Abstract
Background
Stimulant medications for the treatment of attention-deficit/hyperactivity disorder have a history of safe and effective use; however, concerns exist that they may adversely affect growth trajectories in children and adolescents.
Objective
The objective of this study was to evaluate the longer-term effects of lisdexamfetamine dimesylate on weight, height, body mass index and pubertal development in children and adolescents with attention-deficit/hyperactivity disorder.
Methods
Children and adolescents aged 6–17 years with attention-deficit/hyperactivity disorder took open-label lisdexamfetamine dimesylate (30, 50 or 70 mg/day) in this open-label 2-year safety and efficacy study. Safety evaluations included treatment-emergent adverse events, measurement of weight, height and body mass index, and self-reported pubertal status using Tanner staging.
Results
The safety analysis population comprised all enrolled participants (N = 314) and 191 (60.8%) completed the study. Weight decrease was reported as a treatment-emergent adverse event in 63 participants (20.1%) and two participants (0.6%) discontinued the study as a result of treatment-emergent adverse events of weight decrease. Growth retardation of moderate intensity was reported as a treatment-emergent adverse event for two participants. From baseline to the last on-treatment assessment, there were increases in mean weight of 2.1 kg (standard deviation 5.83) and height of 6.1 cm (standard deviation 4.90), and a body mass index decrease of 0.5 kg/m2 (standard deviation 1.72). Mean weight, height and body mass index z-scores decreased over the first 36 weeks of the study and then stabilised. Changes from baseline to the last on-treatment assessment in mean z-scores for weight, height and body mass index were significantly less than zero (− 0.51, − 0.24 and − 0.59, respectively; nominal p < 0.0001). The proportion of participants with a z-score of < − 1 ranged from 5.1% (baseline) to 22.1% (week 84) for weight, 8.2% (baseline) to 12.6% (week 96) for height, and 8.3% (baseline) to 28.8% (week 96) for body mass index. Thirteen participants (4.1%) shifted to a weight below the fifth percentile at the last on-treatment assessment from a higher weight category at baseline. At the last on-treatment assessment, most participants remained at their baseline Tanner stage or had shifted higher.
Conclusions
Findings from this comprehensive examination of growth outcomes associated with lisdexamfetamine dimesylate treatment over 2 years were consistent with previous studies of stimulant medications. Whilst mean weight and height increased over the course of the study, there was a small but transient reduction in mean weight, height and body mass index z-scores. A small increase in the proportion of participants in the lowest weight and body mass index categories highlights the importance of the regular monitoring of weight and height. There was no evidence of delayed onset of puberty.
ClinicalTrials.gov identifier
NCT01328756.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This 2-year clinical study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder provides the most in-depth analysis to date of the longer-term effect of lisdexamfetamine dimesylate on growth in this population |
Over the 2-year study, there was an increase in mean weight and height, and a modest reduction in the mean body mass index. Mean z-scores for weight, height and body mass index decreased over the first 36 weeks of the study and then stabilised at this reduced level |
No clinical concerns of lisdexamfetamine dimesylate treatment on pubertal status were observed |
1 Introduction
The psychostimulants amphetamine and methylphenidate (MPH) effectively reduce the core symptoms of attention-deficit/hyperactivity disorder (ADHD) in children and adolescents [1,2,3,4]. Lisdexamfetamine dimesylate (LDX) is a long-acting amphetamine prodrug approved in USA for the treatment of children, adolescents and adults with ADHD [5]. Lisdexamfetamine dimesylate is approved in some European countries for use in children aged 6 years and older when their response to previous MPH therapy is considered to be clinically inadequate, as well as in adults with ADHD of at least moderate severity [6, 7]. The efficacy and safety of LDX has been evaluated in a series of short-term randomised controlled trials [8,9,10,11,12] and longer term studies of up to 15 months duration [13,14,15,16]. Two of the most frequently reported treatment-emergent adverse events (TEAEs) in the longer term studies are decreased appetite (reported by 21–33% of study participants) and weight loss (16–18%). Accordingly, and in line with other stimulant medications, LDX prescribing information recommends that weight, height and body mass index (BMI) are closely monitored [5, 17].
SPD489-404 (ClinicalTrials.gov identifier: NCT01328756) was a phase IV, single-arm open-label study and was the first to evaluate the safety and efficacy of LDX in children and adolescents with ADHD over a period of 2 years. Improvements in symptoms were maintained throughout the 2 years of the study, with a mean (95% confidence intervals) change in the ADHD Rating Scale IV (ADHD-RS-IV) total score from baseline to the last on-treatment assessment (LOTA) of − 25.8 (− 27.0, − 24.5) [18]. Overall, 89.8% of participants reported at least one TEAE, with more than half (54%) reporting a decreased appetite and 20% experiencing a decrease in weight [18]. Effects on growth and sexual development were predefined safety outcomes in SPD489-404. We now report in detail, changes in height, weight and BMI, at both the group and individual level, as well as describing pubertal development (based on Tanner staging) over the 2-year duration of SPD489-404.
2 Methods
The study was conducted in accordance with current applicable regulations, International Conference on Harmonization Good Clinical Practice Guideline E6 (1996), European Union Clinical Trials Directive 2001/20/EC (2001) and its updates, and local ethical and legal requirements. The study protocol was approved by an independent ethics committee/institutional review board and regulatory agency in each centre (as appropriate). Each patient’s parent/legal guardian provided written informed consent, and assent was obtained from each participant (as applicable) before taking part in the study. The study was conducted between 7 July, 2011 and 30 September, 2014 at 35 sites in ten European countries (Belgium, Germany, Hungary, Italy, the Netherlands, Poland, Romania, Spain, Sweden, and the UK).
2.1 Study Design and Participants
Participants were enrolled either directly into this study or had taken part in a previous LDX trial (SPD489-317 [ClinicalTrials.gov identifier: NCT01106430], SPD489-325 [NCT00763971] or SPD489-326 [NCT00784654]). Individuals eligible to take part were children aged 6–12 years and adolescents aged 13–17 years at the time of consent for directly enrolled participants or at the time of their consent into one of the previous studies. Participants were excluded if they had been terminated from a previous LDX study for protocol non-adherence or non-compliance or had experienced an adverse event leading to discontinuation, a medication-related serious adverse event or a clinically significant adverse event in a previous LDX study. Patients whose current ADHD medication provided effective control of symptoms with acceptable tolerability were also excluded. Additional inclusion and exclusion criteria are described in Coghill et al. [18]. All participants were required to meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition—Text RevisionTM criteria for a primary diagnosis of ADHD and to have an ADHD-RS-IV total score of at least 28 at the baseline visit (week 0). With the exception of oppositional defiant disorder, participants with a comorbid psychiatric diagnosis were excluded, as were those who were significantly underweight (defined as a BMI below the third percentile). The plan was to enrol approximately 300 participants. The sample size was not based on statistical considerations as this was an open-label and uncontrolled study.
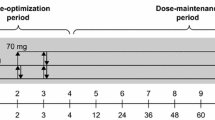
Participants received a once-daily dose of LDX (30, 50 or 70 mg) for up to 104 weeks, comprising a 4-week dose-optimisation period followed by a 100-week dose-maintenance period. During dose optimisation, weekly dose adjustments of 20 mg/day (from a starting dose of 30 mg/day at week 0) were allowed, until an acceptable response (defined as a 30% reduction in the ADHD-RS-IV total score together with a Clinical Global Impression-Improvement score of 1 or 2 with tolerable side effects) was obtained with an LDX dose between 30 and 70 mg/day. If necessary, dose adjustments were also permitted during the maintenance period. Participants were instructed to take their LDX dose each morning upon awakening (at approximately 07:00 h).
2.2 Assessments
After the baseline assessment (week 0), visits were scheduled every week during the dose-optimisation period (visits 1–4; weeks 1–4). During the maintenance period, visits were scheduled 12 weeks (± 7 days) after baseline (visit 0, week 0) and subsequently every 12 weeks until week 96 (visit 12). The final visit (visit 13) was scheduled at week 104, with a safety follow-up visit at 28–30 days (Fig. 1).
The primary objective of SPD489-404 was to evaluate the long-term safety of LDX. Safety evaluations included TEAEs (assessed at all visits), weight (all visits), height (baseline and visits 5–13; weeks 0, 12, 24, 36, 48, 60, 72, 84, 96 and 104) and pubertal status (baseline and visits 5, 7, 9, 11 and 13; weeks 0, 12, 36, 60, 84 and 104). These safety outcomes were also evaluated at an early termination visit in participants who discontinued the study prematurely. A TEAE of decreased weight was based on weight loss as perceived by the participant or their carer rather than an objective measurement of actual weight by the investigators.
Pubertal status was self-reported using an instrument based on the Tanner scales, which was validated in male and female children and adolescents aged 12–16 years [19]. Participants were shown two series of drawings that depicted the five stages of development of external sexual characteristics, genitalia and pubic hair in male individuals [20] and breasts and pubic hair in female individuals [21]. Participants were required to select the drawing that most closely represented their stage of development, with stage 1 indicating preadolescence and stage 5 indicating sexual maturity.
2.3 Analyses
All safety outcomes were assessed in the safety analysis population, defined as all participants who took at least one dose of LDX during the study. For visits where height was not measured, BMI was calculated using the last available height measurement (last observation carried forward). Changes from baseline in weight, height and BMI were summarised as mean, standard deviation (SD) and range. A potentially clinically important (PCI) change in weight was predefined, based on clinical experience and practice [22], as an increase or decrease of ≥ 7% from baseline measured at any visit. Weight, height and BMI percentiles and z-scores were derived using the Centers for Disease Control and Prevention (CDC) growth charts and were summarised using predefined categories (< 5th, ≥ 5th to < 95th, and ≥ 95th percentiles) and z-scores (< − 2, ≥ − 2 to < − 1, ≥ − 1 to < 1, ≥ 1 to ≤ 2, and > 2) [23]. Weight, height and BMI z-scores were also represented graphically by time using mean, median, interquartile range and 1.5 times the interquartile range.
3 Results
3.1 Participant Disposition and Baseline Characteristics
All 314 enrolled participants [children, n = 202 (64.3%); adolescents, n = 112 (35.7%)] received at least one dose of LDX and were included in the safety analysis population [18]. Of these, 124 individuals (39.5%) had participated in an antecedent LDX study and 190 (60.5%) were enrolled directly. The full 2-year study was completed by 191 participants (60.8%). Reasons for early discontinuation by more than 10% of participants were withdrawal by the individual (n = 41, 13.1%) and adverse events (n = 39, 12.4%) [18].
Baseline demographics and disease characteristics are summarised by Coghill et al. [18] and provided in Table 1. At baseline, six participants (1.9%) were classified as being underweight (BMI < 5th percentile). The mean daily dose of LDX across the study was 51.08 mg (SD 14.352) and the mean duration of exposure to LDX was 555.3 days (SD 253.50, maximum possible exposure 730 days). Further details of LDX dosing and exposure are available in the supplementary information of Coghill et al. [18]. Overall, 303/314 participants (96.5%) were adherent to treatment (based on tablets dispensed minus returned; adherence designated as 80–120%). One participant had adherence > 120%.
3.2 Growth-Related Treatment-Emergent Adverse Events
As reported previously based on participant and/or carer report, overall, TEAEs were reported for 282 participants (89.8%) [18]. Weight decrease was reported as a TEAE for 63 participants [20.1%; children, n = 33 (16.3%); adolescents, n = 30 (26.8%)], and two participants (0.6%; both children) discontinued the study as a result of weight decrease TEAEs. Growth retardation of moderate intensity was reported as a TEAE for two participants (0.6%; one child and one adolescent).
3.3 Changes in Weight, Height and Body Mass Index
From baseline to LOTA, there was a mean weight increase of 2.1 kg (SD 5.83; range, − 20 to + 34 kg), a mean height increase of 6.1 cm (SD 4.90; range, − 1 to 20 cm) and a mean BMI decrease of 0.5 kg/m2 (SD 1.72; range, − 7 to + 10 kg/m2). The mean weight decreased by up to 1.9 kg from baseline during the first 24 weeks and then increased throughout the remainder of the study (Fig. 2a). Mean height increased throughout the study (Fig. 2b). There was a small decrease from baseline in BMI, which reached a nadir (− 1.2 kg/m2) at weeks 24 and 36 before beginning to recover towards the baseline value (Fig. 2c). Changes in weight, height and BMI z-scores and percentiles are presented below.
Changes from baseline (BL) in a weight, b height and c body mass index (BMI), and z-score box plots for d weight, e height and f BMI at each study visit and last on-treatment assessment (LOTA) [safety analysis population; N = 314]. In parts a–c, the filled diamond represents the mean, the box represents the standard deviation and the whiskers indicate the range (minimum, maximum). In parts d–f, the filled diamond represents the mean, the box represents the interquartile range, the whiskers indicate 1.5 times the interquartile range and the filled circle represents outliers (values outside 1.5 times the interquartile range). The z-scores were derived using Centers for Disease Control and Prevention growth charts [23]. For calculation of the BMI, for visits where height was not measured or if height was missing, the last observation carried forward value for height was used
A PCI decrease in weight (≥ 7% loss from baseline) was reported for 112/313 participants (35.8%) at any study visit. The largest number of PCI decreases in weight from baseline was reported at week 24 [72/272 (26.5%)]. At LOTA, a PCI decrease in weight from baseline was reported for 33/313 participants (10.5%). A PCI increase in weight from baseline at any study visit was reported in 129/313 participants (41.2%). The largest proportion of PCI increases in weight from baseline was observed at week 104 [99/189 (52.4%)]. At LOTA, a PCI increase in weight from baseline was reported for 119/313 participants (38.0%).
3.4 Changes in Weight, Height and Body Mass Index Z-Scores
During the first 36 weeks of the study, mean z-scores for weight, height and BMI declined by 0.52, 0.15 and 0.63, respectively, and then remained generally stable to LOTA (Figs. 2d–f). Changes from baseline to LOTA in mean z-scores for weight, height and BMI were significantly less than zero (− 0.51, − 0.24 and − 0.59, respectively; nominal p < 0.0001; Table S1 of the Electronic Supplementary Material [ESM]). Figure 3 shows the proportions of participants in each z-score category (< – 2, ≥ – 2 to < – 1, ≥ – 1 to < 1, ≥ 1 to ≤ 2, and > 2) for weight, height and BMI at each study visit. Throughout the study and at LOTA, the majority of participants were within 1 SD of the CDC mean (i.e. were in the ≥ – 1 to < 1 category) for weight (range, 62.7% at baseline and visit 11–72.2% at visit 7), height (range, 53.8% at baseline to 63.6% at visit 12) and BMI (range, 58.7% at visit 1–64.3% at visit 5). Proportions of participants with a z-score of < – 1 ranged from 5.1% at baseline to 22.1% at visit 11 for weight, 8.2% at baseline to 12.6% at visit 12 for height and 8.3% at baseline to 28.8% at visit 12 for BMI (Fig. 3). Proportions of participants with weight, height or BMI z-scores 2 SD or more below the CDC population norm ranged from 0.6% at baseline to 3.9% at visit 11 for weight, 0.5% at visit 13 to 1.5% at visit 11 for height and 1.3% at baseline to 6.4% at visit 11 for BMI.
Similarly, at LOTA, most participants remained within their baseline percentile category (< 5th, ≥ 5th to < 95th, ≥ 95th) for weight [276/314 (87.9%)], height [263/314 (83.8%)] and BMI [275/314 (87.6%)] (Table 2). At LOTA, the increase in the number (%) of participants in the < 5th percentile category compared with baseline was 13 (4.1%) for weight, 3 (1.0%) for height and 26 (8.3%) for BMI. Of the 13 participants in the < 5th percentile category at LOTA for weight, four had weight decrease reported as a TEAE, eight completed the study, two withdrew and three discontinued the study owing to an adverse event (weight gain poor, n = 1; orthostatic hypotension, n = 1; and irritability, n = 1).
3.5 Tanner Staging
At baseline, approximately 30% of all participants were at Tanner stage 1, based on self-reported pubic hair and genitalia development (male individuals) or pubic hair and breast development (female individuals) (Table 3). Almost all participants who were at Tanner stage 1 at baseline were aged 6–12 years (female individuals, 100%; male individuals, > 97%), with approximately half of all children aged 6–12 years being at Tanner stage 1 at baseline. In contrast, most participants who were at Tanner stage 4 or 5 at baseline were adolescents aged 13–17 years. The majority of adolescents (male individuals, > 66%; female individuals, > 78%) were at Tanner stages 4 or 5 at baseline.
Mean age at each Tanner stage at LOTA for male and female individuals is summarised in Table S2 of the ESM. Sexual maturation, as indicated by shifts to a higher Tanner stage from baseline to LOTA, was reported by nearly half of the participants of all ages (pubic hair development: 49.0% male individuals, 44.4% female individuals; genital/breast development: 46.7% male individuals, 39.7% female individuals) (Table 3). In participants aged 6–12 years, proportions of participants shifting to a higher stage at LOTA than baseline were 50.3% for male individuals and 57.1% for female individuals based on pubic hair development, and 48.4% for male individuals and 54.3% for female individuals based on genital/breast development. In participants aged 13–17 years, the proportions of participants reporting shifts from a lower to a higher Tanner stage were 46.4% for male individuals and 28.6% for female individuals based on pubic hair development, and 43.4% for male individuals and 21.4% for female individuals based on genital/breast development.
Approximately half of all participants remained at the same Tanner stage from baseline to LOTA (pubic hair development: 46.5% male individuals, 47.6% female individuals; genital/breast development: 48.0% male individuals, 58.7% female individuals). In participants aged 6–12 years, the proportions of participants remaining at the same Tanner stage from baseline to LOTA were 46.6% for male individuals and 37.1% for female individuals based on pubic hair development, and 47.2% for male individuals and 45.7% for female individuals based on genital/breast development. In the 13–17 year age group, 46.4% of male individuals and 60.7% of female individuals remained at the same Tanner stage at LOTA as at baseline based on pubic hair development and 49.4% of male individuals and 75.0% of female individuals based on genital/breast development.
Shifts to a lower Tanner stage were reported by less than 8% of all participants (pubic hair development: 4.5% male individuals, 7.9% female individuals; genital/breast development: 5.3% male individuals, 1.6% female individuals). In participants aged 6–12 years, 3.1% of male individuals and 5.7% of female individuals shifted from a higher to a lower Tanner stage based on pubic hair development, and 4.3% of male individuals and 0.0% of female individuals shifted to a lower Tanner stage based on genital/breast development. In the 13–17 year age group, 7.1% of male individuals and 10.7% of female individuals shifted from a higher to a lower Tanner stage based on pubic hair development, and 7.2% of male individuals and 3.6% of female individuals based on genital/breast development.
4 Discussion
Regulators have recommended that, to address a relative lack of data about certain potential aspects of the longer term clinical safety of ADHD medications, clinical trials should assess, among other factors, growth, alterations in weight and sexual maturation [24]. Here, we report the weight, height and BMI data from the first 2-year clinical study of LDX. These data provide the most in-depth analysis to date of the longer term effects of LDX on weight, height and BMI in children and adolescents with ADHD. In addition, this was the first study to examine the impact of LDX treatment on pubertal development. Over the 2-year study, there was an increase in mean weight and height, and a modest reduction in mean BMI. Z-scores decreased over the first half of the study and then stabilised for weight, height and BMI. Similar proportions of participants were within 1 SD of the CDC population norms for weight, height and BMI at both baseline and LOTA. However, the proportion of participants who were more than 1 SD below the CDC population norms increased from baseline to LOTA for weight, height and BMI. A small proportion of participants (≤ 7% at any study visit) were recorded with values more than 2 SD below the CDC population norm for weight, height and BMI. In addition, more than 80% of participants remained within their baseline percentile category for weight, height and BMI at LOTA, and some experienced PCI changes in weight of ≥ 7% from baseline. While increases in weight would be expected over the course of the 2-year study, a decrease in weight of ≥ 7% from baseline occurred in approximately one-tenth of participants at LOTA. Overall, at LOTA, most participants had progressed higher or remained at their baseline Tanner stage, suggesting that longer term LDX treatment was not associated with any clinically concerning trends in pubertal development.
The findings on growth are consistent with those of a previous study that demonstrated that children with ADHD receiving LDX for up to 15 months had statistically significant mean reductions from baseline in weight, height and BMI z-scores compared with CDC population norms, with the greatest reductions in weight gain seen within the first 6 months [14]. In the previous study, the greatest impacts on growth were observed in the heaviest and tallest children, those who had not previously received stimulant treatment and those with a greater cumulative exposure to LDX [14]. The findings of the current study are also in line with longer term investigations of other psychostimulants on growth outcomes in children and adolescents with ADHD, which have consistently reported deficits in weight, height and BMI compared with age-adjusted population norms during the initial stages of stimulant treatment. The treatment of children with ADHD with transdermal MPH for up to 36 months was associated with small but statistically significant delays in weight, height and BMI attainment that occurred mainly in the first year and attenuated over time [25]. In this study, growth deficits were more frequent among the heaviest and tallest children, and in those who had not previously received stimulant therapy. Slight decreases in mean weight were also observed in the first 4 months of a 21-month study of osmotic-release oral system MPH in children with ADHD [26, 27]. These weight changes then stabilised and were considered to be clinically insignificant. The decrease in growth z-scores was smaller in individuals who had received previous stimulant therapy than in those not previously treated [26]. Treatment with extended-release mixed amphetamine salts was associated with reductions in expected height and weight gains in a 30-month study in children with ADHD [27]. Again, nearly all of the growth deficits occurred in the first year of treatment. [27].
While the evidence is compelling that the early stages of stimulant treatment are associated with a modest reduction in weight gain, less is known about the long-term effects of stimulant treatment on growth [28]. In a naturalistic 10-year prospective study in children with ADHD, stimulant therapy (using an amphetamine product, MPH product or pemoline) had no effect on growth [29]. More recently, a 16-year follow-up study of 515 individuals with ADHD found extended use of stimulant medication to be associated with a suppression of adult height (in most cases, the stimulant medication was immediate-release MPH) [30]. A meta-analysis of longitudinal studies in children with ADHD receiving stimulants for at least 1 year found significant delays in weight and height attainment during the early stages of treatment, but these effects attenuated over time [28]. This meta-analysis also found that in most head-to-head studies of MPH vs. amphetamine, no differences were observed between these two stimulants in their effects on growth suppression, perhaps because both stimulants increase brain extracellular dopamine concentration, and increased dopamine levels may be associated with inhibition of the growth hormone through dopamine D2 receptors in the pituitary [31].
Attention-deficit hyperactivity disorder itself may be associated with dysregulated growth and may, therefore, confound the interpretation of growth data from uncontrolled studies of ADHD medications [32, 33]. In a cross-sectional analysis of 62,887 children and adolescents, those with ADHD who were not currently receiving medication had 1.5 times the odds of being overweight compared with those without ADHD, with the authors suggesting that impulsivity and poor regulation of behaviour may increase the risk of developing poor eating patterns [34]. In the same study, children and adolescents who were currently receiving medication for ADHD were 1.6 times more likely to be underweight than those without ADHD. However, a 10-year longitudinal case–control study in 522 children found no association between ADHD and dysregulated growth [29], and in the Multimodal Treatment Study of Children with ADHD, newly medicated individuals had a similar mean weight z-score at baseline to non-medicated children, although the mean height z-score was lower for the newly medicated group at baseline and at each subsequent assessment [35]. Notably, in the present study, as well as in certain other studies in children and adolescents with ADHD [14, 25], mean z-scores at baseline were above the CDC population mean. It may be that individuals with ADHD are heavier and taller than the age-adjusted population norms, or it may be that the CDC norms developed in the year 2000 are no longer fully representative of the normal population.
This was the first study to explore whether LDX therapy impairs pubertal development in children and adolescents with ADHD; however, interpretation of our findings is inhibited because of a lack of good quality normative data about pubertal development and associated population norms for Tanner staging for comparison. In a study of American adolescents without ADHD, Tanner stage 2 was achieved in the age range of 11.9–12.3 years for male individuals and 11.2–11.9 years for female individuals, stage 3 at 13.2–13.9 years for boys and 12.4–12.7 years for female individuals, stage 4 at 14.3–14.7 years for boys and 13.1–13.4 years for female individuals and stage 5 at 15.1–15.3 years for male individuals and 14.5–14.6 years for female individuals [36]. These data are broadly consistent with the findings from SPD489-404, in which all participants who were at Tanner stage 1 at LOTA were aged 12 years or younger and most participants aged 13 years or older were at Tanner stages 2–5, suggesting that pubertal development is broadly similar in children and adolescents with and without ADHD.
In SPD489-404, the observation that the majority of participants remained at their baseline Tanner stage or progressed higher does not suggest that LDX treatment has a clinically concerning impact on pubertal development. The possibility, however, that staying at the same Tanner stage may represent a delay in pubertal development in some individuals cannot be discounted. These findings are broadly consistent with data from the 3-year follow-up of the Multimodal Treatment Study of Children with ADHD, which found no statistically significant differences in sexual development between children with or without ADHD, and that stimulant medication had no apparent effect on sexual development [37]. However, a study of boys with ADHD aged 12–15 years who had received stimulant treatment for more than 3 years demonstrated that those in the 14–15 years age group were significantly behind untreated controls in height and pubertal development [38].
Pubertal regression has been reported in certain hormonal conditions such as hypopituitarism but is generally uncommon [39]. In a study designed to validate the Pubertal Development Scale in 253 healthy adolescent male and female individuals, the authors concluded that inaccuracies in self-assessment were responsible for the 9.8% of male individuals and 6.4% of female individuals who reported a decrease in sexual development [40]. The tendency for individuals to overestimate their development during the early stages of puberty and underestimate it during the later stages [41] supports the notion that the small number of shifts from a higher to a lower Tanner stage in SPD489-404 may be the result of inaccurate self-reporting. It should be noted that Tanner staging has only been validated in 12- to 16-year-old individuals, suggesting that assessment of sexual development in individuals below this age range may not be accurately assessed by the instrument. However, the occasional regression of pubertal development in SPD489-404 cannot be entirely excluded.
The strengths of this study include the large number of participants enrolled at multiple sites, its 2-year duration, the range of approaches used to measure growth and the assessment of pubertal development. Important limitations are the open-label design, lack of a control arm and potential inconsistencies in the self-assessment of pubertal development. In addition, recruitment from antecedent studies is likely to have enriched the study population with individuals with good tolerability to LDX and for whom the changes in growth described here may have already taken place. Furthermore, the study design does not permit further exploratory analyses between growth and other parameters such as age and puberty. A substantial proportion of the participants had received prior stimulant medications, which may have masked the full impact of the initiation of LDX treatment on growth in stimulant-naïve participants; however, the number of participants who were treatment naïve was too small to permit a robust subgroup analysis. Analysis of male and female subgroups was also not possible because the study population included relatively few female individuals.
5 Conclusions
Over the course of this 2-year study of LDX in children and adolescents with ADHD, mean weight and height increased. Although deficits compared with CDC population norms were observed in weight, height and BMI, these occurred early in the study and then stabilised. Most participants remained at their baseline Tanner stage or shifted to a higher stage. There was no evidence of a delayed onset of puberty, although a possible delay in the onset of puberty in some individuals cannot be discounted. These findings enhance our understanding of the effects of LDX on growth and pubertal development, and support recommendations for the regular monitoring of growth and development of children and adolescents receiving stimulants in clinical practice [17, 42].
References
A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder: the MTA Cooperative Group. Multimodal treatment study of children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073–86.
Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European clinical guidelines for hyperkinetic disorder: first upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I7–30.
Huang YS, Tsai MH. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs. 2011;25(7):539–54.
Chan E, Fogler JM, Hammerness PG. Treatment of attention-deficit/hyperactivity disorder in adolescents: a systematic review. JAMA. 2016;315(18):1997–2008.
Shire US Inc. Vyvanse® (lisdexamfetamine dimesylate): US prescribing information. Revised Jan 2017.
Shire Pharmaceuticals Ltd. Elvanse® Adult (lisdexamfetamine dimesylate): UK summary of product characteristics. Revised Oct 2016.
Shire Pharmaceuticals Ltd. Elvanse® (lisdexamfetamine dimesylate): UK summary of product characteristics. Revised Oct 2016.
Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29(3):450–63.
Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(4):395–405.
Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23(10):1208–18.
Wigal T, Greenhill L, Chuang S, McGough J, Vitiello B, Skrobala A, et al. Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1294–303.
Findling RL, Wigal SB, Bukstein OG, Boellner SW, Abikoff HB, Turnbow JM, et al. Long-term tolerability of the methylphenidate transdermal system in pediatric attention-deficit/hyperactivity disorder: a multicenter, prospective, 12-month, open-label, uncontrolled, phase III extension of four clinical trials. Clin Ther. 2009;31(8):1844–55.
Findling RL, Childress AC, Krishnan S, McGough JJ. Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectr. 2008;13(7):614–20.
Faraone SV, Spencer TJ, Kollins SH, Glatt SJ. Effects of lisdexamfetamine dimesylate treatment for ADHD on growth. J Am Acad Child Adolesc Psychiatry. 2010;49(1):24–32.
Findling RL, Cutler AJ, Saylor K, Gasior M, Hamdani M, Ferreira-Cornwell MC, et al. A long-term open-label safety and effectiveness trial of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(1):11–21.
Coghill DR, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson CS, et al. Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: randomized-withdrawal study design. J Am Acad Child Adolesc Psychiatry. 2014;53(6):647.e1–657.e1.
Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54(3):227–46.
Coghill DR, Banaschewski T, Soutullo CA, Hernandez-Otero I, Nagy P, Zuddas A, et al. Long-term safety and efficacy of lisdexamfetamine dimesylate in children and adolescents with ADHD: a phase IV, 2-year, open-label study in Europe. CNS Drugs. 2017;31:625–38.
Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–80.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23.
Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303.
Goldenberg K. Weight change. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworth Publishers, a division of Reed Publishing; 1990.
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190.
European Medicines Agency (EMA): EMEA/CHMP/EWP/431734/2008 Guideline on the clinical investigation of medicinal products for the treatment of attention deficit hyperactivity disorder (ADHD) London: EMA; 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/08/WC500095686.pdf. Accessed 04 Aug 2017.
Faraone SV, Giefer EE. Long-term effects of methylphenidate transdermal delivery system treatment of ADHD on growth. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1138–47.
Spencer TJ, Faraone SV, Biederman J, Lerner M, Cooper KM, Zimmerman B. Does prolonged therapy with a long-acting stimulant suppress growth in children with ADHD? J Am Acad Child Adolesc Psychiatry. 2006;45(5):527–37.
Faraone SV, Biederman J, Monuteaux M, Spencer T. Long-term effects of extended-release mixed amphetamine salts treatment of attention-deficit/hyperactivity disorder on growth. J Child Adolesc Psychopharmacol. 2005;15(2):191–202.
Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994–1009.
Biederman J, Spencer TJ, Monuteaux MC, Faraone SV. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157(4):635–40 (640.e1).
Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, et al. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry. 2017;58(6):663–78.
De Zegher F, Van Den Berghe G, Devlieger H, Eggermont E, Veldhuis JD. Dopamine inhibits growth hormone and prolactin secretion in the human newborn. Pediatr Res. 1993;34(5):642–5.
Spencer TJ, Biederman J, Harding M, O’Donnell D, Faraone SV, Wilens TE. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996;35(11):1460–9.
Faraone SV, Lecendreux M, Konofal E. Growth dysregulation and ADHD: an epidemiologic study of children in France. J Atten Disord. 2012;16(7):572–8.
Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122(1):e1–6.
Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1015–27.
Lee PA. Normal ages of pubertal events among American males and females. J Adolesc Health Care. 1980;1(1):26–9.
Greenfield B, Hechtman L, Stehli A, Wigal T. Sexual maturation among youth with ADHD and the impact of stimulant medication. Eur Child Adolesc Psychiatry. 2014;23(9):835–9.
Poulton AS, Melzer E, Tait PR, Garnett SP, Cowell CT, Baur LA, et al. Growth and pubertal development of adolescent boys on stimulant medication for attention deficit hyperactivity disorder. Med J Aust. 2013;198(1):29–32.
Tun P, Phillips RE, Warrell DA, Moore RA, Tin NuS, Myint L, et al. Acute and chronic pituitary failure resembling Sheehan’s syndrome following bites by Russell’s viper in Burma. Lancet. 1987;2(8562):763–7.
Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–33.
Schlossberger NM, Turner RA, Irwin CE Jr. Validity of self-report of pubertal maturation in early adolescents. J Adolesc Health. 1992;13(2):109–13.
National Institute for Health and Clinical Excellence (NICE). The NICE guideline on diagnosis and management of ADHD in children, young people and adults. London: NICE; 2013. http://www.nice.org.uk/guidance/cg72. Accessed 04 Aug 2017.
Acknowledgements
The authors thank the participants and investigators involved in this study. Under the direction of the authors and funded by Shire International GmbH, Dr.Jim Purvis of Oxford PharmaGenesis, Oxford, UK provided writing assistance for this publication. Editorial assistance in formatting, proofreading, copy editing and fact checking was also provided by Oxford PharmaGenesis. Tamara Werner-Kiechle from Shire International GmbH reviewed and edited the manuscript for scientific accuracy. Although employees of the sponsor were involved in the design, collection, analysis, interpretation and fact checking of information, the content of this manuscript, the interpretation of the data and the decision to submit the manuscript for publication in CNS Drugs were made by the authors independently. Dr. Yan served as the statistical expert for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Shire Development LLC. Writing and editing assistance for this paper was provided by Oxford PharmaGenesis, funded by Shire International GmbH. Shire International GmbH also funded open access.
Conflict of interest
Brian Yan is an employee of Shire and owns stock or stock options. The following authors have received compensation for serving as consultants or speakers for, or they or the institutions they work for have received research support or royalties from, the companies or organisations indicated: Tobias Banaschewski (Actelion, CIP Medien, Hexal Pharma, Hogrefe, Kohlhammer, Lilly, Medice, Novartis, Otsuka, Oxford University Press, Shire, Vifor Pharma); David R. Coghill (Eli Lilly, Janssen-Cilag, Medice, Novartis, Oxford University Press, Shire, Vifor Pharma); Mats Johnson (Eli Lilly, Evolan, Ginsana, PCM Scientific, New Nordic, Shire, Vifor Pharma); Peter Nagy (Lilly, Otsuka, Medice, Shire); Isabel Hernández Otero (Alicia Koplowitz Foundation, Eli Lilly, Forest, Janssen-Cilag, Junta de Andalucía, Roche, Shire, Shire Pharmaceuticals Iberica S.L., Sunovion); César A. Soutullo (Alicia Koplowitz Foundation, Editorial Médica Panamericana, Eli Lilly, Fundación Caja Navarra, Lundbeck, Mayo Eds, Medice/Juste, NeuroTech Solutions, Rubiò, Shire); and Alessandro Zuddas (Angelini, EduPharma, Lilly, Lundbeck, Otsuka, Oxford University Press, Roche, Shire, Takeda, Vifor Pharma).
Ethics approval
This study was approved by the appropriate ethics committees and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments, as well as other applicable local ethical and legal requirements.
Consent to participate
Written informed consent was obtained from all individual participants included in the study. Informed consent and assent, where applicable, was documented (on an appropriate form approved by the ethics committee) by the dated signature of the participant and the participant’s legally authorised representative/parents, as applicable.
Additional information
This article discusses data derived from a study described in an article available at https://doi.org/10.1007/s40263-017-0443-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Banaschewski, T., Johnson, M., Nagy, P. et al. Growth and Puberty in a 2-Year Open-Label Study of Lisdexamfetamine Dimesylate in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. CNS Drugs 32, 455–467 (2018). https://doi.org/10.1007/s40263-018-0514-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0514-8