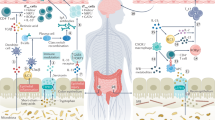
Abstract
It has now been established that a perturbation in gut microbiome composition exists in multiple sclerosis (MS) and its interplay with the immune system and brain could potentially contribute to the development of the disease and influence its course. The effects of the gut microbiota on the disease may be mediated by direct interactions between bacteria and immune cells or through interactions of products of bacterial metabolism with immune and CNS cells. In this review article we summarize the ways in which the gut microbiome of people with MS differs from controls and how bacterial metabolites can potentially play a role in MS pathogenesis, and examine approaches to alter the composition of the gut microbiota potentially alleviating gut dysbiosis and impacting the course of MS.
Similar content being viewed by others
References
Oksenberg JR, Baranzini SE, Sawcer S, Hauser SL. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet. 2008;9(7):516–26. https://doi.org/10.1038/nrg2395.
Mumford CJ, Wood NW, Kellar-Wood H, Thorpe JW, Miller DH, Compston DA. The British Isles survey of multiple sclerosis in twins. Neurology. 1994;44(1):11–5. https://doi.org/10.1212/wnl.44.1.11.
Islam T, Gauderman WJ, Cozen W, Hamilton AS, Burnett ME, Mack TM. Differential twin concordance for multiple sclerosis by latitude of birthplace. Ann Neurol. 2006;60(1):56–64. https://doi.org/10.1002/ana.20871.
Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC, Canadian Collaborative Study Group. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA. 2003;100(22):12877–82. https://doi.org/10.1073/pnas.1932604100.
Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7(3):268–77. https://doi.org/10.1016/S1474-4422(08)70042-5.
Ascherio A. Environmental factors in multiple sclerosis. Expert Rev Neurother. 2013;13(sup2):3–9. https://doi.org/10.1586/14737175.2013.865866.
Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–73. https://doi.org/10.1016/S1474-4422(14)70267-4.
Rook GW, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54(3):317–20. https://doi.org/10.1136/gut.2004.053785.
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. https://doi.org/10.1038/nature12331.
Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci. 2011;108(Supplement 1):4615–22. https://doi.org/10.1073/pnas.1000082107.
Croxford JL, Miyake S. Immunoregulation of multiple sclerosis by gut environmental factors. Clin Exp Neuroimmunol. 2015;6(4):362–9. https://doi.org/10.1111/cen3.12252.
Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–41. https://doi.org/10.1038/nature10554.
Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–50. https://doi.org/10.4049/jimmunol.0900747.
Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173(6):1714–23. https://doi.org/10.2353/ajpath.2008.080622.
Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. https://doi.org/10.1038/ncomms12015.
Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang FH, Willocq V, et al. Gut microbiome in progressive multiple sclerosis. Ann Neurol. 2021;89(6):1195–211. https://doi.org/10.1002/ANA.26084.
Durazzi F, Sala C, Castellani G, Manfreda G, Remondini D, De Cesare A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci Rep. 2021;11(1):3030. https://doi.org/10.1038/s41598-021-82726-y.
Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019. https://doi.org/10.3389/FIMMU.2019.00277.
Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7):2485S-2493S. https://doi.org/10.1093/JN/133.7.2485S.
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9. https://doi.org/10.1074/JBC.M211609200.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014. https://doi.org/10.1126/SCITRANSLMED.3009759.
Pérez-Pérez S, Domínguez-Mozo MI, Alonso-Gómez A, Medina S, Villarrubia N, Fernández-Velasco JI, et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ. 2020;8:e10220. https://doi.org/10.7717/peerj.10220.
Moles L, Delgado S, Gorostidi-Aicua M, Sepúlveda L, Alberro A, Iparraguirre L, et al. Microbial dysbiosis and lack of SCFA production in a Spanish cohort of patients with multiple sclerosis. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.960761.
Chen T, Noto D, Hoshino Y, Mizuno M, Miyake S. Butyrate suppresses demyelination and enhances remyelination. J Neuroinflamm. 2019;16(1):165. https://doi.org/10.1186/s12974-019-1552-y.
Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067-1080.e16. https://doi.org/10.1016/j.cell.2020.02.035.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. https://doi.org/10.1111/J.1574-6968.2009.01514.X.
Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. https://doi.org/10.1111/1462-2920.13589.
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429. https://doi.org/10.1371/journal.pone.0137429.
Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, et al. Gut microbiota in early pediatric multiple sclerosis: a case–control study. Eur J Neurol. 2016;23(8):1308–21. https://doi.org/10.1111/ene.13026.
Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med Off Publ Am Fed Clin Res. 2015;63(5):729–34. https://doi.org/10.1097/JIM.0000000000000192.
Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol. 2017;8:1141. https://doi.org/10.3389/fmicb.2017.01141.
Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol. 2020;11:1390. https://doi.org/10.3389/fimmu.2020.01390.
Takewaki D, Suda W, Sato W, Takayasu L, Kumar N, Kimura K, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci. 2020;117(36):22402–12. https://doi.org/10.1073/PNAS.2011703117.
Levi I, Gurevich M, Perlman G, Magalashvili D, Menascu S, Bar N, et al. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiome-metabolome analysis. Cell Rep Med. 2021. https://doi.org/10.1016/J.XCRM.2021.100246.
Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat Rev Immunol. 2007;7(10):817–23. https://doi.org/10.1038/nri2163.
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–9. https://doi.org/10.1038/nature06881.
Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013;65(4):1148. https://doi.org/10.1124/PR.113.007823.
Neavin DR, Liu D, Ray B, Weinshilboum RM. The role of the aryl hydrocarbon receptor (AHR) in immune and inflammatory diseases. Int J Mol Sci. 2018;19(12):3851. https://doi.org/10.3390/IJMS19123851.
Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor BV, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7(1):1–9. https://doi.org/10.1038/srep41473.
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97. https://doi.org/10.1038/nm.4106.
Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–8. https://doi.org/10.1038/s41586-018-0119-x.
Nourbakhsh B, Bhargava P, Tremlett H, Hart J, Graves J, Waubant E. Altered tryptophan metabolism is associated with pediatric multiple sclerosis risk and course. Ann Clin Transl Neurol. 2018;5(10):1211–21. https://doi.org/10.1002/ACN3.637.
Fitzgerald KC, Smith MD, Kim S, Sotirchos ES, Kornberg MD, Douglas M, et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell Rep Med. 2021;2(10):100424. https://doi.org/10.1016/J.XCRM.2021.100424.
Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand JW, Heim CE, et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22(1):25-37.e6. https://doi.org/10.1016/J.CHOM.2017.06.007.
Zelante T, Iannitti RG, Cunha C, DeLuca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. https://doi.org/10.1016/J.IMMUNI.2013.08.003.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–24. https://doi.org/10.1016/J.CHOM.2018.05.003.
Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. https://doi.org/10.1038/srep28484.
Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. https://doi.org/10.1016/J.CMET.2016.05.005.
Bhargava P, Smith MD, Mische L, Harrington E, Fitzgerald KC, Martin K, et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Investig. 2020;130(7):3467–82. https://doi.org/10.1172/JCI129401.
Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53. https://doi.org/10.1016/S1097-2765(00)80348-2.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5. https://doi.org/10.1126/SCIENCE.284.5418.1362.
Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–40. https://doi.org/10.1074/JBC.M209706200.
Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramírez L. TUDCA: an agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. J Cell Physiol. 2017;232(8):2231–45. https://doi.org/10.1002/JCP.25742.
Lewis ND, Patnaude LA, Pelletier J, Souza DJ, Lukas SM, King FJ, et al. A GPBAR1 (TGR5) small molecule agonist shows specific inhibitory effects on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS One. 2014;9(6):e100883. https://doi.org/10.1371/JOURNAL.PONE.0100883.
Ho PP, Steinman L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc Natl Acad Sci. 2016;113(6):1600–5. https://doi.org/10.1073/PNAS.1524890113.
Hucke S, Herold M, Liebmann M, Freise N, Lindner M, Fleck AK, et al. The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an IL-10-dependent fashion. Acta Neuropathol. 2016;132(3):413–31. https://doi.org/10.1007/S00401-016-1593-6.
Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414(6863):555–8. https://doi.org/10.1038/35107092.
Mazmanian SK, Cui HL, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–18. https://doi.org/10.1016/j.cell.2005.05.007.
Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–5. https://doi.org/10.1038/nature07008.
Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–95. https://doi.org/10.1038/mi.2010.29.
Rojas OL, Pröbstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. 2019;176(3):610-624.e18. https://doi.org/10.1016/j.cell.2018.11.035.
Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, et al. Gut microbiota–specific iga+ B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020. https://doi.org/10.1126/SCIIMMUNOL.ABC7191.
Miyauchi E, Kim SW, Suda W, Kawasumi M, Onawa S, Taguchi-Atarashi N, et al. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature. 2020;585(7823):102–6. https://doi.org/10.1038/s41586-020-2634-9.
Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185(7):4101–8. https://doi.org/10.4049/JIMMUNOL.1001443.
Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5(2):e9009. https://doi.org/10.1371/JOURNAL.PONE.0009009.
Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269–77. https://doi.org/10.1016/J.CELREP.2017.07.031.
Shahi SK, Freedman SN, Murra AC, Zarei K, Sompallae R, Gibson-Corley KN, et al. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front Immunol. 2019. https://doi.org/10.3389/FIMMU.2019.00462.
Shahi SK, Jensen SN, Murra AC, Tang N, Guo H, Gibson-Corley KN, et al. Human commensal Prevotella histicola ameliorates disease as effectively as interferon-beta in the experimental autoimmune encephalomyelitis. Front Immunol. 2020. https://doi.org/10.3389/FIMMU.2020.578648.
Quigley EMM, Gajula P. Recent advances in modulating the microbiome. F1000Research. 2020;9:46. https://doi.org/10.12688/f1000research.20204.1.
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. https://doi.org/10.1126/science.1208344.
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nat 2012 4887410. 2012;488(7410):178-184. doi:https://doi.org/10.1038/nature11319
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013;505(7484):559–63. https://doi.org/10.1038/nature12820.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. https://doi.org/10.1186/s12967-017-1175-y.
Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742–53. https://doi.org/10.1038/s41579-019-0256-8.
Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging. 2016;8(7):1416–31. https://doi.org/10.18632/AGING.100994.
Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018;27(6):1222-1235.e6. https://doi.org/10.1016/j.cmet.2018.05.006.
Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–85. https://doi.org/10.1038/nrn3820.
Hoare S, Lithander F, van der Mei I, Ponsonby AL, Lucas R. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: results from the Ausimmune Study. Mult Scler J. 2016;22(7):884–92. https://doi.org/10.1177/1352458515604380.
Gu Y, Vorburger RS, Gazes Y, Habeck CG, Stern Y, Luchsinger JA, et al. White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Ann Neurol. 2016;79(6):1014–25. https://doi.org/10.1002/ana.24674.
Gharagozloo M, Gris KV, Mahvelati T, Amrani A, Lukens JR, Gris D. NLR-dependent regulation of inflammation in multiple sclerosis. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2017.02012.
Di Majo D, Cacciabaudo F, Accardi G, Gambino G, Giglia G, Ferraro G, et al. Ketogenic and modified mediterranean diet as a tool to counteract neuroinflammation in multiple sclerosis: nutritional suggestions. Nutrients. 2022;14(12):2384. https://doi.org/10.3390/nu14122384.
Jensen SN, Cady NM, Shahi SK, Peterson SR, Gupta A, Gibson-Corley KN, et al. Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. Sci Adv. 2021;7(28):eabd4595. https://doi.org/10.1126/sciadv.abd4595.
Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci. 2017;114(40):10713–8. https://doi.org/10.1073/pnas.1711235114.
Valizadeh S, Seghinsara AM, Chollou KM, Bahadori A, Abbaszadeh S, Taghdir M, et al. The efficacy of probiotics in experimental autoimmune encephalomyelitis (an animal model for MS): a systematic review and meta-analysis. Lett Appl Microbiol. 2021;73(4):408–17. https://doi.org/10.1111/LAM.13543.
Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83(6):1147–61. https://doi.org/10.1002/ana.25244.
Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA. 2017;114(40):10719–24. https://doi.org/10.1073/pnas.1711233114.
Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr Edinb Scotl. 2017;36(5):1245–9. https://doi.org/10.1016/j.clnu.2016.08.015.
Fleming J, Isaak A, Lee J, Luzzio C, Carrithers M, Cook T, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler J. 2011;17(6):743–54. https://doi.org/10.1177/1352458511398054.
Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61(2):97–108. https://doi.org/10.1002/ANA.21067.
Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):1–11. https://doi.org/10.1186/S13073-016-0300-5.
Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388-1405.e21. https://doi.org/10.1016/j.cell.2018.08.041.
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-1423.e16. https://doi.org/10.1016/j.cell.2018.08.047.
Li K, Wei S, Hu L, Yin X, Mai Y, Jiang C, et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediat Inflamm. 2020;2020:e2058272. https://doi.org/10.1155/2020/2058272.
Borody T, Leis S, Campbell J, Torres M, Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am J Gastroenterol. 2011;106:S352. https://doi.org/10.14309/00000434-201110002-00942.
Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):459. https://doi.org/10.1212/NXI.0000000000000459.
Engen PA, Zaferiou A, Rasmussen H, Naqib A, Green SJ, Fogg LF, et al. Single-arm, non-randomized, time series, single-subject study of fecal microbiota transplantation in multiple sclerosis. Front Neurol. 2020;11(September):1–11. https://doi.org/10.3389/fneur.2020.00978.
Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. https://doi.org/10.1016/j.chom.2018.05.012.
Sand IK, Zhu Y, Ntranos A, Clemente JC, Cekanaviciute E, Brandstadter R, et al. Disease-modifying therapies alter gut microbial composition in MS. Neurol Neuroimmunol Neuroinflamm. 2019. https://doi.org/10.1212/NXI.0000000000000517.
Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3(7):e1700492. https://doi.org/10.1126/sciadv.1700492.
Forbes JD, Chen CY, Knox NC, Marrie RA, El-Gabalawy H, de Kievit T, et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome. 2018;6(1):221. https://doi.org/10.1186/s40168-018-0603-4.
Ventura RE, Iizumi T, Battaglia T, Liu M, Perez-Perez GI, Herbert J, et al. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci Rep. 2019;9(1):1–10. https://doi.org/10.1038/s41598-019-52894-z.
Choileáin SN, Kleinewietfeld M, Raddassi K, Hafler DA, Ruff WE, Longbrake EE. CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J Transl Autoimmun. 2020;3:100032. https://doi.org/10.1016/j.jtauto.2019.100032.
Reynders T, Devolder L, Valles-Colomer M, Van Remoortel A, Joossens M, De Keyser J, et al. Gut microbiome variation is associated to multiple sclerosis phenotypic subtypes. Ann Clin Transl Neurol. 2020;7(4):406–19. https://doi.org/10.1002/acn3.51004.
Tremlett H, Zhu F, Arnold D, Bar-Or A, Bernstein CN, Bonner C, et al. The gut microbiota in pediatric multiple sclerosis and demyelinating syndromes. Ann Clin Transl Neurol. 2021;8(12):2252–69. https://doi.org/10.1002/acn3.51476.
Cantoni C, Lin Q, Dorsett Y, Ghezzi L, Liu Z, Pan Y, et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine. 2022;76:103798. https://doi.org/10.1016/j.ebiom.2021.103798.
Zhou X, Baumann R, Gao X, Mendoza M, Singh S, Katz Sand I, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467-3486.e16. https://doi.org/10.1016/j.cell.2022.08.021.
Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023;15(1):1. https://doi.org/10.1186/s13073-022-01148-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partly supported by a Harry Weaver Neuroscience Scholar Award from the National MS Society to Pavan Bhargava.
Conflict of interest
DCL has no conflicts of interest to declare. PB is the principal investigator of a trial of TUDCA supplementation in progressive MS supported by the National Multiple Sclerosis Society.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
DCL and PB planned and prepared the manuscript. Both authors agreed on the finalized version of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ladakis, D.C., Bhargava, P. The Role of Gut Dysbiosis and Potential Approaches to Target the Gut Microbiota in Multiple Sclerosis. CNS Drugs 37, 117–132 (2023). https://doi.org/10.1007/s40263-023-00986-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-00986-w