Abstract
OnabotulinumtoxinA (Botox®; a formulation of botulinum toxin type A (BoNT/A)] is indicated for the prevention of headaches in adults with chronic migraine (CM) in numerous countries, including those of Europe. In clinical trials, intramuscular administration of BoNT/A (155–195 units at 12-week intervals) to patients with CM was generally well tolerated and associated with sustained and clinically meaningful improvements in multiple assessments of headache symptoms, headache-related impact and/or disability and migraine-specific health-related quality of life over a period of 1 year (in the pivotal PREEMPT 1 and 2 studies) and 2 years (in the phase IV COMPEL study). The efficacy and safety of BoNT/A therapy have been confirmed in a number of large, prospective, real-world studies conducted in Europe, including the 2-year REPOSE study. Intramuscular BoNT/A has also demonstrated greater clinical utility than the oral prophylactic medication topiramate in a clinical practice setting (FORWARD study).
Video abstract
Video abstract (MP4 141598 kb)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Q&A, including a peer-reviewed video abstract, can be found at https://doi.org/10.6084/m9.figshare.13218869. |
Administered intramuscularly every 3 months |
Effective in CM patients with or without acute medication overuse |
Effective in CM patients who have or have not previously used recognized prophylactic medications |
Neck pain, (facial) muscle weakness and eyelid ptosis are the most common treatment-related adverse events |
1 What is the rationale for using onabotulinumtoxinA in chronic migraine?
Chronic migraine [CM; defined as ≥ 15 headache days/month for > 3 months (within the last 12 months), with ≥ 8 migraine days/month [1]] affects ≈ 1–2% of the general population [2]. It usually evolves from episodic migraine (EM; defined as < 15 headache days/month) and is associated with a greater symptomatic and socioeconomic burden, as well as higher healthcare resource utilization (HRU), compared with EM [3].
The first-line treatment of CM is pharmacological [4]; it is based on the use of acute medications to relieve or ameliorate the symptoms of a migraine attack that has already begun, and the use of preventative therapies to reduce the frequency, duration and severity of attacks (thereby limiting the need for acute medications, as these may be causing concurrent medication overuse headache) [3].
Various oral medications [e.g. β-adrenoreceptor antagonists (β-blockers), anticonvulsants, antidepressants and calcium channel blockers (CCBs)] are used for the prophylaxis of CM [4]. However, topiramate, the only oral agent currently approved for the prophylaxis of migraine headache in Europe, is not specifically licenced for the prophylaxis of CM in this region [5]. Moreover, shortcomings of these oral preventatives in terms of efficacy, tolerability and adherence have led to the development and subsequent approval of novel therapeutic modalities for the treatment of CM in the form of onabotulinumtoxinA [Botox®; a formulation of botulinum toxin type A (BoNT/A)] [3, 6] and, more recently, monoclonal antibody calcitonin gene receptor peptide (CGRP) antagonists (e.g. erenumab, fremanezumab and galcanezumab) [4, 7].
Intramuscular administration of BoNT/A has been approved for the prevention of headaches in adults with CM in numerous countries worldwide, including those of Europe and North America [3, 8]. However, the exact wording of the indication may vary between countries, and local prescribing information should be consulted for specific details [3].
Prescribing information pertaining to the use of BoNT/A for the prophylaxis of CM in the UK [9] (as a representative of prescribing information in European countries) is summarized in Table 1. This article discusses the efficacy and tolerability of BoNT/A in the prevention of CM, primarily from a European perspective.
2 How does onabotulinumtoxinA work in chronic migraine?
Although the exact mechanism(s) of action are still being investigated, extracranial administration of BoNT/A is believed to prevent headaches in patients with CM by inhibiting peripheral sensitization and, indirectly, central sensitization within the trigeminovascular system, both of which are postulated to be involved in migraine pathophysiology and chronification (i.e. conversion of EM to CM) [3, 10, 11].
The intraneuronal target for BoNT/A is synaptosomal-associated fusion attachment protein (SNAP‐25), which is one of the essential proteins of the soluble N‐ethylmaleimide‐sensitive fusion attachment protein receptor (SNARE) complex; BoNT/A cleaves SNAP-25, thereby disrupting SNARE-mediated vesicle trafficking [10]. Preclinical evidence indicates pericranial injections of BoNT/A in the trigeminally-innervated cranio-facial-cervical region may reduce peripheral sensitization by modulating two SNARE-dependent processes:
-
Decreasing the release of pro-inflammatory and excitatory neurotransmitters and neuropeptides (e.g. substance P, CGRP and glutamate) from primary afferent fibres that transmit nociceptive pain; and
-
Decreasing the upregulation of pain-sensitive ion channels [e.g. transient receptor potential vanilloid 1 (TRPV1) receptor and P2X3 purinergic receptor)] on nociceptive nerve terminals and cell bodies [10].
3 What is the clinical efficacy of onabotulinumtoxinA in chronic migraine?
The short- and longer-term efficacy of intramuscular BoNT/A for headache prophylaxis in adults with CM has been demonstrated over a period of 1 year in two pivotal phase III studies (PREEMPT 1 [12] and 2 [13]; together comprising the PREEMPT clinical programme [14, 15]) and over a period of 2 years in a phase IV study (COMPEL) [16].
3.1 In the PREEMPT clinical programme
The multicentre PREEMPT 1 and multinational PREEMPT 2 studies, which were otherwise identical in design (apart from the designation of the primary and secondary endpoints), enrolled a combined total of 1384 patients (86% females; mean age 41 years; 65% acute analgesic/medication overusers) who had ≥ 15 headache days/month (with headache lasting ≥ 4 h/day), with ≥ 50% of the days being migraine/probable migraine days [14, 15].
The method whereby BoNT/A was administered in these studies (referred to as the ‘PREEMPT paradigm’) is the approved dosing regimen in Europe (see Table 1). Patients received up to five treatment cycles: two with BoNT/A 155–195 U or placebo during the 24-week, randomized, double-blind phase (at weeks 0 and 12) and three with BoNT/A 155–195 U during the subsequent open-label extension phase (at weeks 24, 36 and 48) [12,13,14,15].
3.1.1 Versus placebo
Two treatment cycles of BoNT/A significantly improved all assessments of headache symptoms, including the monthly frequency of headache days (primary endpoint), compared with placebo, according to a predefined pooled analysis of the two PREEMPT trials (Table 2). At week 24, significantly (p < 0.001) more BoNT/A recipients than placebo recipients were responders in terms of achieving a clinically meaningful (i.e. ≥ 50%) improvement in the monthly frequency of headache days (47% vs 35%), moderate to severe headache days (49% vs 38%) and migraine days (48% vs 36%), as well as in the monthly cumulative hours of headache on headache days (50% vs 39%) [14, 15]. Improvements in headache symptoms in BoNT/A-treated patients were reflected in reduced triptan consumption, albeit overall analgesic/medication consumption was not significantly decreased relative to placebo (Table 2). A post hoc pooled analysis indicated that BoNT/A therapy had an early onset of action, with significant (p < 0.05 vs placebo) reductions in headache and migraine days/week apparent at week 1, and persisting from week 3 onwards, after the first treatment cycle [17].
BoNT/A therapy also significantly improved headache-related impact, assessed using the Headache Impact Test-6 (HIT-6), and migraine-specific health-related quality of life (HR-QOL), assessed using the Migraine Specific Quality of Life Questionnaire (MSQ), compared with placebo, according to the predefined pooled analysis (Table 2). Significant (p < 0.001) and clinically meaningful improvements in HIT-6 and MSQ role-restrictive (RR), role-preventive (RP) and emotional-functioning (EF) domain scores favouring BoNT/A over placebo were seen at all assessment points through week 24 [14, 15].
In additional pooled analyses [18,19,20,21,22,23,24] (post hoc [18, 21, 22, 24], where stated):
-
BoNT/A improved (p < 0.05 vs placebo) headache symptoms in patients who were or were not acute medication overusers at baseline [19, 23], those who had or had not previously used recognized prophylactic medications [23], and those who had previously failed to respond to ≥ 3 preventative therapies [20]
-
BoNT/A increased (p < 0.001 vs placebo) responder rates at week 24 in terms of the proportions of patients who achieved clinically meaningful improvements in monthly headache-day frequency (45% vs 34%), average daily headache severity (36% vs 22%), HIT-6 score (41% vs 25%) and MSQ RR domain score (59% vs 40%), as well as the proportions of patients who achieved at least one (72% vs 57%), at least two (54% vs 37%), at least three (34% vs 20%) or all four (20% vs 9%) of these outcomes [18]
-
Among patients who did not achieve a clinically meaningful improvement in monthly headache-day frequency (i.e. monthly headache-day frequency non-responders), assessments of headache severity [21, 24], headache-related impact (HIT-6 score) [24] and migraine-specific HR-QOL (all three MSQ domain scores) [24] at week 24 were significantly improved with BoNT/A (all p < 0.01 vs placebo)
-
More BoNT/A-treated than placebo-treated patients achieved treatment-controlled CM, defined as < 15 headache days/month during weeks 13–24 (56.1% vs 49.1%; p = 0.01) [22]. BoNT/A recipients, as compared with placebo recipients, who achieved treatment-controlled CM reported significantly (p ≤ 0.017) fewer headache days/month (7.4 vs 6.8), as well as greater (p ≤ 0.012) – and clinically meaningful – improvements in HIT-6 and MSQ RR, RP and EF domain scores at all assessments through week 24 [22].
3.1.2 Over repeated treatment cycles
Cumulative prophylactic effects were seen over successive BoNT/A treatment cycles in the PREEMPT trials [15, 17, 25]. In the predefined pooled analysis [15], all assessments of headache symptoms, acute analgesic/medication use, headache-related impact and migraine-specific HR-QOL continued to improve relative to baseline during the open-label extension phase, both in those who had previously received BoNT/A (early treatment) and those who had previously received placebo (late treatment) during the double-blind phase (Table 2). Nonetheless, multiple assessments of headache symptoms still significantly favoured the early treatment group over the late treatment group at week 56 (Table 2), indicating that patients who started treatment earlier (i.e. had been treated for longer) had better outcomes at that time point.
A post hoc pooled analysis indicated that a high proportion (61%) of patients treated with BoNT/A throughout achieved sustained treatment-controlled CM, defined as < 15 headache days/month during all 6 months of the extension phase, i.e. weeks 25–56 [22]. BoNT/A-treated patients who achieved sustained treatment-controlled CM reported a mean of 5.0 headache days/month, as well as clinically meaningful improvements in HIT-6 and MSQ RP, RR and EF domain scores at all assessments through week 56 [22].
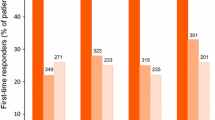
Importantly, another post hoc pooled analysis [26] indicated that at least two or three treatment cycles may be needed to determine responsiveness to BoNT/A therapy. For example, 22% of the 349 BoNT/A recipients who were monthly headache-day frequency non-responders after the first cycle became monthly headache-day frequency responders after the second cycle; 26% of the 271 BoNT/A recipients who were monthly headache-day frequency non-responders after the first and second cycles became monthly headache-day frequency responders after the third cycle. Results for responder rates for other outcomes, including monthly moderate to severe headache-day frequency and HIT-6 score, were similar [26].
3.2 In the COMPEL study
The multinational COMPEL study [16] enrolled 716 patients (85% females; mean age 43 years; 64% acute analgesic/medication overusers) who had ≥ 15 headache days/month (with headache lasting ≥ 4 h/day). They received up to nine cycles of BoNT/A 155 U (i.e. using only the fixed-site, fixed-dose approach of the PREEMPT paradigm; see Table 1) during the 108-week open-label treatment intervention phase (at weeks 0, 12, 24, 36, 48, 60, 72, 84 and 96). Just over one-half (52.1%) completed all nine treatment cycles [16].
The COMPEL study showed the continuing benefit of treatment with BoNT/A over a 2-year period, thereby substantiating and extending the findings of the PREEMPT clinical programme [16]. BoNT/A therapy significantly and progressively decreased the number of headache days/month at all assessment time points throughout the study, including week 24 (first post-baseline assessment) and week 108 (fourth and final post-baseline assessment; primary endpoint) (Table 3). Similarly, there were significant and progressive reductions in the number of moderate to severe headache days/month, as well as significant and sequential improvements in headache-related impact (HIT-6 score) (Table 3).
In other analyses of BoNT/A efficacy in COMPEL [16, 27,28,29,30,31,32,33,34,35,36,37] (post hoc [27, 35, 36], where stated):
-
Assessments of migraine-related disability (MIDAS score) [28] and migraine-specific HR-QOL (all three MSQ domain scores) were improved (p < 0.0001 vs baseline) at week 108 [28]
-
Comorbid symptoms of depression [measured using the 9-item Patient Health Questionnaire (PHQ-9)] and anxiety (7-item Generalized Anxiety Disorder Assessment), and associated symptoms of sleep disturbance (Pittsburgh Sleep Quality Index) and fatigue (Fatigue Severity Scale), were improved (p < 0.0001 vs baseline) at week 108; similar improvements were seen at all earlier assessments [29]
-
Assessments of headache symptoms [16, 30,31,32,33,34], headache-related impact (HIT-6 score) [16, 30, 33], migraine-related disability (MIDAS score) [31, 32, 34] and migraine-specific HR-QOL (all three MSQ domain scores) [32, 34] were improved to a similar extent in patients with or without a history of acute medication overuse [30, 31], those receiving or not receiving oral preventive treatments at baseline [16], those with or without daily headaches at baseline [32, 33], and those with or without allodynia at baseline [34]
-
The proportion of monthly headache-day frequency responders progressively increased over the course of the study (47%, 54%, 57% and 62% at weeks 24, 60, 84, 108, respectively). Among patients who completed all nine treatment cycles, a high proportion (76%) of monthly headache-day frequency responders at week 24 maintained this response through week 108 [35]
-
Responder rates at week 108 were 62% for monthly headache-day frequency, 59% for HIT-6 score, 75% for MIDAS score and 66% for MSQ RR domain score; 87%, 72%, 52% and 27% of patients achieved at least one, at least two, at least three or all four of these outcomes, respectively [27]
-
The proportion of patients who achieved treatment-controlled CM (defined as < 15 headache days/month in any of the 4-week periods ending at weeks 24, 60, 84 and 108) was high and increased progressively over the course of the study (56%, 69%, 70% and 74% at weeks 24, 60, 84 and 108, respectively) [36]
-
Sustained treatment-controlled CM (defined as < 15 headache days/month in all of the 4-week periods ending at weeks 24, 60, 84 and 108) was achieved by 50% of evaluable patients (n = 289). These individuals reported a reduction in moderate-to-severe headache days/month (−10.4 , −11.0, −11.4 and −11.9 at weeks 24, 60, 84 and 108, respectively; all p < 0.001vs baseline); the majority were monthly headache-day frequency responders (78%, 88%, 88% and 89% at weeks 24, 60, 84 and 108, respectively) [36].
4 What is the effectiveness of onabotulinumtoxinA in clinical practice?
The effectiveness of BoNT/A administered in accordance with the PREEMPT paradigm as a real-world preventative therapy for CM has been demonstrated in a number of large, prospective, observational studies conducted in routine clinical practice in Europe [38,39,40,41,42,43], notably the multinational REPOSE study [38] (Table 4).
4.1 In the REPOSE study
Consistent with the observed efficacy of BoNT/A in the PREEMPT and COMPEL studies, the REPOSE study showed the sustained effectiveness of BoNT/A in the preventive treatment of CM over a period of up to 2 years [38].
Exclusion criteria were receipt of any botulinum toxin type A serotype in the previous 26 weeks and concurrent enrolment in a CM post-authorisation safety study (hereafter referred to as ‘CM-PASS’; see Sect. 5). Enrolled patients received at least one BoNT/A treatment cycle. Most had previously received oral preventative therapies [e.g. β-blockers (72%), antidepressants (70%), antiepileptics (70%) and CCBs (30%)]; these could be continued throughout the study period. The majority (90%) of patients were BoNT/A-naïve, i.e. they had not previously received BoNT/A for CM [38].
BoNT/A significantly and progressively decreased the number of headache days/month at all assessment time points throughout the study (i.e. administration visits 2 through 8) (Table 4), when administered largely as recommended in the summary of product characteristics and following the PREEMPT paradigm. Similarly, there were significant (p < 0.001 vs baseline) and progressive improvements in migraine-specific HR-QOL (all three MSQ domain scores) and generic quality of life (as assessed using the EuroQol 5-Dimenion Questionnaire) at all assessment time points [38].
4.2 In other prospective studies
Treatment with up to five cycles of BoNT/A has resulted in responder rates of ≈50–80%, depending on the definition used, and sustained beneficial effects have been seen in responders who have continued treatment for up to 2–3 years (Table 4).
Long-term outcomes are available for responders in the largest study of the real-world effectiveness of BoNT/A therapy to date, which has evaluated 972 patients (who have received a total of 5745 treatment cycles) over a period of 8 years at a single centre in the UK (Hull Migraine Clinic) [39, 44]. At year 5, 44 of 186 patients who were responders after the second cycle were still receiving—and benefitting from—treatment. Moreover, 105 had stopped treatment after achieving < 10 headache days/month for 3 consecutive months (modified positive stopping rule) and continued to fulfil the criteria for EM. The remaining 37 had stopped treatment for other reasons (e.g. resistance or pregnancy) or were lost to follow-up [44].
Treatment with eight cycles of BoNT/A at the higher dose of 195 U (i.e. using the full follow-the-pain approach of the PREEMPT paradigm; n = 132 [45]) resulted in greater (p < 0.05) reductions in headache days, migraine days and HIT-6 score at all assessment time points over the 2-year study period relative to BoNT/A at the lower dose of 155 U (i.e. using only the fixed-site, fixed-dose approach; n = 143 [46]), based on an indirect comparison of the results of two (separate) studies conducted at the same single centre in Italy [45]. Analgesic intake was also significantly (p < 0.001) reduced with the higher versus the lower dose at all assessments time points from month 6 onwards [45].
Consistent with experience in Europe, an interim analysis of a Canadian, multicentre, prospective, observational study (PREDICT) [47] showed that four cycles of BoNT/A (≈ 170 U/cycle) significantly (p < 0.0001 vs baseline) improved migraine-specific HR-QOL (all three MSQ domain scores) in BoNT/A-naïve CM patients (n = 196 enrolled).
4.2.1 Versus topiramate
The comparative effectiveness of BoNT/A and topiramate for the preventative treatment of CM in routine clinical practice (in the USA) has been evaluated in a randomized, open-label, multicentre, post-authorization study (FORWARD) [48]. Adults with CM were randomized to receive either three cycles of BoNT/A 155 U (i.e. using only the fixed-site, fixed-dose approach of the PREEMPT paradigm) or immediate-release topiramate 50–100 mg/day for 36 weeks; those who discontinued topiramate could cross-over to BoNT/A and remain in the study until week 48.
The proportion of patients achieving a ≥ 50% improvement in the monthly frequency of headache days at week 32 (primary outcome measure) was significantly higher among those initially randomized to BoNT/A than those initially randomized to topiramate [40% vs 12%; adjusted odds ratio, 4.9 (95% CI 2.7–9.1); p < 0.001]. This primary analysis of the comparative effectiveness of the two treatments used a baseline observation carried forward approach to impute missing values for any reason [e.g. discontinuation due to lack of efficacy or adverse events (AEs)]; in this regard, the proportion of patients who completed the randomized treatment period was much higher among those initially assigned to BoNT/A (86% of 140 patients) than those initially assigned to topiramate (20% of 142 patients). In terms of the comparative efficaciousness of the two treatments, responder rates did not differ significantly between patients remaining on BoNT/A and those remaining on topiramate in a sensitivity analysis that used pro-rated observed data (57% vs 68%).
BoNT/A was superior (p ≤ 0.024) to topiramate on all secondary and other outcomes [48, 49], including the ≥ 50% headache-day frequency responder rate at week 12 (46% vs 29%; post hoc analysis of observed data) [48] and assessments of headache impact (HIT-6 score at week 30) [49] and depression (PHQ-9 score at week 36) [49].
Among the 80 patients initially assigned to topiramate who crossed-over to BoNT/A, the ≥ 50% headache-day frequency responder rates at weeks 32 and 48 (exploratory outcomes) were 39% and 28%, respectively [48].
5 What is the tolerability of onabotulinumtoxinA in chronic migraine?
Injection of up to five cycles of BoNT/A (155–195 U/cycle) at 12-week intervals is generally well tolerated, according to pooled analyses of the 56-week PREEMPT clinical programme [3, 14, 15, 25]. BoNT/A recipients mostly reported AEs that were mild or moderate in severity and resolved without sequelae; they infrequently discontinued therapy due to AEs (Table 5).
The overall incidence of treatment-related AEs (TRAEs) in BoNT/A recipients was higher than that for placebo recipients (Table 5). However, the incidence rates for individual TRAEs, which included neck pain, muscular weakness (e.g. facial paresis), eyelid ptosis, musculoskeletal pain, injection-site pain, headache and musculoskeletal stiffness, were consistent with the known pharmacology and established tolerability profile of BoNT/A when injected into head and neck muscles; no new safety events were observed, either in the 24-week double-blind phase or the 32-week open-label phase [3, 14, 15]. The overall rate of TRAEs in BoNT/A recipients progressively decreased with repeated treatments, being 48%, 37%, 38%, 26% and 19% after the first, second, third, fourth and fifth cycles of BoNT/A, respectively [25]. Neck pain (4.3%), muscular weakness (1.6%), injection site pain (2.1%), and eyelid ptosis (1.9%) were the most frequently reported TRAEs in patients who received all five cycles of onabotulinumtoxinA in the PREEMPT clinical programme [25]. Only one BoNT/A recipient in the pooled PREEMPT studies experienced a serious TRAE (migraine requiring hospitalization); no deaths were reported [14].
Tolerability findings over a period of 1 year in the PREEMPT clinical programme are supported and extended by those of the 2-year (108-week) COMPEL study [16, 50], as well as those of several real-world studies from Europe, including the 64-week CM-PASS study [51] and the 24-month REPOSE study [38] (see Sect. 4). The overall rates of treatment-emergent AEs (TEAEs) and TRAEs, as well as the incidences of individual TRAEs, reported in COMPEL, CM-PASS and REPOSE are generally comparable to those reported in the open-label phase of the pooled PREEMPT studies (Table 5); no new safety concerns or cumulative tolerability issues have been identified [16, 38, 50, 51]. Only one BoNT/A recipient in the COMPEL study experienced a serious TRAE (generalized rash); no deaths were reported [16].
CM-PASS is the largest observational study to date to examine the safety of BoNT/A for the preventative treatment of CM in routine clinical practice [51]. The majority (86%) of the 1160 patients (84% women; median age 46.6 years; 24.7% medication overusers) enrolled at 58 centres across Germany, Spain, Sweden and the UK, had a diagnosis of CM or transformed (i.e. chronified) migraine at baseline, although nearly half (48%) were BoNT/A-naïve. Similar to the pooled PREEMPT and COMPEL studies, only one BoNT/A recipient in CM-PASS reported a serious TRAE (worsening of migraine); neither of the two observed fatal adverse events were considered related to treatment [51]. Special interest TRAEs included worsening of migraine (reported in 4.0% of patients), intractable migraine (0.4%), hypersensitivity (0.9%) and dysphagia (0.3%); the incidence rates of intractable migraine and dysphagia (secondary and primary outcome measures, respectively) were 1.6 and 0.4 per 1000 person-months [52].
Against a background of inadequate data on the use of BoNT/A in pregnancy (see Table 1), outcomes in 45 pregnant CM patients exposed to BoNT/A have been reported recently [53]. Among the 32 patients that consented to continue treatment during their pregnancy, there was one miscarriage and 32 full-term deliveries of healthy newborns with normal birthweight and no congenital malformations [53].
Like other BoNT/A products, BoNT/A exhibits a low immunogenic potential [3].
5.1 Versus topiramate
Intramuscular administration of BoNT/A as per the PREEMPT paradigm had a more favourable tolerability profile than oral administration of topiramate in terms of the rates of AEs and discontinuations due to AEs in the open-label FORWARD study [48]. Specifically:
-
TEAEs were reported in 48% of patients initially randomized to, or who crossed-over to, BoNT/A (n = 220) compared with 79% of patients initially randomized to topiramate (n = 142); TRAEs were reported in 17% and 70% of patients, respectively
-
Five (4%) of the 140 patients initially randomized to BoNT/A compared with 72 (51%) of the patients initially randomized to topiramate discontinued treatment due to adverse events
-
The most common TRAEs with BoNT/A were neck pain (4%), musculoskeletal pain (2%), migraine (1%) and blurred vision (1%); the most common TRAEs with topiramate were paresthesia (29%), cognitive disorder (12%), fatigue (12%), nausea (12%), decreased appetite (11%), dizziness (11%) and attention disturbance (8%) [48].
6 What is the current clinical position of onabotulinumtoxinA in chronic migraine?
BoNT/A is one of the most widely utilized preventive medications for CM [54, 55]; it continues to be a central component of clinical practice in the era of CGRP antagonists [54]. BoNT/A is an effective and generally well tolerated treatment, as demonstrated in clinical trials (PREEMPT 1 and 2; COMPEL) and confirmed in real-world studies (e.g. REPOSE and CM-PASS). In terms of reducing the number of days with headache, the favourable effect of BoNT/A appears early, although it also appears to accumulate with successive treatment cycles, at least initially, suggesting that maximum benefit may require multiple administrations [17]. At least half of the patients treated with BoNT/A throughout the PREEMPT or COMPEL studies of 1 and 2 years’ duration, respectively, achieved sustained treatment-controlled CM (i.e. they no longer met the criteria for CM) while continuing therapy.
Beyond headache-day reduction, BoNT/A therapy is associated with clinically meaningful improvements in assessments of headache severity, headache-related impact (including migraine-related disability) and migraine-specific HR-QOL, including in monthly headache-day frequency non-responders. Accordingly, headache-day reduction as a sole outcome measure may not be appropriate to assess responsiveness to BoNT/A. Treatment with BoNT/A also reportedly reduces HRU (e.g. in the COMPEL [37] and REPOSE [56] studies) and improves work productivity (e.g. in the PREDICT [47] and FORWARD [49] studies). Importantly, the results of studies evaluating onabotulinumtoxinA (Botox®) are specific to this particular formulation of BoNT/A and cannot be extrapolated to other commercially available formulations of BoNT/A.
European Headache Federation (EHF) guidelines concerning the use of BoNT/A in clinical practice are mainly based on expert opinon (Table 6). Consistent with data from the PREEMPT clinical programme, the EHF recommends attempting at least two to three cycles of BoNT/A before categorizing patients as responders or non-responders. However, like the National Institute for Health and Care Excellence (NICE) in the UK, it recommends that a monthly headache-day frequency responder be defined using less stringent criteria than used in the PREEMPT trials, i.e. a ≥ 30% (rather than ≥ 50%) reduction in headache days [55]. Short disease duration, high serum CGRP levels and (in women) polymorphisms in genes encoding CGRP and TRPV1 are among the potential predictors of responsiveness that have been identified, based on data collected in clinical practice [3]. Of note, the EHF acknowledges that the optimal definition of a de novo non-responder to BoNT/A has still to be determined [55].
Regarding how long treatment should be continued in responders, the EHF has adopted the modified stopping rule proposed by Gooriah and Ahmed [57], which is more stringent than the positive stopping rule proposed by NICE (i.e. the patient has to have fulfilled criteria for EM for 3 consecutive months) [20]. The EHF recommends re-evaluating patients 4–5 months after stopping BoNT/A to ensure the patient continues to fulfil the criteria for EM (Table 6).
According to the EHF, patients should be given realistic expectations about their treatment [55]. This includes advising that BoNT/A therapy may improve, but does not cure, their CM, and that a positive clinical effect may wear off before their next treatment cycle [55]. ‘BoNT/A wear off’ has been defined as a good initial, but short-lasting (8–10 weeks), response to treatment [58]; it is a widely recognized, albeit underexplored, phenomenon [58, 59]. Various strategies to counteract BoNT/A wear-off have been suggested. These include increasing the dose in subsequent cycles (up to the maximum of 195 U) or offering prophylactic bridging therapies (e.g. peripheral nerve blocks) between injections [58, 59]; however, the optimal approach remains to be determined [60].
Head-to-head comparisons between BoNT/A (administered as per the PREEMPT paradigm) and other medications approved for the prevention of migraine/CM are limited to the real-world FORWARD study, in which BoNT/A therapy demonstrated greater clinical utility than topiramate, largely due to tolerability issues associated with the latter (Sects. 4.2.1 and 5.1). On the basis of this result, the logic of using topiramate ahead of BoNT/A in the treatment of CM could be queried [48], although it was also observed that the two treatments were similarly efficacious in those patients who remained on them and, moreover, that the effectiveness of BoNT/A in patients who received it after failing topiramate was comparable to that in patients who received it from the outset (Sect. 4.2.1). Pending the availability of direct comparisons, the effectiveness of BoNT/A and CGRP antagonists for the prevention of CM has been [61] or is being [62] compared indirectly in network meta-analyses. According to the completed analysis [61], the efficacy of BoNT/A is seemingly comparable to that of erenumab and fremanezumab. The results of the ongoing analysis [62] are awaited with interest.
Change history
31 March 2022
A peer-reviewed video abstract was retrospectively added to this publication
16 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40263-020-00786-6
21 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40263-021-00828-7
References
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorder, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599–609.
Frampton JE, Silberstein S. OnabotulinumtoxinA: a review in the prevention of chronic migraine. Drugs. 2018;78(5):589–600.
Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20(1):92.
Janssen Sciences Ireland UC. Topamax 100 mg film-coated tablets: summary of product characteristics 2020. https://www.hpra.ie. Accessed 2 Sep 2020.
Whitcup SM, Turkel CC, Degryse RE, et al. Development of onabotulinumtoxinA for chronic migraine. Ann NY Acad Sci. 2014;1329:67–80.
Bendtsen L, Ashina M, Reuter U, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6.
AdisInsight. Drug Profile Onabotulinum toxin A—AbbVie. https://adisinsight.springer.com. Accessed 2 Sep 2020.
Allergan Pharmaceuticals Ireland. BOTOX 50, 100, 200 Allergan units, powder for solution for injection: summary of product characteristics. 2020. https://www.hpra.ie. Accessed 20 Jul 2020.
Burstein R, Blumenfeld AM, Silberstein SD, et al. Mechanism of action of onabotulinumtoxinA in chronic migraine: a narrative review. Headache. 2020;60(7):1259–72.
Gago-Veiga AB, Santos-Lasaosa S, Cuadrado ML, et al. Evidence and experience with onabotulinumtoxinA in chronic migraine: recommendations for daily clinical practice. Neurologia (Engl Ed). 2019;34(6):408–17.
Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803.
Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14.
Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–36.
Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358–73.
Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19(1):13.
Dodick DW, Silberstein SD, Lipton RB, et al. Early onset of effect of onabotulinumtoxinA for chronic migraine treatment: analysis of PREEMPT data. Cephalalgia. 2019;39(8):945–56.
Diener HC, Dodick DW, Lipton RB, et al. Benefits beyond headache days with onabotulinumtoxinA treatment: a pooled PREEMPT analysis. Pain Ther. 2020;9(2):683–94.
Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331(1–2):48–56.
National Institute for Health and Care Excellence. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine. Technology appraisal guidance [TA260]. 2012. https://www.nice.org.uk. Accessed 25 Aug 2020.
Matharu M, Halker R, Pozo-Rosich P, et al. The impact of onabotulinumtoxinA on severe headache days: PREEMPT 56-week pooled analysis. J Headache Pain. 2017;18:78.
Silberstein S, Diener HC, Dodick DW, et al. Sustained benefits of onabotulinumtoxinA treatment in chronic migraine: results from a PREEMPT pooled analysis [abstract no. 004 plus poster]. In: American Academy of Neurology Annual Meeting, 2020.
Medicines and Healthcare Products Regulatory Agency. Botox (Botulinum toxin type A): PL 00426/0074-0105; PL 00426/0118-0025; PL 00426/0119-0007: UKPAR. https://webarchive.nationalarchives.gov.uk. Accessed 12 Oct 2020.
Silberstein S, Diener HC, Dodick DW, et al. The impact of onabotulinumtoxinA vs. placebo on efficacy outcomes in headache day responder and nonresponder patients with chronic migraine. Pain Ther. 2020;9(2):695–707.
Aurora SK, Dodick DW, Diener HC, et al. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014;129(1):61–70.
Silberstein SD, Dodick DW, Aurora SK, et al. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86(9):996–1001.
Blumenfeld A, Diener HC, Lipton RB, et al. Clinically meaningful benefits of onabotulinumtoxinA beyond headache days in chronic migraine: analysis of the COMPEL and pooled PREEMPT studies [abstract no 009 plus poster]. In: American Academy of Neurology Annual Meeting. 2020.
Blumenfeld A, Stark R, Manack Adams A, et al. Efficacy and safety of onabotulinumtoxinA in an open-label study for the prophylactic treatment of chronic migraine in adult patients: COMPEL [abstract no. P2.178]. Neurology. 2017:88(16 Suppl 1).
Blumenfeld AM, Tepper SJ, Robbins LD, et al. Effects of onabotulinumtoxinA treatment for chronic migraine on common comorbidities including depression and anxiety. J Neurol Neurosurg Psychiatry. 2019;90(3):353–60.
Tepper SJ, Wilson MC, Rothrock JF, et al. Effect of onabotulinumtoxinA on the frequency and impact of headaches in patients with chronic migraine with or without a history of acute pain medication overuse: results of the COMPEL study [abstract no. P27]. J Headache Pain. 2018;19(Suppl 1):58.
Tepper SJ, Wilson M, Orejudos A, et al. The long-term efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine in patients with medication overuse: results of the COMPEL study [abstract no. PF26]. Headache. 2018;58(Suppl 2):93–4.
Young WB, Ivan Lopez J, Rothrock JF, et al. Effects of onabotulinumtoxinA treatment in chronic migraine patients with and without daily headache at baseline: results from the COMPEL study. J Headache Pain. 2019;20(1):12.
Ivanlopez J, Blumenfeld AM, Young WB, et al. Effects of onabotulinumtoxinA treatment on headache frequency, intensity, and impact in patients with and without daily headaches at baseline: a COMPEL subanalysis [abstract no. MTIS2018-095]. Cephalalgia. 2018;38(Suppl 1):79–81.
Young WB, Ivan Lopez J, Rothrock JF, et al. Effects of onabotulinumtoxinA treatment in patients with and without allodynia: results of the COMPEL study. J Headache Pain. 2019;20(1):10.
Rothrock JF, Luo L, Eross EJ, et al. Responder rates to onabotulinumtoxinA in patients with chronic migraine: a post hoc analysis of the COMPEL study [abstract no. 006 plus poster]. In: American Academy of Neurology Annual Meeting, 2020.
Blumenfeld A. Benefits of long-term onabotulinumtoxinA treatment in chronic migraine: results from the COMPEL study [abstract no. 009 plus poster]. In: American Academy of Neurology Annual Meeting, 2020.
Rothrock JF. Healthcare resource utilization in adult patients treated with onabotulinumtoxinA for chronic migraine: results from the COMPEL study [abstract no. 003 plus poster]. In: American Academy of Neurology Annual Meeting, 2020.
Ahmed F, Gaul C, Garcia-Monco JC, et al. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. 2019;20(26):1–14.
Ahmed F, Buture A, Tanvir T, et al. Hull prospective analysis of onabotulinumtoxinA (Botox) in the treatment of chronic migraine; a real-life data in 972 patients; updated results on over 8 years of experience [abstract no. IHC-PO-423]. Cephalalgia. 2019;39(1 Suppl):276–7.
Andreou AP, Trimboli M, Al-Kaisy A, et al. Prospective real-world analysis of onabotulinumtoxinA in chronic migraine post-National Institute for Health and Care Excellence UK technology appraisal. Eur J Neurol. 2018;25(8):1069-e83.
Corbelli I, Bernetti L, Verzina A, et al. Sustained efficacy and safety of onabotulinum toxin type A in chronic migraine patients: a multicentric prospective real-life study [abstract]. Neurol Sci. 2019;40(Suppl 2):S248.
Dominguez C, Pozo-Rosich P, Torres-Ferrus M, et al. OnabotulinumtoxinA in chronic migraine: predictors of response. A prospective multicentre descriptive study. Eur J Neurol. 2018;25(2):411–6.
Torres-Ferrus M, Gallardo VJ, Alpuente A, et al. Influence of headache pain intensity and frequency on migraine-related disability in chronic migraine patients treated with onabotulinumtoxinA. J Headache Pain. 2020;21(1):88.
Ahmed F, Buture A, Tanvir T, et al. Five year outcome on 310 patients receiving onabotulinumtoxinA for chronic migraine; data from Hull Migraine Clinic [abstract no. IHC-PO-419]. Cephalalgia. 2019;39(1 Suppl):274.
Negro A, Curto M, Lionetto L, et al. A two years open-label prospective study of onabotulinumtoxinA 195 U in medication overuse headache: a real-world experience. J Headache Pain. 2015;17:1.
Negro A, Curto M, Lionetto L, et al. OnabotulinumtoxinA 155 U in medication overuse headache: a two years prospective study. Springerplus. 2015;4:826.
Boudreau G, Becker WJ, Graboski C, et al. Impact of onabotulinumtoxinA on quality of life, health resource utilization, and work productivity in people with chronic migraine: interim results from a prospective, observational study (PREDICT) [abstract no. PF27]. Headache. 2018;58(Suppl 2):94.
Rothrock JFMAA, Lipton RB, et al. FORWARD study: evaluating the comparative effectiveness of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache. 2019;59(10):1700–13.
Blumenfeld AM, Patel AT, Turner IM, et al. Patient-reported outcomes from a 1-year, real-world, head-to-head comparison of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. J Prim Care Community Health. 2020. https://doi.org/10.1177/2150132720959936.
Winner PK, Blumenfeld AM, Eross EJ, et al. Long-term safety and tolerability of onabotulinumtoxinA treatment in patients with chronic migraine: results of the COMPEL study. Drug Saf. 2019;42(8):1013–24.
Matharu M, Pascual J, Nilsson Remahl I, et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia. 2017;37(14):1384–97.
US National Institutes of Health. ClinicalTrials.gov identifier NCT01432379. 2016. https://clinicaltrials.gov. Accessed 12 Aug 2020.
Wong H-T, Khalil M, Ahmed F. OnabotulinumtoxinA for chronic migraine during pregnancy: a real world experience on 45 patients. J Headache Pain. 2020;21:129.
Wang Y-F. OnabotulinumtoxinA injection in the treatment of chronic migraine. Prog Brain Res. 2020;255:171–206.
Bendtsen L, Sacco S, Ashina M, et al. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain. 2018;19(91):1–10.
Kollewe K, Antonakakis A, Kiszka M, et al. Reduction in healthcare resource utilisation in patients treated with onabotulinumtoxinA for chronic migraine: results from the REPOSE study [abstract no. HC-DP-012]. Cephalalgia. 2019;39(1 Suppl):21–2.
Gooriah R, Ahmed F. OnabotulinumtoxinA for chronic migraine: a critical appraisal. Ther Clin Risk Manag. 2015;11:1003–13.
Quintas S, Garcia-Azorin D, Heredia P, et al. Wearing off response to onabotulinumtoxinA in chronic migraine: analysis in a series of 193 patients. Pain Med. 2019;20(9):1815–21.
Khan FA, Mohammed AE, Poongkunran M, et al. Wearing off effect of onabotulinumtoxinA near the end of treatment cycle for chronic migraine: a 4-year clinical experience. Headache. 2020;60(2):430–40.
Ruscheweyh R, Athwal B, Gryglas-Dworak A, et al. Wear-off of onabotulinumtoxinA effect over the treatment interval in chronic migraine: a retrospective chart review with analysis of headache diaries. Headache. 2020;60(8):1673–82.
Institute for Clinical and Economic Review. Calcitonin gene-related peptide (CGRP) inhibitors as preventive treatments for patients with episodic or chronic migraine: effectiveness and value: final evidence report. https://icer-review.org. Accessed 8 Sep 2020.
She T, Chen Y, Tang T, et al. Calcitonin gene-related peptide antagonists versus botulinum toxin A for the preventive treatment of chronic migraine protocol of a systematic review and network meta-analysis: a protocol for systematic review. Medicine. 2020;99:5.
Acknowledgements
The manuscript was reviewed by: A. Blumenfeld, Headache Center of Southern California, The Neurology Center, Carlsbad, CA, USA; P. Martelletti, Department of Clinical and Molecular Medicine, Sapienza University, Rome, Italy; J. Rothrock, Department of Neurology, George Washington University School of Medicine, Washington, DC, USA; R. J. Stark, Monash University and Alfred Hospital, Melbourne, VIC, Australia; Y-F. Wang, Department of Neurology, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan. During the peer review process, the manufacturer of onabotulinumtoxinA was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
James Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
Enhanced material for this Adis Drug Q&A, including a video abstract, can be found at https://doi.org/10.6084/m9.figshare.13218869.
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Frampton, J.E. OnabotulinumtoxinA in Chronic Migraine: A Profile of Its Use. CNS Drugs 34, 1287–1298 (2020). https://doi.org/10.1007/s40263-020-00776-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00776-8