/s41386-018-0148-z.
Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6(5):484–90. https://doi.org/10.1038/nn1043.
Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26(8–9):907–13. https://doi.org/10.1023/a:1012376215967.
Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci. 2010;32(8):1300–9. https://doi.org/10.1111/j.1460-9568.2010.07331.x.
Roth FC, Draguhn A. GABA metabolism and transport: effects on synaptic efficacy. Neural Plast. 2012;2012:805830. https://doi.org/10.1155/2012/805830
Spellman T, Liston C. Toward circuit mechanisms of pathophysiology in depression. Am J Psychiatry. 2020;177(5):381–90. https://doi.org/10.1176/appi.ajp.2020.20030280.
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75–90. https://doi.org/10.1016/j.neuron.2019.03.013.
Northoff G, Sibille E. Cortical GABA neurons and self-focus in depression: a model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19(9):959. https://doi.org/10.1038/mp.2014.108.
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82(8):549–59. https://doi.org/10.1016/j.biopsych.2017.05.024.
Fogaça MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87. https://doi.org/10.3389/fncel.2019.00087.
Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19(9):966–77. https://doi.org/10.1038/mp.2014.68.
Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry. 2013;4:104. https://doi.org/10.3389/fpsyt.2013.00104.
Pandya M, Palpagama TH, Turner C, Waldvogel HJ, Faull RL, Kwakowsky A. Sex- and age-related changes in GABA signaling components in the human cortex. Biol Sex Differ. 2019;10(1):5. https://doi.org/10.1186/s13293-018-0214-6.
Gold BI, Bowers MBJ, Roth RH, Sweeney DW. GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry. 1980;137(3):362–4. https://doi.org/10.1176/ajp.137.3.362.
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–42. https://doi.org/10.1038/mp.2011.113.
Seney ML, Tripp A, McCune S, Lewis DA, Sibille E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2015;73:213–9. https://doi.org/10.1016/j.nbd.2014.10.005.
Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2007;32(2):471–82. https://doi.org/10.1038/sj.npp.1301234.
Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CAJ. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry. 2018;84(8):582–90. https://doi.org/10.1016/j.biopsych.2018.01.027.
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–37. https://doi.org/10.1016/j.biopsych.2006.09.020.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatr. 2015;72(6):603–11. https://doi.org/10.1001/jamapsychiatry.2015.0071.
Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–81. https://doi.org/10.1176/jnp.9.3.471.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–7. https://doi.org/10.1073/pnas.0812686106.
Prévot T, Sibille E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol Psychiatry. 2021;26(1):151–67. https://doi.org/10.1038/s41380-020-0727-3.
Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91(2):260–92. https://doi.org/10.1016/j.neuron.2016.06.033.
Murayama M, Pérez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457(7233):1137–41. https://doi.org/10.1038/nature07663.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–8. https://doi.org/10.1038/nature10360.
Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014;39(9):2252–62. https://doi.org/10.1038/npp.2014.76.
Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–7. https://doi.org/10.1038/nn2001.
Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J Neurosci Off J Soc Neurosci. 2013;33(47):18566–73. https://doi.org/10.1523/JNEUROSCI.1973-13.2013.
Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–9. https://doi.org/10.1016/j.neuroimage.2012.09.029.
Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjaǹska M, Doyon J, et al. GABA in the insula—a predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–8. https://doi.org/10.1016/j.neuroimage.2013.04.042.
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–7. https://doi.org/10.1126/science.2422758.
Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196. https://doi.org/10.1016/j.ynstr.2019.100196.
Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109–22. https://doi.org/10.1016/j.neubiorev.2012.10.005.
Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–9. https://doi.org/10.1038/nature05324.
Sugasawa Y, Cheng WWL, Bracamontes JR, Chen ZW, Wang L, Germann AL, et al. Site-specific effects of neurosteroids on GABAA receptor activation and desensitization. Czajkowski CM, Aldrich RW, editors. Elife [Internet]. 2020;9:e55331. https://doi.org/10.7554/eLife.55331
Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson IM. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br J Pharmacol. 2011;162(2):311–27. https://doi.org/10.1111/j.1476-5381.2010.01059.x.
Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–75. https://doi.org/10.1038/nrn1703.
Alvarez LD, Pecci A, Estrin DA. In search of GABA(A) receptor’s neurosteroid binding sites. J Med Chem. 2019;62(11):5250–60. https://doi.org/10.1021/acs.jmedchem.8b01400.
Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4(5):759–65. https://doi.org/10.1016/0896-6273(90)90202-q.
Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl-currents. Mol Pharmacol. 1991;39(6):691–6.
Puia G, Ravazzini F, Castelnovo LF, Magnaghi V. PKCε and allopregnanolone: functional cross-talk at the GABAA receptor level. Front Cell Neurosci. 2015;9:83. https://doi.org/10.3389/fncel.2015.00083.
Reddy DS, Apanites LA. Anesthetic effects of progesterone are undiminished in progesterone receptor knockout mice. Brain Res. 2005;1033(1):96–101. https://doi.org/10.1016/j.brainres.2004.11.026.
Poisbeau P, Keller AF, Aouad M, Kamoun N, Groyer G, Schumacher M. Analgesic strategies aimed at stimulating the endogenous production of allopregnanolone. Front Cell Neurosci. 2014;8:174. https://doi.org/10.3389/fncel.2014.00174.
Kapur J, Joshi S. Progesterone modulates neuronal excitability bidirectionally. Neurosci Lett. 2021;744:135619. https://doi.org/10.1016/j.neulet.2020.135619
Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. 2014;113:79–87. https://doi.org/10.1016/j.pneurobio.2013.09.003.
Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230(2):151–88. https://doi.org/10.1007/s00213-013-3276-5.
Antonoudiou P, Colmers PLW, Walton NL, Weiss GL, Smith AC, Nguyen DP, et al. Allopregnanolone mediates affective switching through modulation of oscillatory states in the basolateral amygdala. Biol Psychiatry. 2022;91(3):283–93. https://doi.org/10.1016/j.biopsych.2021.07.017.
Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Hum Brain Mapp. 2014;35(7):3249–61. https://doi.org/10.1002/hbm.22399.
Dornellas APS, Macedo GC, McFarland MH, Gómez-A A, O’Buckley TK, Da Cunha C, et al. Allopregnanolone decreases evoked dopamine release differently in rats by sex and estrous stage. Front Pharmacol. 2020;11:608887. https://doi.org/10.3389/fphar.2020.608887
Syan SK, Minuzzi L, Costescu D, Smith M, Allega OR, Coote M, et al. Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil Steril. 2017;107(5):1246-1255.e4. https://doi.org/10.1016/j.fertnstert.2017.03.021.
Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, Shaffer SA, Villamarin V, Tan Y, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2019;44(3):546–54. https://doi.org/10.1038/s41386-018-0242-2.
Beauchamp MH, Ormerod BK, Jhamandas K, Boegman RJ, Beninger RJ. Neurosteroids and reward: allopregnanolone produces a conditioned place aversion in rats. Pharmacol Biochem Behav. 2000;67(1):29–35. https://doi.org/10.1016/s0091-3057(00)00299-9.
Fish EW, Whitman BJ, DiBerto JF, Robinson JE, Morrow AL, Malanga CJ. Effects of the neuroactive steroid allopregnanolone on intracranial self-stimulation in C57BL/6J mice. Psychopharmacology. 2014;231(17):3415–23. https://doi.org/10.1007/s00213-014-3600-8.
Peart DR, Andrade AK, Logan CN, Knackstedt LA, Murray JE. Regulation of cocaine-related behaviours by estrogen and progesterone. Neurosci Biobehav Rev. 2022;135:104584. https://doi.org/10.1016/j.neubiorev.2022.104584
Meltzer-Brody S, Kanes SJ. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress. 2020;12:100212. https://doi.org/10.1016/j.ynstr.2020.100212
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet (London, England). 2018;392(10152):1058–70. https://doi.org/10.1016/S0140-6736(18)31551-4.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet (London, England). 2017;390(10093):480–9. https://doi.org/10.1016/S0140-6736(17)31264-3.
Nguyen AJ, Hoyer E, Rajhans P, Strathearn L, Kim S. A tumultuous transition to motherhood: Altered brain and hormonal responses in mothers with postpartum depression. J Neuroendocrinol. 2019;31(9):e12794. https://doi.org/10.1111/jne.12794
Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J Neuroendocrinol. 2014;26(10):665–84. https://doi.org/10.1111/jne.12183.
Vignato J, Perkhounkova Y, McCarthy AM, Segre LS. Pain and depression symptoms during the third trimester of pregnancy. MCN Am J Matern Nurs [Internet]. 2020;45(6).
Zhang L, Wang L, Cui S, Yuan Q, Huang C, Zhou X. Prenatal depression in women in the third trimester: prevalence, predictive factors, and relationship with maternal-fetal attachment. Front Public Health. 2020;8:602005. https://doi.org/10.3389/fpubh.2020.602005
Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910(1–2):55–66. https://doi.org/10.1016/s0006-8993(01)02565-3.
Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305(2):541–8. https://doi.org/10.1124/jpet.102.045120.
Beddig T, Reinhard I, Kuehner C. Stress, mood, and cortisol during daily life in women with premenstrual dysphoric disorder (PMDD). Psychoneuroendocrinology. 2019;109:104372. https://doi.org/10.1016/j.psyneuen.2019.104372
Petersen N, London ED, Liang L, Ghahremani DG, Gerards R, Goldman L, et al. Emotion regulation in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2016;19(5):891–8. https://doi.org/10.1007/s00737-016-0634-4.
Lee EE, Nieman LK, Martinez PE, Harsh VL, Rubinow DR, Schmidt PJ. ACTH and cortisol response to Dex/CRH testing in women with and without premenstrual dysphoria during GnRH agonist-induced hypogonadism and ovarian steroid replacement. J Clin Endocrinol Metab. 2012;97(6):1887–96. https://doi.org/10.1210/jc.2011-3451.
Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Bäckström T, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131–7. https://doi.org/10.1007/s00737-013-0327-1.
Rasgon N, McGuire M, Tanavoli S, Fairbanks L, Rapkin A. Neuroendocrine response to an intravenous l-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144–9. https://doi.org/10.1016/s0015-0282(99)00452-5.
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788–97. https://doi.org/10.1016/s0006-3223(00)01044-1.
Huang Y, Zhou R, Wu M, Wang Q, Zhao Y. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160–8. https://doi.org/10.3109/10253890.2014.999234.
Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M, et al. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2004;18(2):79–87. https://doi.org/10.1080/09513590310001652955.
Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci Off J Soc Neurosci. 2011;31(50):18198–210. https://doi.org/10.1523/JNEUROSCI.2560-11.2011.
Almeida FB, Pinna G, Barros HMT. The role of HPA axis and allopregnanolone on the neurobiology of major depressive disorders and PTSD. Int J Mol Sci. 2021;22(11). https://doi.org/10.3390/ijms22115495
Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213(1–2):63–72. https://doi.org/10.1007/s00429-008-0192-2.
Miklós IH, Kovács KJ, Herman JP, Cullinan WE, Ziegler DR, Tasker JG. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Eur J Neurosci. 2002;113(3):381–5. https://doi.org/10.1046/j.1460-9568.2002.02133.x.
Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16(3):381–5. https://doi.org/10.1046/j.1460-9568.2002.02133.x.
Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62(1):265–71. https://doi.org/10.1016/0306-4522(94)90330-1.
Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227–36. https://doi.org/10.1176/appi.ajp.2014.14070918.
Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37(1–3):110–5. https://doi.org/10.1016/s0165-0173(01)00129-1.
Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, et al. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95(22):13284–9. https://doi.org/10.1073/pnas.95.22.13284.
Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375(1–3):225–35. https://doi.org/10.1016/s0014-2999(99)00232-0.
Boero G, Pisu MG, Biggio F, Muredda L, Carta G, Banni S, et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology. 2018;133:242–53. https://doi.org/10.1016/j.neuropharm.2018.01.045.
Brunton PJ, Russell JA. Allopregnanolone and suppressed hypothalamo–pituitary–adrenal axis stress responses in late pregnancy in the rat. Stress. 2011;14(1):6–12. https://doi.org/10.3109/10253890.2010.482628.
Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, et al. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98(1):122–33. https://doi.org/10.1111/j.1471-4159.2006.03850.x.
Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. https://doi.org/10.1016/j.neuron.2008.06.019.
Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925–33. https://doi.org/10.1016/j.biopsych.2006.12.019.
Miller A, Vo H, Huo L, Roca C, Schmidt PJ, Rubinow DR. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788–94. https://doi.org/10.1016/j.jpsychires.2010.01.013.
Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression—a pilot study. Arch Womens Ment Health. 2013;16(6):499–509. https://doi.org/10.1007/s00737-013-0373-8.
Costas J, Gratacòs M, Escaramís G, Martín-Santos R, de Diego Y, Baca-García E, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. 2010;44(11):717–24. https://doi.org/10.1016/j.jpsychires.2009.12.012.
Mehta D, Newport DJ, Frishman G, Kraus L, Rex-Haffner M, Ritchie JC, et al. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol Med. 2014;44(11):2309–22. https://doi.org/10.1017/S0033291713003231.
Dubey N, Hoffman JF, Schuebel K, Yuan Q, Martinez PE, Nieman LK, et al. The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder. Mol Psychiatry. 2017;22(8):1172–84. https://doi.org/10.1038/mp.2016.229.
Li HJ, Goff A, Rudzinskas SA, Jung Y, Dubey N, Hoffman J, et al. Altered estradiol-dependent cellular Ca(2+) homeostasis and endoplasmic reticulum stress response in premenstrual dysphoric disorder. Mol Psychiatry. 2021. https://doi.org/10.1038/s41380-021-01144-8.
Marrocco J, Einhorn NR, Petty GH, Li H, Dubey N, Hoffman J, et al. Epigenetic intersection of BDNF Val66Met genotype with premenstrual dysphoric disorder transcriptome in a cross-species model of estradiol add-back. Mol Psychiatry. 2020;25(3):572–83. https://doi.org/10.1038/s41380-018-0274-3.
Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, et al. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72(6):499–504. https://doi.org/10.1016/j.biopsych.2012.03.032.
Comasco E, Hahn A, Ganger S, Gingnell M, Bannbers E, Oreland L, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450–8. https://doi.org/10.1002/hbm.22486.
Ullah A, Long X, Mat WK, Hu T, Khan MI, Hui L, et al. Highly recurrent copy number variations in GABRB2 associated with schizophrenia and premenstrual dysphoric disorder. Front psychiatry. 2020;11:572. https://doi.org/10.3389/fpsyt.2020.00572.
Bertone-Johnson ER, Ronnenberg AG, Houghton SC, Nobles C, Zagarins SE, Takashima-Uebelhoer BB, et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum Reprod. 2014;29(9):1987–94. https://doi.org/10.1093/humrep/deu170.
Yama K, Asari Y, Ono A, Machida M, Miura J. Plasma interleukin-10 levels are altered in women with severe premenstrual syndrome: a preliminary study. Women’s Heal Rep (New Rochelle, NY). 2020;1(1):73–9. https://doi.org/10.1089/whr.2019.0010.
Crowley T, Cryan JF, Downer EJ, O’Leary OF. Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun. 2016;54:260–77. https://doi.org/10.1016/j.bbi.2016.02.001.
Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107(6):2580–5. https://doi.org/10.1073/pnas.0915139107.
Tian J, Middleton B, Kaufman DL. GABA(A)-receptor agonists limit pneumonitis and death in murine coronavirus-infected mice. Viruses. 2021;13(6). https://doi.org/10.3390/v13060966
Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6(11):e946. https://doi.org/10.1038/tp.2016.212
Clements RJ, McDonough J, Freeman EJ. Distribution of parvalbumin and calretinin immunoreactive interneurons in motor cortex from multiple sclerosis post-mortem tissue. Exp Brain Res. 2008;187(3):459–65. https://doi.org/10.1007/s00221-008-1317-9.
Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav Immun. 2013;28:218–26. https://doi.org/10.1016/j.bbi.2012.11.012.
Giovanoli S, Weber L, Meyer U. Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain Behav Immun. 2014;40:48–54. https://doi.org/10.1016/j.bbi.2014.04.005.
Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4(9):490–503. https://doi.org/10.1038/ncpneuro0883.
Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, et al. Allopregnanolone and mood disorders. Prog Neurobiol. 2014;113:88–94. https://doi.org/10.1016/j.pneurobio.2013.07.005.
Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac Rev. 2022;11:11. https://doi.org/10.12703/r/11-11
Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186(3):323–33. https://doi.org/10.1007/s00213-005-0168-3.
Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49(5):573–86. https://doi.org/10.1016/j.neuropharm.2005.04.026.
Timby E, Bäckström T, Nyberg S, Stenlund H, Wihlbäck ACN, Bixo M. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls-a pilot study. Psychopharmacology. 2016;233(11):2109–17. https://doi.org/10.1007/s00213-016-4258-1.
Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87. https://doi.org/10.1007/s11920-015-0628-3.
Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2007;32(10):2190–8. https://doi.org/10.1038/sj.npp.1301351.
Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61(8):741–56. https://doi.org/10.1037/0003-066X.61.8.741.
Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35(1):105–35. https://doi.org/10.1038/npp.2009.109.
Kenwood MM, Kalin NH, Barbas H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology. 2022;47:1141. https://doi.org/10.1038/s41386-021-01216-x.
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. https://doi.org/10.1016/j.smrv.2009.04.002.
Aue T, Okon-Singer H. Expectancy biases in fear and anxiety and their link to biases in attention. Clin Psychol Rev. 2015;42:83–95. https://doi.org/10.1016/j.cpr.2015.08.005.
Marjoribanks J, Brown J, O’Brien PMS, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;2013(6):CD001396. https://doi.org/10.1002/14651858.CD001396.pub3
Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529–34. https://doi.org/10.1056/NEJM199506083322301
Yonkers KA, Halbreich U, Freeman E, Brown C, Endicott J, Frank E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. A randomized controlled trial. Sertraline Premenstrual Dysphoric Collaborative Study Group. JAMA. 1997;278(12):983–8.
Steinberg EM, Cardoso GMP, Martinez PE, Rubinow DR, Schmidt PJ. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531–40. https://doi.org/10.1002/da.21959.
Kornstein SG, Pearlstein TB, Fayyad R, Farfel GM, Gillespie JA. Low-dose sertraline in the treatment of moderate-to-severe premenstrual syndrome: efficacy of 3 dosing strategies. J Clin Psychiatry. 2006;67(10):1624–32. https://doi.org/10.4088/jcp.v67n1020.
Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512–7. https://doi.org/10.1073/pnas.96.23.13512.
Trauger JW, Jiang A, Stearns BA, LoGrasso PV. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451–9. https://doi.org/10.1021/bi026109w.
Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9(1):24–30. https://doi.org/10.1016/j.coph.2008.12.006.
Devall AJ, Santos JM, Fry JP, Honour JW, Brandão ML, Lovick TA. Elevation of brain allopregnanolone rather than 5-HT release by short term, low dose fluoxetine treatment prevents the estrous cycle-linked increase in stress sensitivity in female rats. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25(1):113–23. https://doi.org/10.1016/j.euroneuro.2014.11.017.
Gracia CR, Freeman EW, Sammel MD, Lin H, Sheng L, Frye C. Allopregnanolone levels before and after selective serotonin reuptake inhibitor treatment of premenstrual symptoms. J Clin Psychopharmacol US 2009;403–5. https://doi.org/10.1097/JCP.0b013e3181ad8825
Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161(2):368–70. https://doi.org/10.1176/appi.ajp.161.2.368.
Freeman EW, Kroll R, Rapkin A, Pearlstein T, Brown C, Parsey K, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10(6):561–9. https://doi.org/10.1089/15246090152543148.
Graham CA, Sherwin BB. A prospective treatment study of premenstrual symptoms using a triphasic oral contraceptive. J Psychosom Res. 1992;36(3):257–66. https://doi.org/10.1016/0022-3999(92)90090-o.
Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106(3):492–501. https://doi.org/10.1097/01.AOG.0000175834.77215.2e.
Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72(6):414–21. https://doi.org/10.1016/j.contraception.2005.08.021.
Eisenlohr-Moul TA, Girdler SS, Johnson JL, Schmidt PJ, Rubinow DR. Treatment of premenstrual dysphoria with continuous versus intermittent dosing of oral contraceptives: results of a three-arm randomized controlled trial. Depress Anxiety. 2017;34(10):908–17. https://doi.org/10.1002/da.22673.
Halbreich U, Freeman EW, Rapkin AJ, Cohen LS, Grubb GS, Bergeron R, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85(1):19–27. https://doi.org/10.1016/j.contraception.2011.05.008.
Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, et al. 5α-Reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41(4):1093–102. https://doi.org/10.1038/npp.2015.246.
Bixo M, Ekberg K, Poromaa IS, Hirschberg AL, Jonasson AF, Andréen L, et al. Treatment of premenstrual dysphoric disorder with the GABA(A) receptor modulating steroid antagonist Sepranolone (UC1010)-A randomized controlled trial. Psychoneuroendocrinology. 2017;80:46–55. https://doi.org/10.1016/j.psyneuen.2017.02.031.
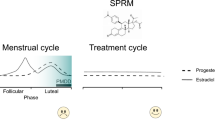
Comasco E, Kopp Kallner H, Bixo M, Hirschberg AL, Nyback S, de Grauw H, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: a proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178(3):256–65. https://doi.org/10.1176/appi.ajp.2020.20030286.
Bäckström T, Das R, Bixo M. Positive GABA(A) receptor modulating steroids and their antagonists: implications for clinical treatments. J Neuroendocrinol. 2022;34(2):e13013. https://doi.org/10.1111/jne.13013.
Bäckström T, Ekberg K, Hirschberg AL, Bixo M, Epperson CN, Briggs P, et al. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology. 2021;133:105426. https://doi.org/10.1016/j.psyneuen.2021.105426.
Kaltsouni E, Fisher PM, Dubol M, Hustad S, Lanzenberger R, Frokjaer VG, et al. Brain reactivity during aggressive response in women with premenstrual dysphoric disorder treated with a selective progesterone receptor modulator. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2021;46(8):1460–7. https://doi.org/10.1038/s41386-021-01010-9.
Dubol M, Epperson CN, Lanzenberger R, Sundström-Poromaa I, Comasco E. Neuroimaging premenstrual dysphoric disorder: a systematic and critical review. Front Neuroendocrinol. 2020;57:100838. https://doi.org/10.1016/j.yfrne.2020.100838.
Rabe T, Saenger N, Ebert AD, Roemer T, Tinneberg HR, De Wilde RL, et al. Selective progesterone receptor modulators for the medical treatment of uterine fibroids with a focus on ulipristal acetate. Biomed Res Int. 2018;2018:1374821. https://doi.org/10.1155/2018/1374821.
Whitaker LHR, Williams ARW, Critchley HOD. Selective progesterone receptor modulators. Curr Opin Obstet Gynecol [Internet]. 2014;26(4).
Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–39. https://doi.org/10.1016/j.yfrne.2008.02.001.
Beckley EH, Scibelli AC, Finn DA. Progesterone receptor antagonist CDB-4124 increases depression-like behavior in mice without affecting locomotor ability. Psychoneuroendocrinology. 2011;36(6):824–33. https://doi.org/10.1016/j.psyneuen.2010.11.004.
Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, et al. Actions of steroids: new neurotransmitters. J Neurosci Off J Soc Neurosci. 2016;36(45):11449–58. https://doi.org/10.1523/JNEUROSCI.2473-16.2016.
Mani SK, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Front Endocrinol (Lausanne). 2012;3:7. https://doi.org/10.3389/fendo.2012.00007.
Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96(2):162–71. https://doi.org/10.1159/000339822.
Paulmurugan R, Tamrazi A, Massoud TF, Katzenellenbogen JA, Gambhir SS. In vitro and in vivo molecular imaging of estrogen receptor α and β homo- and heterodimerization: exploration of new modes of receptor regulation. Mol Endocrinol. 2011;25(12):2029–40. https://doi.org/10.1210/me.2011-1145.
Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–29. https://doi.org/10.1056/NEJMra022219.
Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125(3):411–4. https://doi.org/10.1016/j.cell.2006.04.021.
Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev [Internet]. 2004;25(1):45–71. https://doi.org/10.1210/er.2003-0023.
Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437–52. https://doi.org/10.2147/CIA.S66690.
Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110(10):1524–8. https://doi.org/10.1111/j.1464-410X.2012.10968.x.
Adashi EY. Clomiphene citrate: mechanism(s) and site(s) of action—a hypothesis revisited. Fertil Steril. 1984;42(3):331–44. https://doi.org/10.1016/s0015-0282(16)48069-6.
Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. Steroids. 2003;68(10–13):1005–11. https://doi.org/10.1016/s0039-128x(03)00130-2.
Hild SA, Reel JR, Hoffman LH, Blye RP. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000;15(4):822–9. https://doi.org/10.1093/humrep/15.4.822.
Communal L, Vilasco M, Hugon-Rodin J, Courtin A, Mourra N, Lahlou N, et al. Ulipristal acetate does not impact human normal breast tissue. Hum Reprod. 2012;27(9):2785–98. https://doi.org/10.1093/humrep/des221.
Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectr. 2008;13(7):566–72. https://doi.org/10.1017/s1092852900016849.
Dimmock PW, Wyatt KM, Jones PW, O’Brien PM. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet (London, England). 2000;356(9236):1131–6. https://doi.org/10.1016/s0140-6736(00)02754-9.
Shah NR, Jones JB, Aperi J, Shemtov R, Karne A, Borenstein J. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder: a meta-analysis. Obstet Gynecol. 2008;111(5):1175–82. https://doi.org/10.1097/AOG.0b013e31816fd73b.
Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586. https://doi.org/10.1002/14651858.CD006586.pub4
de Wit AE, de Vries YA, de Boer MK, Scheper C, Fokkema A, Janssen CAH, et al. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: pairwise and network meta-analysis of randomized trials. Am J Obstet Gynecol. 2021;225(6):624–33. https://doi.org/10.1016/j.ajog.2021.06.090.
Sletten IW, Gershon S. The premenstrual syndrome: A discussion of its pathophysiology and treatment with lithium ion. Compr Psychiatry [Internet]. 1966;7(3):197–206. https://doi.org/10.1016/S0010-440X(66)80015-9
Deleon-Jones FA, Val E, Herts C. MHPG excretion and lithium treatment during premenstrual tension syndrome: a case report. Am J Psychiatry. 1982;139(7):950–2. https://doi.org/10.1176/ajp.139.7.950.
Jackson C, Pearson B, Girdler S, Johnson J, Hamer RM, Killenberg S, et al. Double-blind, placebo-controlled pilot study of adjunctive quetiapine SR in the treatment of PMS/PMDD. Hum Psychopharmacol. 2015;30(6):425–34. https://doi.org/10.1002/hup.2494.
Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16(3):e407-29.
Doll H, Brown S, Thurston A, Vessey M. Pyridoxine (vitamin B6) and the premenstrual syndrome: a randomized crossover trial. J R Coll Gen Pract. 1989;39(326):364–8.
Kendall KE, Schnurr PP. The effects of vitamin B6 supplementation on premenstrual symptoms. Obstet Gynecol. 1987;70(2):145–9.
Moslehi M, Arab A, Shadnoush M, Hajianfar H. The association between serum magnesium and premenstrual syndrome: a systematic review and meta-analysis of observational studies. Biol Trace Elem Res. 2019;192(2):145–52. https://doi.org/10.1007/s12011-019-01672-z.
Arab A, Rafie N, Askari G, Taghiabadi M. Beneficial role of calcium in premenstrual syndrome: a systematic review of current literature. Int J Prev Med. 2020;11:156. https://doi.org/10.4103/ijpvm.IJPVM_243_19.
Thys-Jacobs S, Starkey P, Bernstein D, Tian J. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. Am J Obstet Gynecol. 1998;179(2):444–52. https://doi.org/10.1016/s0002-9378(98)70377-1.
Mortola JF, Girton L, Fischer U. Successful treatment of severe premenstrual syndrome by combined use of gonadotropin-releasing hormone agonist and estrogen/progestin. J Clin Endocrinol Metab. 1991;72(2):252A-252F. https://doi.org/10.1210/jcem-72-2-252.
Mezrow G, Shoupe D, Spicer D, Lobo R, Leung B, Pike M. Depot leuprolide acetate with estrogen and progestin add-back for long-term treatment of premenstrual syndrome. Fertil Steril. 1994;62(5):932–7.
Freeman EW, Sondheimer SJ, Rickels K. Gonadotropin-releasing hormone agonist in the treatment of premenstrual symptoms with and without ongoing dysphoria: a controlled study. Psychopharmacol Bull. 1997;33(2):303–9.
Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28(Suppl 3):1–23. https://doi.org/10.1016/s0306-4530(03)00098-2.
Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Women’s Heal [Internet]. 2013;9(6):537–56. https://doi.org/10.2217/WHE.13.62.
Sundström-Poromaa I, Comasco E. New pharmacological approaches to the management of premenstrual dysphoric disorder. CNS Drugs. 2023;37(5):371–9. https://doi.org/10.1007/s40263-023-01004-9.
Sundström I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 1997;17(6):370–81. https://doi.org/10.1016/S0893-133X(97)00086-9.
Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. https://doi.org/10.1016/b978-0-12-571136-4.50008-5.
Carroll BJ, Ritchie JC, Rogers H, Kim DK. Fast feedback inhibition of adrenocorticotropic hormone secretion by endogenous cortisol in humans. Neuroendocrinology [Internet]. 2019;109(4):299–309. https://doi.org/10.1159/000499662.
Dallman MF, Yates FE. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci. 1969;156(2):696–721. https://doi.org/10.1111/j.1749-6632.1969.tb14008.x.
Sundström-Poromaa I, Comasco E, Sumner R, Luders E. Progesterone—friend or foe? Front Neuroendocrinol. 2020;59:100856. https://doi.org/10.1016/j.yfrne.2020.100856.
Olbert CM, Gala GJ, Tupler LA. Quantifying heterogeneity attributable to polythetic diagnostic criteria: theoretical framework and empirical application. J Abnorm Psychol. 2014;123(2):452–62. https://doi.org/10.1037/a0036068.
Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35(3):121–43. https://doi.org/10.1017/S0140525X11000446.
Carvalho T. New depression drug zuranolone one step closer to FDA ruling. Nat Med US. 2023;49:1032–3. https://doi.org/10.1038/d41591-023-00032-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this manuscript.
Conflicts of Interest
Dr. Rubinow is on the Scientific Advisory Board of (and has received honoraria and stock options from) Sage Therapeutics. He is also on the Scientific Advisory Boards of Sensorium Therapeutics and Embarq Neuro. He also has consulted to Arrivo Therapeutics. Dr. Sikes-Keilp reports no conflicts of interest.
Ethics approval
No ethics approval for this review was required.
Consent to participate
Not applicable.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
No software application or custom code was used in the generation of this manuscript.
Author contributions
CSK and DRR both made substantial contributions to the written manuscript, including conceptualization, drafting, and critical review for important intellectual content. Both authors provided final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sikes-Keilp, C., Rubinow, D.R. GABA-ergic Modulators: New Therapeutic Approaches to Premenstrual Dysphoric Disorder. CNS Drugs 37, 679–693 (2023). https://doi.org/10.1007/s40263-023-01030-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01030-7