Abstract
Background
(Es)ketamine and monoamine oxidase inhibitors (MAOIs), e.g., tranylcypromine, are therapeutic options for treatment-resistant major depression. Simultaneous administration is currently not recommended because of concern about hypertensive crises.
Objective
Our objective was to evaluate whether changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) during esketamine administration differed between patients who concomitantly received tranylcypromine and those who did not.
Methods
This was a retrospective cohort study utilizing cardiovascular monitoring data from inpatients treated for severe depression in unipolar, bipolar, and schizoaffective disorder. Primary outcomes were change in mean BP and HR during the first hour after intravenous or subcutaneous esketamine administration compared with baseline, controlled for confounders. Secondary analyses quantify differences in absolute BP during esketamine treatment and comparisons of BP peaks, temporal effects, and intraindividual comparisons before and after tranylcypromine initiation.
Results
Our analysis included 509 esketamine administrations in 43 patients, 14 of whom concomitantly received tranylcypromine. Controlling for creatinine and age, mean ± standard deviation (SD) BP changes were significantly increased by concomitant tranylcypromine treatment (ΔSBP: F[1,503] = 86.73, p < 0.001; ΔDBP: F[1,503] = 55.71, p < 0.001), but HR remained unaffected. Mean SBP change during esketamine administration was 2.96 ± 18.11 mmHg in patients receiving tranylcypromine (TCP+) and −8.84 ± 11.31 mmHg in those who did not (TCP−). Changes in DBP were −2.81 ± 11.20 mmHg for TCP+ and −10.77 ± 9.13 mmHg for TCP−. Moreover, we found a significant dose–response relationship between tranylcypromine dose and BP (SBP: B = 0.35, standard error [SE] = 0.12, 95% confidence interval [CI] 0.12–0.60, p = 0.004; adjusted R2 = 0.11, p = 0.008; DBP: B = 0.21, SE = 0.08, 95% CI 0.06–0.36, p = 0.007; adjusted R2 = 0.08; p = 0.023).
Conclusions
Although statistically significant changes in BP were identified in patients receiving tranylcypromine and esketamine, these changes were clinically insignificant. Thus, combining esketamine and this MAOI appears to be safe at standard doses. The dose–response relationship calls for caution with higher doses of tranylcypromine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Concomitant use of tranylcypromine and esketamine did not cause clinically significant hypertensive crises. |
A dose–response relationship between daily dosages given in milligrams of tranylcypromine and blood pressure during esketamine administrations indicated that caution might be necessary at high doses of tranylcypromine. |
1 Background
Depression is a leading contributor to the global burden of disease, and its relative share is predicted to further increase [1]. Depressive episodes mostly arise in the context of unipolar and bipolar affective disorder. Notably, most antidepressant treatments have a latency until they take effect, and there are a large number of nonresponders [2]. Considering the scope of this growing problem, effective and safe treatment options are urgently needed.
One novel approach to treating treatment-resistant depression (TRD) is the use of ketamine and its enantiomer esketamine. (Es)ketamine has been used as an anesthetic for decades. In 2000, the first randomized controlled cross-over study demonstrated its antidepressant properties in humans [3]. Ever since, research has established (es)ketamine as a novel, fast-acting antidepressant, especially in TRD. In addition, an antisuicidal effect has been observed that appears to be independent of depression severity [4, 5]. An intranasal formulation of esketamine was recently approved by the US FDA and by the European Medicines Agency (EMA) for adults with TRD based on several phase II/III studies [6,7,8,9,10,11]. (Es)ketamine’s exact mechanism of action as an antidepressant is only partially understood. Current hypotheses emphasize its role as a reversible N-methyl-d-aspartate (NMDA) receptor antagonist and activator of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and brain-derived neurotrophic factor (BNDF) [12]. Common short-term side effects include anxiety, dissociative states, and transient increases in blood pressure (BP) and heart rate (HR) [13].
Conversely, irreversible monoamine oxidase inhibitors (MAOIs) such as tranylcypromine have been used as antidepressant medications since the 1950s. Their mechanism of action is to increase the presynaptic concentration of neurotransmitters by inhibiting the enzymatic inactivation of monoamine neurotransmitters by monoamine oxidase. There are two isoforms of monoamine oxidase: MAO-A and MAO-B. Inhibiting MAO-A increases presynaptic serotonin and noradrenaline, whereas inhibiting MAO-B increases dopamine concentration. Different types of MAOI can be characterized by selectivity and type of binding (reversible/irreversible). Amongst the MAOIs, tranylcypromine is commonly used (although the extent of usage varies by country). It is irreversible and nonselective and thus increases the synaptic concentrations of all three neurotransmitters assumed (amongst others) to be involved in the pathophysiology of depression [14]. While MAOIs, especially tranylcypromine, have robust antidepressant efficacy, their use has decreased drastically since alternative antidepressants have become available because they require dietary restrictions and cause potentially severe side effects. Oral consumption of tyramine can lead to hypertensive crises in individuals treated with tranylcypromine. Nowadays, tranylcypromine is mostly recommended as a treatment option in TRD. Evidence suggests that it might be especially beneficial in atypical depression or depression with motor retardation [15].
In theory, individuals with TRD could benefit from the fast-acting antidepressant effect of (es)ketamine in combination with the additional sustained antidepressant properties of MAOIs such as tranylcypromine. The combination is not advised because of a fear of additive toxicity due to an increase in monoaminergic effects on cardiovascular function, especially hypertensive crises [16]. Consequently, clinical data evaluating the combination of the two substances are sparse.
Initially, a case report described cardiovascular parameters in a patient treated with tranylcypromine while receiving ketamine for anesthetic purposes during surgery and observed no hypertensive crises [17]. Later on, case studies reported data on psychiatric patients who received (es)ketamine in subanesthetic doses for TRD while simultaneously taking MAOIs. Two case reports on a total of five patients concomitantly receiving an MAOI (tranylcypromine or phenelzine) and intravenous (es)ketamine for TRD observed no relevant hemodynamic changes [18, 19]. In contrast, three other case studies described significant increases in BP after the administration of (es)ketamine (intravenous and nasal) in subjects treated with MAOIs (tranylcypromine, phenelzine, selegiline) in a total of nine patients [20,21,22]. One of those studies reported several hypertensive episodes, with measurements of up to 180/110 mmHg during ketamine infusions in one patient with preexisting cardiovascular comorbidities and mean increases in maximum BP of 15.8 mmHg systolic and 9.4 mmHg diastolic in three patients concomitantly treated with intravenous ketamine and MAOIs [20]. The second study described increases of maximum BP after nasal esketamine administration of 23 mmHg systolic and 12 mmHg diastolic in one patient [21]. As these increases were transient and asymptomatic, they were deemed clinically irrelevant. Lastly, statistically significant systolic BP (SBP) increases of 3 mmHg without significant differences in diastolic BP (DBP) or HR were found over 66 intravenous ketamine administrations and parallel phenelzine treatment [22]. For an overview of previous studies, please see Table 1 in the electronic supplementary material (ESM).
It is therefore vital to establish the safety profile of a combined treatment before being able to offer concomitant use as a new treatment option to individuals with TRD. However, until now, only a few case reports have suggested that a combination of an MAOI and (es)ketamine might be tolerated. Thus far, no study has looked at a large sample of patients simultaneously taking MAOIs and esketamine and conducted quantitative analyses of cardiovascular parameters, comparing the data derived with that from patients who only received esketamine without MAOIs. Thus, our aim is to provide quantitative clinical data of cardiovascular parameters in patients treated with esketamine and tranylcypromine concomitantly and compare them with data from patients who did not receive tranylcypromine while being treated with esketamine.
2 Methods
2.1 Study Design and Participants
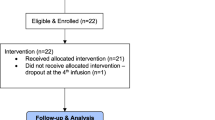
This was a retrospective clinical cohort study. The study was approved by the local ethics committee of the Medical Faculty of the Technical University Dresden (EK 263052019). Routine clinical data were anonymized for evaluation.
Data were collected from patients at the psychiatric inpatient unit of the University Hospital Carl Gustav Carus Dresden in Germany. All patients who had received at least one dose of esketamine for the treatment of a major depressive episode during their hospital stay between 4 October 2016 and 1 May 2020 were included in this analysis. Patients were diagnosed with a major depressive episode in the context of either unipolar depression, bipolar depression, or schizoaffective disorder. Per protocol, esketamine 0.25–0.5 mg/kg body weight was administered, usually starting with 0.25 mg/kg and increasing to the maintenance dose of 0.5 mg/kg. For safety reasons, the first dose was administered intravenously over 60 minutes, and the subsequent doses were given subcutaneously. Afterwards, continuous monitoring occurred via a multiparameter medical monitor for BP, HR, and O2 saturation for at least 1 h after administration. Esketamine was administered twice to thrice weekly. More information on this protocol has been published previously [23].
The primary outcome was to quantify and compare the mean change in SBP (ΔSBP) and DBP (ΔDBP) and change in HR (ΔHR) between baseline measurements and esketamine administration in patients receiving tranylcypromine (TCP+) or not (TCP−), controlled for relevant covariates.
Secondary outcomes were assessed to gain a more granular understanding of clinically relevant cardiovascular dynamics. Mean absolute SBP, DBP, and HR during esketamine administration were compared for TCP+ and TCP−, controlled for covariates. As several patients had newly initiated or discontinued tranylcypromine treatment during a series of esketamine administrations, we conducted a subgroup analysis of these cases and compared intraindividual changes and absolute measures in BP and HR during esketamine administrations before and after initiation of tranylcypromine using descriptive statistics. To account for possible transient sympathomimetic crises that might not be sufficiently captured by mean values, we computed individual maximum BP and HR values over all esketamine administrations for each patient and compared these between TCP+ and TCP−, controlled for covariates. To investigate a potential dose–response relationship for the effect of tranylcypromine, we analyzed the relationship between the daily dose of tranylcypromine at each esketamine administration and the cardiovascular outcome parameters (ΔSBP, ΔDBP, ΔHR, SBP, DBP, HR).
Lastly, we conducted several sensitivity analyses to ensure our assumptions were robust. We checked for possible adaptive effects of cardiovascular parameters during esketamine series by looking for temporal effects in single BP and HR values, as well as the development of mean BP and HR over the course of the administrations in patients not receiving tranylcypromine. Also, as we substituted missing baseline data for some time points, we repeated the main analyses without the substituted values for a sensitivity analysis. Lastly, checking for a potential impact of patients that contributed cases to both conditions, TCP+ and TCP−, we repeated the analyses for the primary outcome variables on patients who belonged to only one of these groups, excluding the subgroup.
2.2 Data Preparation
Anonymized clinical data (age, sex, height, weight, psychiatric diagnoses, preexisting arterial hypertension, medication, creatinine level, standard daily BP and HR readings, BP and HR during esketamine administration, and treatment status of tranylcypromine [including dose]) for each esketamine administration were extracted from the hospital IT system.
As hypertension is said to be a short-term adverse effect of esketamine, we assumed there to be no temporal dynamics or adaptive effects to esketamine over the course of treatment. Hypertensive crises are just as likely to occur at the first dose as at any subsequent dose. Thus, every single time point of esketamine administration can be treated independently; in order not to lose information by aggregating the data, we treated each time point of esketamine administration as a single case for the main analysis.
Our primary outcome variables were all recorded BP and HR readings within 60 minutes after every subcutaneous esketamine administration, or during the 60 minutes of intravenous esketamine administration. Baseline values were assessed collecting the last three standard BP and HR readings before each esketamine administration. In some cases (especially if esketamine was given on successive days in young patients without hypertension), no standard BP readings between esketamine administrations had taken place. For these measurements, we substituted the missing baseline data of the specific time point with the baseline BP readings before the first esketamine administration in that patient.
To separate the cases (TCP+) from the control measurements (TCP−), we checked clinical records for each measurement in every patient as to whether the patient had received tranylcypromine on the day of the esketamine administration and, if so, recorded the dose. Subsequently, we split the data into those receiving (TCP+) and those not receiving (TCP−) tranylcypromine. Where patients were initiated on tranylcypromine during the course of the esketamine series, or tranylcypromine was ended during the esketamine series, cases were split, meaning a single patient provided data for both case (TCP+) and control (TCP−) groups. Patients who received more than one series of esketamine during separate inpatient treatments within the recruitment period were treated as single cases. Single administrations with missing BP readings were excluded from the analysis.
2.3 Statistical Analyses
We calculated mean BP values (SBP and DBP) and HR before (baseline) and during (K+) esketamine treatments for every esketamine administration in every patient in the two groups, TCP+ and TCP−. Mean change in BP (ΔSBP, ΔDBP) and HR (ΔHR) for every esketamine administration in every patient was computed by subtracting the baseline value from the K+ value. We aggregated the data to calculate overall mean BP and HR measures and mean total change in BP and HR for each patient. To account for BP peaks, we identified the three highest BP and HR measures for each patient and from these computed mean maximum BP and HR readings (SBP max, DBP max, HR max). We checked for normal distribution of the data using Kolmogorov–Smirnov tests, Q–Q plots, and histograms. Descriptive statistics were computed using independent sample t-tests and chi-squared tests as appropriate.
We decided a priori to control for preexisting arterial hypertension and creatinine level as an indirect measure of hypertension in the main analysis, as well as relevant other confounders that differ between TCP+ and TCP−. As the variables preexisting arterial hypertension and creatinine levels were highly associated, we only included creatinine level as a covariate in the analyses. Furthermore, in the main analysis, where each time point of esketamine administration was treated as a single case, we included age as a covariate, since the distribution of age was significantly different between TCP+ and TCP−. Body mass index (BMI) was not included because there was no significant correlation with the outcome measures (see Table 2 in the ESM).
For the primary outcome, analyses of covariance (ANCOVAs) were conducted for the change in BP values for TCP+ and TCP− adjusting for potential confounders (creatinine level and age). To illustrate the clinical relevance of our findings, we also report mean absolute BP and HR values for TCP+ and TCP− adjusted for creatinine and age and compare these results using ANCOVAs.
Intraindividual changes in BP and HR between TCP+ and TCP− in the subgroup of patients that changed tranylcypromine status during the course of the esketamine treatment were analyzed descriptively because of the small sample size (n = 9). To test for a dose–response-relationship between milligrams of tranylcypromine and change in BP and HR, we conducted a linear regression analysis on patients taking tranylcypromine. Single transient hypertensive crises were analyzed using ANCOVAs comparing differences in mean maximum values for BP and HR (SBP max, DBP max, HR max) between TCP+ and TCP−, controlling for creatinine and age.
To account for potential adaptive effects or differences in cardiovascular parameters over the course of an esketamine series, we conducted linear mixed models to evaluate changes in primary and secondary outcome parameters over the course of the esketamine administrations. As the sample size in the TCP+ group was very small from the seventh dose onwards, this analysis was only performed on the first six esketamine administrations. For the sensitivity analyses, we repeated the ANCOVAs for differences in ΔSBP, ΔDBP, ΔHR, SBP, DBP, and HR between TCP+ and TCP− on a dataset without the substituted baseline measures. Moreover, we repeated the same analyses for a dataset only including participants that were solely attributable to TCP+ or TCP−, excluding the subgroup that switched tranylcypromine medication status. All analyses were conducted using IBM SPSS, version 26.
3 Results
3.1 Descriptive Statistics
A total of 44 patients received at least one dose of esketamine for the treatment of a major depressive episode between 4 October 2016 and 1 May 2020. One patient was excluded because the primary outcome variables were missing. Thus, 43 patients, aged 19–77 years (mean ± standard deviation 48.1 ± 15.8), with a total of 507 administered doses of esketamine were included in this analysis. Nine patients were initiated on or discontinued tranylcypromine during treatment and so contributed to TCP+ as well as to TCP−. Patient characteristics stratified by TCP+ and TCP− can be found in Table 1. t-tests and chi-squared tests revealed no significant differences between the groups for any of the reported characteristics.
3.2 Primary and Secondary Outcomes
There was a significant effect of tranylcypromine on ΔSBP during esketamine administration (2.96 ± 18.11 mmHg in TCP+ vs. −8.84 ± 11.31 mmHg in TCP−; F[1,503] = 66.89, p < 0.001) as well as on ΔDBP (−2.81 ± 11.20 mmHg for TCP+ vs. −10.77 ± 9.13 for TCP−; F[1,503] = 42.65, p < 0.001). However, receiving tranylcypromine did not yield statistically significant differences in ΔHR (−4.78 ± 6.36 bpm for TCP+ vs. −7.87 ± 9.34 bpm for TCP−; F[1,450] = 1.05, p = 0.306). Results are illustrated in Fig. 1.
Receiving tranylcypromine significantly impacted mean absolute SBP (TCP+: 128.93 ± 18.54 mmHg; TCP−: 119.69 ± 12.49 mmHg; F[1,503] = 29.61, p < 0.001) and DBP after esketamine administration (TCP+: 77.63 ± 11.46 mmHg; TCP−: 75.33 ± 9.68 mmHg; F[1,503] = 7.94, p = 0.005). HR was not significantly impacted by status of tranylcypromine (68.02 ± 8.92 bpm for TCP+ vs. 76.98 ± 12.06 bpm for TCP−; F[1,450] = 2.20, p = 0.139). Absolute BP and HR values during esketamine administration for TCP+ and TCP− are presented in Fig. 2.
3.3 Subgroup Analysis
Nine patients changed tranylcypromine status during the course of the esketamine treatment (eight were initiated on tranylcypromine and one discontinued). Descriptive analyses did not indicate meaningful intraindividual differences for changes in mean BP (ΔSBP: −0.7 ± 9.5 for TCP+ vs. −5.1 ± 11.9 for TCP−; ΔDBP: −8.5 ± 7.1 for TCP+ vs. −9.4 ± 9.4 for TCP−) or changes in HR (−5.7 ± 1.8 for TCP+ vs. −3.9 ± 12.4 for TCP−) during esketamine administration. Intraindividual changes in mean absolute cardiovascular parameters (SBP, DBP, HR) yielded similar outcomes. No major differences regarding medication status of tranylcypromine were found for SBP (125.4 ± 16.7 for TCP+ vs. 121.8 ± 16.2 for TCP−), DBP (78.0 ± 11.3 for TCP+ vs. 74.5 ± 9.4 for TCP−), and HR (74.2 ± 12.2 for TCP+ vs. 72.8 ± 9.9 for TCP−).
3.4 Maximum Measures
Comedication with tranylcypromine did not significantly influence the mean maximum SBP (146.6 ± 25.0 for TCP+ vs. 139.2 ± 16.6 for TCP−; F[1,48] = 1.22, p = 0.276) or mean maximum DBP (89.9 ± 13.5 for TCP+ vs. 92.3 ± 12.3 for TCP−; F[1,48] = 0.31, p = 0.581). Similarly, mean maximum HR was not significantly influenced by tranylcypromine (84.6 ± 10.9 for TCP+ vs. 94.0 ± 14.5 for TCP−; F[1,43] = 1.95, p = 0.170).
3.5 Dose–Response Relationship
The relationship between dose of tranylcypromine and difference in SBP during esketamine administration was significant (B = 0.35; SE 0.12; 95% CI 0.12–0.60; p = 0.004; adjusted R2 = 0.11; p = 0.008). The same held true for the relationship between DBP and tranylcypromine dose (B = 0.21; SE 0.08; 95% CI 0.06–0.36; p = 0.007; adjusted R2 = 0.08; p = 0.023). Lastly, changes in HR during esketamine administration were not significantly related to dose of tranylcypromine (B = −0.002; SE = 0.05; 95% CI −0.11–0.11; p = 0.976). Results are presented in Fig. 3.
Dose of tranylcypromine also showed a significant association with mean absolute SBP (B = 0.42; SE 0.12; 95% CI 0.18–0.66; p = 0.001; adjusted R2 = 0.13; p = 0.003). Conversely, daily dose of tranylcypromine was not significantly correlated with mean absolute DBP (B = 0.14; SE 0.07; 95% CI −0.01–0.28]; p = 0.073; adjusted R2 = 0.14; p = 0.002). Lastly, tranylcypromine dose did not significantly improve predicted HR measures (B = −0.08; SE 0.07; 95% CI −0.21 to −0.05]; p = 0.229, adjusted R2 = 0.22, p < 0.001). Results are presented in Fig. 4.
3.6 Sensitivity Analyses
3.6.1 Temporal Effects
To check for potential adaptive effects of repeated esketamine administration, we looked at temporal effects. We used linear mixed models to analyze the changes in BP and HR over the course of the first six esketamine administrations. No significant gradient in difference in BP (ΔSBP: F[6,42.1] = 0.48; p = 0.821; ΔDBP: F[6,43.6] = 0.44; p = 0.846) and ΔHR (F[6,44.4] = 0.71; p = 0.647) nor mean values (SBP: F[6,42.9] = 0.81; p = 0.566; DBP: F[6,43.2] = 0.37; p = 0.895; HR: F[6,44.6] = 0.13; p = 0.992) was observed.
3.6.2 Substituted Baseline Values
When excluding measurements with substituted baseline values, we accounted for a total of 485 esketamine administrations. Sensitivity analyses revealed no significant differences between the sample with or without substituted baseline values. Results are presented in Table 3 in the ESM.
3.6.3 Subgroup of Patients with Cases for TCP+ and TCP−
There were 34 patients with a total of 375 esketamine administrations who did not change medication status for tranylcypromine over the course of their esketamine series. Sensitivity analysis revealed no differences, and only mean absolute DBP failed to reach statistical significance (F[1,371] = 7.50, p = 0.006), unlike in the full sample. Results can be found in Table 4 in the ESM.
4 Discussion
4.1 Main Study Findings
This is the first study to quantitatively compare cardiovascular parameters in patients concomitantly receiving esketamine and tranylcypromine with those of patients only receiving esketamine. In our clinical cohort, we found that changes in mean SBP and DBP during esketamine administration differed significantly between patients receiving tranylcypromine and those who did not, controlling for serum creatinine concentration and age. A closer look at the results revealed that, unlike previous studies and assumptions, BP decreased during esketamine administration in the control group. However, in patients taking MAOI, SBP increased by roughly 3 mmHg after esketamine administration and DBP decreased but to a lesser extent than in the control group. Mean HR during esketamine administration decreased in both groups and did not differ significantly. These results suggest that the significant differences in BP between the two groups may not be caused by hypertension in patients receiving an MAOI and esketamine but rather by the absence or smaller drop in BP after esketamine administration in these patients. The results for mean absolute cardiovascular parameters showed significantly higher BP and insignificantly lower HR measures in patients taking tranylcypromine than in those who did not. However, the mean absolute BP for both groups, TCP+ and TCP−, was within the normal physiological range as defined by European Society of Cardiology (ESC) and European Society of Hypertension (ESH) guidelines (SBP ≤ 129 mmHg; DBP ≤ 84 mmHg) [24]. Moreover, all individual BP increases were asymptomatic and did not reach the level of hypertensive crises (= grade 3 hypertension according to ESC/ESH guidelines: SBP ≥ 180 mmHg or DBP ≥ 110 mmHg). No patient discontinued esketamine treatment because of hemodynamic events.
Supplementary analyses further aided in elucidating different aspects of the data. Making use of the fact that nine individuals changed tranylcypromine medication status during their esketamine series, we investigated potential intraindividual differences of cardiovascular parameters. As the sample size of this subgroup was small, only descriptive analyses were conducted. No clinically relevant differences in mean changes in cardiovascular parameters or in mean absolute parameters between the esketamine administrations with or without concomitant tranylcypromine medication were observed. These results indicate that tranylcypromine did not seem to have a relevant impact on cardiovascular parameters during esketamine administration. To account for possible short transient hypertension, mean maximum measures were analyzed and revealed no significant differences between groups. Thus, there seemed to be no differences in transient episodes of hypertension after esketamine administration in patients concomitantly receiving an MAOI. For the practicing physician, it therefore seems reasonable to conclude that co-administering tranylcypromine and esketamine appears to be safe.
However, the data do suggest a significant dose–response relationship between tranylcypromine dose and absolute SBP in patients receiving tranylcypromine. Higher doses of tranylcypromine were related to higher SBP during esketamine administration (Fig. 4). These changes in SBP were within the physiological range. Nonetheless, it would be prudent from a clinical viewpoint to use the combined treatment more cautiously in patients receiving tranylcypromine doses > 40 mg/day.
We conducted several sensitivity analyses to ascertain that our a priori assumptions did not distort the results. There were no effects of time on single BP and HR measures and no development of mean BP and HR was found over the course of the administrations. Thus, treating each esketamine administration as an individual case in looking at short-term adverse side effects of the combination of esketamine and an MAOI should not have influenced the results. Furthermore, sensitivity analyses revealed no cause for concern about the substituted missing baseline values or the subgroup of patients contributing cases to TCP+ and TCP− for BP data.
4.2 Strengths and Limitations
This study presents the largest sample of patients concomitantly treated with (es)ketamine and an MAOI to date. Moreover, the resolution of the data was very high, containing information on dose of esketamine and tranylcypromine for every single esketamine administration in every patient. Thus, one of the main strengths of this study is the large number of included esketamine administrations. Also, this is the first study to provide quantitative analyses on cardiovascular parameters in patients receiving esketamine and an MAOI compared with that for patients receiving esketamine only.
As this is a retrospective analysis of clinical data, the data are not as standardized and complete as they would be in a predesigned clinical study. Some of the data for either baseline measures or esketamine administrations were missing. Moreover, the number of recorded cardiovascular measurements during esketamine administrations varied notably. Also, despite presenting the largest sample to date, the number of patients taking tranylcypromine was still rather small (n = 14). The comparison between patients in TCP+ and TCP− revealed no significant group differences for sample characteristics (Table 1); however, comparing esketamine administrations between TCP+ and TCP−, most of the sample characteristics differed significantly (Table 2 in the ESM). This is likely because patients in the TCP− group received, on average, more esketamine doses (TCP+ 6.29 vs. TCP− 11.79), which led to a distorted representation of the sample characteristics of individual esketamine administrations. We can only speculate about reasons why individuals in TCP+ received on average fewer esketamine doses. Either there might be a synergistic effect of esketamine and tranylcypromine, leading to faster recovery and discontinuation of esketamine, or the opposite, that patients on tranylcypromine did not respond well to esketamine and it was thus stopped. Without further data on depression scores, this cannot be sufficiently answered.
To fully account for the high granularity of data, we conducted a differentiated data analysis, not only viewing the data at the patient level but also analyzing every esketamine administration as an individual case. Moreover, we utilized the subgroup of patients who were initiated on or discontinued tranylcypromine during the esketamine series to depict intraindividual changes, thereby controlling for other physiological variables that may have differed between the groups.
To ensure that mean cardiovascular parameters did not mask transient peaks, we compared mean maximum values, which confirmed the conclusion that the coadministration of the two compounds is probably safe.
4.3 Comparison with Other Studies
Studies on cardiovascular parameters during (es)ketamine administration in subanesthetic doses for the treatment of depression have typically reported increases in BP and HR. Although the extent of the BP increases differs (with one study reporting mean increases of 19.6 mmHg systolic and 13.4 mmHg diastolic in 205 intravenous ketamine doses in 97 patients [25] and another study finding increases of 3.28 mmHg systolic and 3.17 mmHg diastolic in 684 intravenous ketamine administrations in 66 patients [26]), they were all transient and responded well to treatment if treatment was at all necessary. Thus, the overall cardiovascular risk of (es)ketamine administration for depression was evaluated as low and acceptable by the authors, still stressing the importance of clinical monitoring during infusions [25, 26].
Curiously, our control group exhibited decreases in BP after receiving esketamine (mean ΔSBP −8.84 mmHg, mean ΔDBP −10.77 mmHg). One possible explanation for these different results might be the mode of administration. Previous studies mostly report on intravenous administration of (es)ketamine. Our patients received intravenous esketamine for the first dose; if no adverse side effects were experienced, all successive administrations were subcutaneous. A double-blind, placebo-controlled pilot study compared different routes of ketamine administration (intravenous, subcutaneous, and intramuscular) and concluded that subcutaneous ketamine administrations had the least hemodynamic side effects [27]. This might be, in part, due to the lower peak ketamine plasma concentrations after subcutaneous or intramuscular administration compared with intravenous administrations. However, a study on cardiovascular changes in 394 subcutaneous esketamine administrations in 70 patients reported mean BP increases of 4.35 mmHg systolic and 4.26 mmHg diastolic 30 minutes after administration [28], thereby also indicating small BP increases after subcutaneous esketamine administration.
Another explanation for the BP decreases in our sample might be the different settings in which the cardiovascular parameters were assessed. At baseline, cardiovascular parameters were collected during the day, sitting down in the nursing station. After an esketamine administration, our patients had to lie down on their bed in a calm atmosphere for an hour while they were monitored. Thus, baseline parameters in our sample might be higher in comparison as patients were less relaxed than they were during the esketamine administrations, which could explain the decreases in BP found in this study.
Similar to the results regarding cardiovascular changes after ketamine administration in general, case reports on patients concomitantly receiving ketamine and an MAOI inconsistently reported transient increases in cardiovascular parameters after (es)ketamine administration [20,21,22]. Two studies describe BP increases that range between 15.8 mmHg systolic and 9.4 mmHg diastolic [20], and 23 mmHg systolic and 12 mmHg diastolic [21]. Another study reported no ‘relevant hemodynamic changes’ [18] but failed to provide exact data. Lastly, a recent study analyzed 22 ketamine infusions in three patients and did not find statistically significant differences in BP or HR after ketamine infusions [19]. Our data show small increases in SBP (∼ 3 mmHg) and a decrease in DBP and HR in patients following administration of esketamine while on tranylcypromine. This deviates slightly from reports in previous studies. Again, these slight differences might be explained by mode of administration, because all other studies report intravenous [17,18,19,20, 22] or nasal [21] application of (es)ketamine, which might lead to bigger hemodynamic changes, or by differences in setting between baseline and esketamine BP readings. Generally, it is important to note that these factors might slightly limit the comparability of our BP change measurements to previous study results of BP change. In contrast, results for mean absolute BP during (es)ketamine administrations were very similar between our patients in the TCP+ group (128.9/77.6 mmHg) and previously reported cases with concomitant (es)ketamine and MAOI treatment (mean BP: 123/80 mmHg [19]; mean average of maximum BP: 143.2/85.6 mmHg [20]; highest (es)ketamine BP 128/78.9 mmHg [22]; BP range during (es)ketamine treatment: 99–135/60–82 mmHg [21]). We thus feel confident that our absolute data are fully comparable to other studies.
Overall, it is important to keep in mind that the aim of our study was to compare BP in TCP+ and TCP−. Both factors that might hamper comparisons between our study and previous studies (mode of administration, settings of BP measurements) are not relevant for our main research question, as these factors were consistent for all our patients. As no previous study investigating differences between TCP+ to TCP− exists, we cannot compare these findings to previous results.
5 Conclusions
Overall, our results suggest that, although there are statistically significant differences in cardiovascular parameters in individuals treated with subcutaneous esketamine and tranylcypromine, these differences are not clinically relevant. The combination of (es)ketamine and MAOIs for the treatment of major depressive episodes may be a valid option, especially in TRD. It is important to note that an intranasal formulation of esketamine has been approved by the FDA and EMA. Our data are based on off-label (mainly) subcutaneous use of esketamine. Although it seems possible that the coadministration of intranasal esketamine and an MAOI may lead to similar outcomes to those reported here, more research is needed. We hope the approval of intranasal esketamine might encourage more research on this important topic. Generally, clinicians may feel reassured about administering (es)ketamine subcutaneously if they are concerned about hemodynamic adverse events (e.g., in patients with preexisting hypertension), because this mode of administration exhibited the least hemodynamic side effects and, to date, is seldom used. However, caution should be used at doses exceeding tranylcypromine 40 mg/day. Future research, ideally large prospective clinical trials, are needed to further confirm the safety of this combination of antidepressant medications.
References
Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21:10.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4.
Wilkinson ST, Ballard ED, Ph D, Bloch MH, Mathew SJ. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–8.
Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry Society of Biological Psychiatry. 2009;66:522–6.
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2018;75:139–48.
Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2019;76:893–903.
Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22:616–30.
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–38.
Wajs E, Leah A, Morrison R, Daly E, Lane R, Lim P, et al. Long-term safety of esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: SUSTAIN-2 phase 3 study. Eur Neuropsychopharmacol. 2019;29:S44–5.
Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28:121–41.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. https://doi.org/10.1016/S2215-0366(17)30272-9.
Köhler S, Stöver LA, Bschor T. MAO-Hemmer als Behandlungsoption der therapieresistenten Depression: Anwendung, Wirksamkeit und Besonderheiten. Fortschritte der Neurol Psychiatr. 2014;82:228–38.
Ulrich S, Ricken R, Buspavanich P, Schlattmann P, Adli M. Efficacy and adverse effects of tranylcypromine and tricyclic antidepressants in the treatment of depression: a systematic review and comprehensive meta-analysis. J Clin Psychopharmacol. 2020;40:63–74.
Janssen Pharmaceutica NV. Spravato EPAR Product information [Internet]. Sprav. EPAR Prod. Inf. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/spravato#product-information-section. Accessed 30 July 2020.
Doyle DJ. Ketamine induction and monoamine oxidase inhibitors. J Clin Anesth. 1990;2:324–5.
Bartova L, Vogl SE, Stamenkovic M, Praschak-Rieder N, Naderi-Heiden A, Kasper S, et al. Combination of intravenous S-ketamine and oral tranylcypromine in treatment-resistant depression: a report of two cases. Eur Neuropsychopharmacol. 2015;25:2183–4.
Bottemanne H, Bonnard E, Claret A, Petit AC, Gaillard R, Fossati P. Ketamine and monoamine oxidase inhibitor combination: utility, safety, efficacy? J Clin Psychopharmacol. 2020;40:636–8.
Katz RB, Toprak M, Wilkinson ST, Sanacora G, Ostroff R. Concurrent use of ketamine and monoamine oxidase inhibitors in the treatment of depression: a letter to the editor. Gen Hosp Psychiatry. 2018;54:62–4.
Dunner DL, Fugate RM, Demopulos CM. Safety and efficacy of esketamine nasal spray in a depressed patient who was being treated with tranylcypromine: a case report. Neurol Psychiatry Brain Res. 2020;36:30–1.
Wang JCC, Swainson J. The concurrent treatment with intravenous ketamine and an irreversible monoamine oxidase inhibitor for treatment-resistant depression without hypertensive crises. J Clin Psychopharmacol. 2020;40:515–7. https://doi.org/10.1097/JCP.0000000000001244.
Ritter P, Findeis H, Bauer M. Ketamine in the treatment of depressive episodes. Pharmacopsychiatry. 2020;53:45–50.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). Blood Press. 2018;36:2284–309.
Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76:247–52.
Riva-Posse P, Reiff CM, Edwards JA, Job GP, Galendez GC, Garlow SJ, et al. Blood pressure safety of subanesthetic ketamine for depression: a report on 684 infusions. J Affect Disord. 2018;236:291–7.
Loo CK, Gálvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016;134:48–56.
Del Sant LC, Sarin LM, Magalhães EJM, Lucchese AC, Tuena MA, Nakahira C, et al. Effects of subcutaneous esketamine on blood pressure and heart rate in treatment-resistant depression. J Psychopharmacol. 2020;34:1155–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. This report represents independent research supported by Bundesministerium für Bildung und Forschung (BMBF) grant tC2020_03_MED (transCampus/TUD/BMBF), as well as the National Institute for Health Research (AHY and NIHR Clinician Scientist Award JR CS-2017-17- 007) and NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. PR received grants from the BMBF. The views expressed are those of the author(s) and not necessarily those of the BMBF, NHS, NIHR, or the Department of Health.
Conflict of interest
AHY gave paid lectures and is on advisory boards for the following companies: AstraZeneca, Eli Lilly, Lundbeck, Sunovion, Servier, LivaNova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, and COMPASS and serves as a consultant to Johnson & Johnson and LivaNova. JR was paid by COMPASS Pathways to attend trial-related meetings and conferences to present the results of research using psilocybin and served as a paid consultant to Beckley PsyTech and Clerkenwell Health. MB has received institutional funding/grant support from Deutsche Forschungsgemeinschaft (DFG), BMBF, and the European Commission; has received speaker honoraria and/or travel compensation from Aristo, Hexal AG, Janssen Pharmaceutica NV, Janssen-Cilag, and Sunovion; has served on advisory boards or received honoraria consultancy from GH Research, Janssen-Cilag, neuraxpharm, Novartis, Sandoz, Shire International GmbH, Sumitomo Dainippon, Sunovion, and Takeda. The authors assert that the mentioned companies had no influence over the content of this article. VML, CS, HF, and PR have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Our study complies with the Declaration of Helsinki and the study protocol was approved by the local ethics committee of the Medical Faculty of the Technical University Dresden (EK 263052019).
Consent
Not applicable, as anonymized routine clinical data were used.
Availability of data and material
The dataset can be obtained from the corresponding author upon reasonable request.
Code availability
SPSS Syntax codes are available upon request.
Author contributions
All authors contributed to the study design and interpretation of the data. Material preparation and data collection were performed by VML and HF, and data analysis was performed by CS and VML. The first draft of the manuscript was written by VML; all authors commented on subsequent versions of the manuscript and approved the final manuscript and the decision to submit it for publication. All authors agree to be accountable for the data reported in the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ludwig, V.M., Sauer, C., Young, A.H. et al. Cardiovascular Effects of Combining Subcutaneous or Intravenous Esketamine and the MAO Inhibitor Tranylcypromine for the Treatment of Depression: A Retrospective Cohort Study. CNS Drugs 35, 881–892 (2021). https://doi.org/10.1007/s40263-021-00837-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00837-6