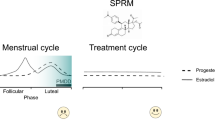
Abstract
Premenstrual dysphoric disorder (PMDD) is a sex-specific cyclic mood disorder characterized by heterogenous mood and physical symptoms during the luteal phase of the menstrual cycle. Currently available treatment options for PMDD are limited and suboptimal. With advanced understanding in pathophysiology of PMDD, the interaction between the gamma-aminobutyric acid (GABA) system and allopregnanolone, a neuroactive steroid derivative of progesterone, have been proposed as a potentially effective target for the treatment of PMDD. Although further research is needed, early clinical findings of new targeted treatments, such as dutasteride, isoallopregnanolone and ulipristal acetate, have demonstrated promising results in women with PMDD.
Similar content being viewed by others
References
Sikes-Keilp C, Rubinow DR. GABA-ergic modulators: new therapeutic approaches to premenstrual dysphoric disorder. CNS Drugs. 2023;37(8):679–93.
Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac Rev. 2022;11:11.
Hantsoo L, Payne JL. Towards understanding the biology of premenstrual dysphoric disorder: from genes to GABA. Neurosci Biobehav Rev. 2023;149: 105168.
Sundström-Poromaa I, Comasco E. New pharmacological approaches to the management of premenstrual dysphoric disorder. CNS Drugs. 2023;37(5):371–9.
Steiner M, Ravindran AV, LeMelledo JM, et al. Luteal phase administration of paroxetine for the treatment of premenstrual dysphoric disorder: a randomized, double-blind, placebo-controlled trial in Canadian women. J Clin Psychiatry. 2008;69(6):991–8.
Kornstein SG, Pearlstein TB, Fayyad R, et al. Low-dose sertraline in the treatment of moderate-to-severe premenstrual syndrome: efficacy of 3 dosing strategies. J Clin Psychiatry. 2006;67(10):1624–32.
Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized clinical trial. JAMA Psychiat. 2015;72(10):1037–44.
de Wit AE, de Vries YA, de Boer MK, et al. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: pairwise and network meta-analysis of randomized trials. Am J Obstet Gynecol. 2021;225(6):624–33.
Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;2:CD006586.
Martinez PE, Rubinow DR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41(4):1093–102.
Bixo M, Ekberg K, Poromaa IS, et al. Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist sepranolone (UC1010)-A randomized controlled trial. Psychoneuroendocrinology. 2017;80:46.
Bäckström T, Ekberg K, Hirschberg AL, et al. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology. 2021;133: 105426.
Comasco E, Kopp Kallner H, Bixo M, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: a proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178:256.
Kaltsouni E, Fisher PM, Dubol M, et al. Brain reactivity during aggressive response in women with premenstrual dysphoric disorder treated with a selective progesterone receptor modulator. Neuropsychopharmacology. 2021;46(8):1460–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Young-A Heo, a salaried employee of Adis International Ltd/Springer Nature and an editor of Drugs & Therapy Perspectives, was not involved in any publishing decisions for the manuscript and declares no declare no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heo, YA. Expand the treatment horizon of premenstrual dysphoric disorder with targeted therapeutic approaches. Drugs Ther Perspect 40, 27–30 (2024). https://doi.org/10.1007/s40267-024-01046-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-024-01046-z