Abstract
Background
Here we review the safety and tolerability profile of lisdexamfetamine dimesylate (LDX), the first long-acting prodrug stimulant for the treatment of attention-deficit/hyperactivity disorder (ADHD).
Methods
A PubMed search was conducted for English-language articles published up to 16 September 2013 using the following search terms: (lisdexamfetamine OR lisdexamphetamine OR SPD489 OR Vyvanse OR Venvanse OR NRP104 NOT review [publication type]).
Results
In short-term, parallel-group, placebo-controlled, phase III trials, treatment-emergent adverse events (TEAEs) in children, adolescents, and adults receiving LDX were typical for those reported for stimulants in general. Decreased appetite was reported by 25–39 % of patients and insomnia by 11–19 %. The most frequently reported TEAEs in long-term studies were similar to those reported in the short-term trials. Most TEAEs were mild or moderate in severity. Literature relating to four specific safety concerns associated with stimulant medications was evaluated in detail in patients receiving LDX. Gains in weight, height, and body mass index were smaller in children and adolescents receiving LDX than in placebo controls or untreated norms. Insomnia was a frequently reported TEAE in patients with ADHD of all ages receiving LDX, although the available data indicated no overall worsening of sleep quality in adults. Post-marketing survey data suggest that the rate of non-medical use of LDX was lower than that for short-acting stimulants and lower than or equivalent to long-acting stimulant formulations. Small mean increases were seen in blood pressure and pulse rate in patients receiving LDX.
Conclusions
The safety and tolerability profile of LDX in individuals with ADHD is similar to that of other stimulants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In short-term clinical trials of the prodrug stimulant lisdexamfetamine dimesylate (LDX), treatment-emergent adverse events (TEAEs) in children, adolescents, and adults were typical of those reported for stimulant medications, with decreased appetite and insomnia the most frequently reported TEAEs. TEAEs in long-term studies were similar to those reported in the short-term trials. Most TEAEs were mild or moderate in severity. |
Data related to four specific safety concerns associated with stimulant medications were reviewed in patients receiving LDX. Gains in weight, height, and body mass index were smaller in children and adolescents receiving LDX than in placebo controls or untreated norms. Insomnia was a frequently reported TEAE in children and adolescents with ADHD receiving LDX, but the drug was not associated with an overall worsening of sleep quality in adults. Post-marketing survey data suggested that the rate of non-medical use of LDX was lower than that for short-acting stimulants and lower than or equivalent to long-acting stimulant formulations. Small mean increases were seen in blood pressure and pulse rate in patients receiving LDX. |
1 Introduction
Stimulants are recommended by European and North American guidelines as a first-line medication option for children and adolescents (aged 6–17 years) with attention-deficit/hyperactivity disorder (ADHD) [1–3], and are also recommended in some guidelines for the treatment of adults with the disorder [2, 4]. A range of amphetamine (AMP)- and methylphenidate (MPH)-based stimulants, as well as the non-stimulants atomoxetine (ATX), guanfacine, and clonidine, are available for the treatment of ADHD in North America and several European countries [5]. Numerous studies have shown stimulants to be effective in reducing the core symptoms and behavioral impairments associated with ADHD [1, 6]. In a meta-analysis of 32 double-blind, placebo-controlled trials of ADHD medications in patients aged 6–18 years, effect sizes were shown to be significantly greater for stimulants than for non-stimulants [7]. A second meta-analysis of 23 double-blind, placebo-controlled trials of stimulant medications for ADHD in children and adolescents found that effect sizes compared with placebo were modestly but statistically significantly greater for AMP-based stimulants than for MPH [8].
Various long-acting AMP- and MPH-based stimulants have been developed, with the aim of relieving ADHD symptoms throughout the day using a once-daily dose [9]. Lisdexamfetamine dimesylate (LDX) is the first long-acting prodrug stimulant for the treatment of ADHD [10]. After ingestion and absorption, LDX is enzymatically hydrolyzed to release the therapeutically active moiety d-AMP, and the essential amino acid lysine [11]. As hydrolysis of LDX occurs mainly in the blood, the generation of d-AMP is unlikely to be affected by either gastrointestinal pH or transit time [12–14]. Pharmacokinetic studies in humans have shown that exposure to d-AMP following oral administration of LDX is monophasic, sustained, and dose-proportional, with low intra- and inter-patient variability [12, 15, 16]. This profile of systematic exposure to d-AMP facilitates dose optimization by reducing the likelihood of sub- or supra-therapeutic levels [17]. The pharmacodynamic properties of LDX are reflected in clinical analog classroom studies and simulated adult workplace studies that have shown that, following a single dose of LDX, therapeutic effects are observed through to the last assessment of the day; 13 h post dose in children and 14 h post dose in adults [18, 19]. In a series of randomized, controlled trials, effect sizes for LDX have been shown to be greater than those for MPH-based stimulants in the treatment of children and adolescents with ADHD [8], and a post hoc analysis of data from a randomized, placebo- and active-controlled phase III clinical trial showed that improvements in the symptoms of ADHD were statistically significantly greater in patients receiving LDX than in those receiving the reference therapy osmotic-release oral system MPH (OROS-MPH) [20].
The safety warnings for LDX are similar to those for other stimulant treatments for ADHD [21]. In this review, we examine the safety and tolerability profile of LDX. We begin by analyzing the treatment-emergent adverse events (TEAEs) and vital signs data recorded in clinical trials of LDX, of both short- and longer-term duration. We then focus down on evidence relating to four specific safety concerns associated with stimulant ADHD pharmacotherapies, namely reduced weight and growth, sleep disruption, abuse liability, and cardiovascular events [3, 5, 22].
2 Methods
A PubMed search was conducted using the following search terms: (lisdexamfetamine OR lisdexamphetamine OR SPD489 OR Vyvanse OR Venvanse OR NRP104 NOT review [publication type]). The final iteration of the search was conducted on 16 September 2013. The search was not limited by publication date but was limited to English language articles. The above search terms were subsequently used in conjunction with the following additional terms (applied individually): AND ADHD, AND abuse liability, AND cardiovascular safety, AND sleep, AND weight, AND growth. Of 129 references identified, 35 contained LDX safety and tolerability data in patients with ADHD (Fig. 1).
3 Results
3.1 Safety and Tolerability in Short-Term Trials
3.1.1 Randomized, Parallel-Group, Double-Blind Trials in Patients with Attention-Deficit/Hyperactivity Disorder (ADHD)
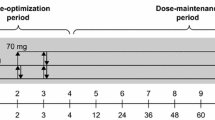
The efficacy and safety of LDX in the treatment of ADHD were evaluated in six randomized, parallel-group, double-blind, phase III trials (Table 1) [23–28]. Three trials (studies 301, 303, and 305), were forced-dose titration studies in which patients were randomized to receive once-daily LDX 30, 50, or 70 mg, or placebo for 4 weeks [23, 24, 26]. In these trials, dose increases followed a predefined schedule: patients randomized to LDX 30 mg received this dose throughout the study; patients randomized to LDX 50 mg received 30 mg/day during week 1 and 50 mg/day during weeks 2–4; patients randomized to LDX 70 mg received 30 mg/day during week 1, 50 mg/day during week 2, and 70 mg/day during weeks 3–4. The remaining three trials utilized dose-optimization protocols: study 403 was placebo controlled, study 325 was placebo and active (OROS-MPH) controlled, and study 317 was a head-to-head comparison of LDX and ATX. In these studies, patients randomized to LDX were individually optimized to LDX 30, 50, or 70 mg/day during weeks 1–4 based on efficacy and tolerability [25, 27, 28]. Patients randomized to the reference treatment OROS-MPH in study 325 were individually optimized to 18, 36, or 54 mg/day (OROS-MPH was administered according to European regulations with a maximum licensed dose of 54 mg/day) [25]. Patients randomized to the control treatment ATX in study 317 were optimized to 0.5–1.2 mg/kg (with a maximum daily dose of 1.4 mg/kg) if under 70 kg in weight, or 40, 80, or 100 mg/day in patients weighing 70 kg or over [27].
The overall rates of TEAEs for LDX-treated patients were generally similar across age groups and were typical of those previously reported for stimulants in general [3, 5, 22]. The overall frequency of TEAEs for LDX-treated patients did not differ greatly between studies with durations of 4 weeks or 7–10 weeks (Table 1). This may have been because most TEAEs are reported to occur within 4 weeks of treatment initiation [18, 19, 23, 24, 29, 30]. It is also possible that the dose-optimized design of studies 317, 325, and 403 may have reduced the rate of TEAEs compared with the forced-dose titration design of the three shorter trials.
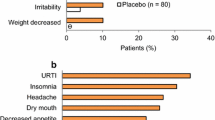
The most common TEAEs reported in patients receiving LDX in these short-term trials are shown in Table 1. In all studies, decreased appetite was the most common TEAE and was reported by ≥25 % (range 25.2–39.0) of patients treated with LDX, irrespective of age. Weight loss was reported in 9.2–21.9 % of children and adolescents receiving LDX, but was not consistently reported as a common TEAE in adult studies. Anorexia was reported in 10.8 % of children and adolescents receiving LDX in study 325 but by 5.1 % or less in the adult studies. Insomnia was common in all age groups, occurring in 11–19 % of LDX-treated patients. Dry mouth was a prominent TEAE in adults treated with LDX (25.7–31.6 %) but was reported in <7 % of children and adolescents. Nausea was reported in 2.5–12.5 % of patients receiving LDX. Although headache and nasopharyngitis were commonly reported TEAEs, their frequency did not differ greatly between the LDX and placebo groups in any trial. With regard to active treatment controls, headache, decreased appetite, and nasopharyngitis were reported by more than 10 % of patients receiving OROS-MPH in study 325, and decreased appetite, fatigue, headache, nausea, and somnolence were reported by more than 10 % of patients receiving ATX in study 317.
Across all studies, the percentage of patients who discontinued treatment owing to a TEAE ranged from 4.3 to 9.2 % in the LDX treatment groups, compared with 1.3–3.6 % in the placebo groups, 7.5 % in the ATX group of study 317, and 1.8 % in the OROS-MPH group of study 325 (Table 1). In the placebo-controlled studies, TEAEs leading to discontinuation in at least 1 % of patients receiving LDX were as follows: ventricular hypertrophy as determined by electrocardiography (ECG), tic, vomiting, psychomotor hyperactivity, insomnia, and rash in study 301 in children; irritability, decreased appetite and insomnia in study 305 in adolescents; insomnia, tachycardia, irritability, hypertension, headache, anxiety, and dyspnea in study 303 in adults; and rectal fissure, fatigue, irritability, influenza, and decreased libido/erectile dysfunction also in adults [21, 23, 28].
In the ATX-controlled study in children and adolescents (study 317), the TEAEs leading to discontinuation were agitation, decreased weight, excoriation, indifference, irritability, somnolence, nausea, and tic in the LDX group, and headache, irritability, epigastric discomfort, fatigue, influenza, malaise, nausea, sedation, somnolence, and upper abdominal pain in the ATX group [27]. In the placebo- and OROS-MPH-controlled study 325, the TEAEs leading to discontinuation were vomiting, anorexia, decreased appetite, angina pectoris, tachycardia, decreased weight, and insomnia in the LDX group, and decreased appetite, irritability, and insomnia in patients treated with OROS-MPH [25]. The case of angina pectoris was a 13-year-old boy who experienced pre-cardiac pain that was considered by the study investigator to be of moderate intensity and did not meet the criteria for a serious TEAE. During the study, this patient had no clinically significant laboratory abnormalities, no treatment or concomitant medications were reported, and all ECGs were normal [25].
No deaths were reported in any of the studies. Serious TEAEs (defined as those that resulted in death, were life threatening, required hospitalization or prolongation of hospitalization, resulted in persistent or significant disability or incapacity, caused congenital abnormality or birth defect, or were considered an important medical event) were reported in two studies. In study 303, there were two serious TEAEs in adults receiving LDX (leg injuries following an automobile accident and post-operative knee pain) but neither were judged to be related to study treatment [23]. Serious TEAEs reported in children and adolescents in study 325 were syncope, gastroesophageal reflux disease, and appendicitis in the LDX group; loss of consciousness, hematoma, and clavicle fracture in the placebo group; and syncope and overdose in the OROS-MPH group [25]. Of these, only the case of overdose in a patient receiving OROS-MPH was considered to be related to study drug. This patient inadvertently took two doses of OROS-MPH on the same day and experienced a non-serious episode of initial insomnia; the overdose was reported to be mild in severity, was resolved, and did not result in a change of dosage or treatment (data on file). It was a requirement of the study 325 protocol that all reported instances of syncope were classified as serious TEAEs, regardless of the intensity or medical significance of the event.
As is typical for stimulant medications, LDX treatment was associated with small mean increases in blood pressure (BP) and pulse rate compared with placebo in all age groups, with the largest mean increases seen with LDX 70 mg (Table 2) [17, 23, 25–28]. LDX treatment was generally not associated with any clinically relevant changes in mean ECG parameters, including corrected QT interval, although clinically meaningful post-baseline ECG findings were observed at week 1 in two adolescent patients receiving LDX in one of the forced-dose studies (QT interval corrected by Fridericia’s formula [QTcF] of 479 and 413 ms, respectively), which led to study drug discontinuation; no other clinically concerning trends in ECG interval assessments were observed [26]. While mean changes in vital signs and ECG parameters were generally not considered to be clinically meaningful, as shown in Table 3, small numbers of patients in studies 317 (children and adolescents), 305 (adolescents), and 303 (adults) were reported to meet outlier criteria for various cardiovascular parameters at least once during the study, supporting the need for careful monitoring of patients during treatment [23, 26, 27, 31]. However, few patients met outlier criteria at more than two study time points (study 303) or at 2 consecutive weeks (study 305) (Table 3), suggesting that the cardiovascular effects of treatment were not sustained [26, 31].
3.1.2 Crossover and Open-Label Trials
In addition to the double-blind, parallel-group trials described above, LDX has also been studied in four short-term, placebo-controlled, crossover studies (two in children, one in college students, and one in adults) and two short-term open-label studies (both in children) [18, 19, 32–35]. In all six trials, patients met Diagnostic and Statistical Manual of Mental Disorders, fourth revision, text revision (DSM-IV-TR) diagnostic criteria for ADHD.
In study 201, children with ADHD (N = 52) received mixed AMP salts extended-release (MAS XR) for a 3-week dose-optimization period, followed by a 3-week, double-blind, crossover period, during which each individual received 1 week of treatment with placebo, 1 week with MAS XR (at the individually optimized dose), and 1 week with LDX (at a dose approximately equivalent to that of MAS XR by AMP base content); the order of treatments was randomized [32]. During the double-blind treatment period, the overall level of TEAEs was low and similar among patients receiving LDX (16 %), MAS XR (18 %), and placebo (15 %) [32]. The most frequent TEAEs (>2 % with any treatment) during the double-blind treatment period for patients receiving LDX, MAS XR, and placebo were insomnia (8, 2, 2 %, respectively), decreased appetite (6, 4, 0 %), anorexia (4, 0, 0 %), upper respiratory tract infection (2, 2, 0 %), upper abdominal pain (0, 4, 2 %), and vomiting (0, 2, 4 %). The second crossover trial in children (N = 117) was a 4-week open-label period, during which the dose of LDX was individually optimized, followed by a randomized, placebo-controlled, 2-way crossover phase (1 week each of LDX or placebo) [18]. The most frequent TEAEs (≥10 %) reported for LDX-treated patients (N = 129) during the 4-week dose-optimization period were decreased appetite (47 %), insomnia (27 %), headache (17 %), irritability (16 %), upper abdominal pain (16 %), and affect lability (10 %). In two short-term, open-label trials in children (7 weeks and 4–5 weeks in duration), the profile of TEAEs was similar to those seen in other studies of LDX and alternative stimulants [34, 35]. Again, the most frequent TEAEs were related to decreased appetite and trouble sleeping.
A 5-week, placebo-controlled, crossover study of LDX in 24 university students aged 18–23 years found the most frequent TEAEs were decreased appetite and trouble sleeping [33]. A second crossover trial in adults (aged 18–55 years; N = 142) consisted of a 4-week, open-label period, during which the dose of LDX was individually optimized to 30, 50, or 70 mg daily, followed by a randomized, placebo-controlled, 2-way crossover phase (1 week each of LDX or placebo) [19]. The most common TEAEs (≥10 %) during dose optimization were decreased appetite (52 %), dry mouth (43 %), headache (28 %), insomnia (26 %), upper respiratory tract infection (14 %), irritability (12 %), and nausea (11 %). During the crossover phase, no newly emergent TEAEs were reported in 5 % or more of adults receiving LDX, and the percentage of patients with any TEAE was lower for LDX-treated individuals (32 %) than those receiving placebo (42 %).
3.2 Safety and Tolerability in Long-Term Studies
The safety and tolerability of LDX over the long term (defined for the purposes of this paper as at least 6 months) has been evaluated in extension studies to four of the randomized, parallel-group, double-blind, placebo-controlled phase III trials described above [29, 30, 36, 37]. In each long-term extension study, patients received open-label, individually dose-optimized LDX (30, 50, or 70 mg taken once daily). The open-label treatment period lasted between 26 and 52 weeks in the study in children and adolescents (study 326) and 52 weeks in the other three long-term studies in children, adolescents, and adults (studies 302, 306, and 304, respectively).
The most common TEAEs reported in the long-term extension studies are shown in Table 4. These are largely similar to those reported in the short-term trials (Table 1), and are consistent with those reported for other stimulants. The overall rate of TEAEs did not differ greatly among age groups. As with the short-term trials, the most common TEAEs for LDX-treated patients across all age groups included decreased appetite (14–33 %), headache (17–21 %), and insomnia (12–20 %). Weight loss was more common in children and adolescents (16–18 %) than in adults (6 %). Anorexia was reported in 15 % of LDX-treated children and adolescents in study 326, but occurred in 5 % or less of patients receiving LDX in the other long-term studies.
Most TEAEs reported in the long-term studies were mild or moderate in severity [29, 30, 36, 37]. Serious TEAEs and TEAEs leading to discontinuation were reported by 1–4 % and 6–16 % of patients receiving LDX, respectively (Table 4). The serious TEAEs reported in the long-term studies in children and in adults (studies 302 and 304) were judged by the study investigator to be unrelated to LDX treatment [29, 30]. In study 326 in children and adolescents, syncope and aggression (two cases of each) were the only serious TEAEs reported in more than one patient during the open-label LDX treatment period [36]. In this study, open-label treatment was followed by randomized treatment withdrawal; no clinically relevant safety signals were associated with the abrupt discontinuation of LDX [36]. In the long-term adolescent study (study 306), of the serious TEAEs, only three episodes of syncope were considered to be related to LDX treatment [37]. In this study, any new onset of syncope was considered an important medical event requiring reporting as a serious TEAE. TEAEs that led to treatment discontinuation included insomnia, aggression, irritability, decreased appetite, and depressed mood [29, 30, 37]. The mean changes in vital signs and corrected QT interval observed during the four extension studies were modest and consistent with the profile of LDX seen in the short-term trials (Table 5).
The safety and efficacy of LDX has also been evaluated in a long-term maintenance-of-efficacy study in adults with ADHD (study 401) [38]. This study enrolled adults aged 18–55 years who had already received at least 6 months of treatment with commercially available LDX. During the initial phase of this study, patients received open-label treatment with their established commercial dose of LDX (30, 50, or 70 mg once daily) for 3 weeks. Of 122 patients who received open-label LDX, 20 % reported a TEAE; headache (2.5 %) and upper respiratory tract infection (2.5 %) were the only TEAEs with a frequency of greater than 2 %. As with study 326, no clinically relevant safety signals were associated with the randomized withdrawal of LDX treatment following open-label treatment in study 401 [36, 38].
3.3 Post-Marketing Safety Data
Published accounts of post-marketing data describing adverse events in patients receiving LDX are limited. Spiller at al. [39] described 28 patients who reported adverse events to one of five poison centers in the USA during the first 10 months of LDX marketing. In most (86 %) of these patients, the adverse reaction occurred within the first week of therapy, with agitation (43 %), tachycardia (39 %), insomnia (29 %), dystonia (29 %), vomiting (18 %), chest pain (14 %), hallucination (11 %), and jitters (11 %) occurring in more than 10 % of the patients. In addition, there are case reports of single instances of alopecia [40] and eosinophilic hepatitis [41] in patients with ADHD treated with LDX, and of chorea [42] and serotonin-like syndrome [43] following accidental ingestion of LDX.
4 Specific Safety Concerns Associated with Stimulant Use
4.1 Weight and Growth
As with other stimulants, monitoring of height and weight in pediatric patients receiving LDX is recommended [21]. Reductions in weight and in expected height gains have been reported in multiple clinical trials assessing the use of stimulants for ADHD treatment; however, the relatively short duration of most studies has limited the available data on the long-term impact of stimulants on growth. A 3-year follow-up of the National Institute of Mental Health Multimodal Treatment Study of ADHD found that stimulant-treated children were shorter by an average of 2.0 cm and lighter by 2.7 kg after 3 years compared with un-medicated children [44]. However, the reductions in growth velocity were greatest in the first year of treatment, then decreased in the second year, and were absent in the third year when compared with un-medicated children.
To evaluate the effects of LDX treatment on growth in children, data were analysed from two North American, 4- to 6-week, short-term studies (studies 301 and 201) [24, 32] and a 52-week, long-term study in children (study 302, which enrolled patients from studies 301 and 201) [29]. In this analysis, the weight, height, and body mass index (BMI) of 281 children (aged 6–13 years) were assessed for up to 15 months and compared with norms from the US Centers for Disease Control (CDC) [45]. It was noted that, at baseline, patients were significantly taller and heavier than expected based on CDC norms. The mean (standard deviation [SD]) duration of LDX treatment was 265 (149) days. Consistent with the known effects of stimulants from other long-term studies [46], compared with expected changes based in CDC norms, gains in weight, height, and BMI in children receiving LDX were statistically significantly reduced, with the greatest rate of weight decrease observed within the first 6 months of treatment [45]. Across all studies, mean weight decreased by 0.2 kg, compared with an expected increase of 3.5 kg. Mean height increased by 3.9 cm, compared with an expected increase of 4.8 cm. Among children with endpoint data obtained at or beyond 12 months, the proportion of children with a BMI below or at the fifth percentile increased from 4 % at baseline to 15 % at endpoint. Growth was most affected in the heaviest and tallest children, for those who had not previously received stimulant treatment and for those with a greater cumulative exposure to LDX [45].
In the 7-week, phase III study in children and adolescents with ADHD (study 325), mean [SD] body weight decreased in the patients receiving LDX (−2.1 [1.9] kg) and OROS-MPH (−1.3 [1.4] kg), compared with an increase (+0.7 [1.0] kg) in patients receiving placebo [25]. Of the 47 patients (LDX, n = 35; OROS-MPH, n = 12) who had a potentially clinically significant decrease in weight at endpoint (≥7 % from baseline), three patients (LDX, n = 2; OROS-MPH, n = 1) moved from healthy weight BMI categories to underweight (defined as BMI less than the 5th percentile) [25]. In the short-term, forced-dose study in adolescents (study 305), the mean (SD) weight changes from baseline at week 4 were −3.0 (2.92), −4.5 (3.91), and −5.2 (3.20) lb for the 30, 50, and 70 mg/day LDX groups, respectively, and +2.3 (2.94) lb for the placebo group (this converts to approximately −1.36 [1.33], −2.05 [1.78], and −2.36 [1.45] kg for the 30, 50, and 70 mg/day LDX groups and +1.05 [1.34] kg in the placebo group). In adolescents receiving LDX 30, 50, and 70 mg for 52 weeks (study 306), mean (SD) changes in weight from baseline to endpoint were −0.1 (3.91), −0.4 (4.80), and −1.9 (6.08) kg, respectively [37]. Of the 171 patients with a healthy weight BMI at baseline, five were categorized as underweight at endpoint; there were no underweight individuals at baseline.
The effects of LDX on weight in adults and changes over the longer term are less certain. In the 52-week study 304 in adults, the mean change in weight from baseline to endpoint was −1.8 kg [30]. An increase in BMI was observed in the one adult who was underweight at baseline. Of the 105 adults with a normal BMI (18–24 kg/m2) at baseline, one patient ended the study as underweight (BMI 17.5 kg/m2 at endpoint) and six ended the study as overweight (BMI 24.0–25.1 kg/m2).
4.2 Sleep
ADHD itself may be associated with sleep disturbances, including difficulties in initiating sleep, reduced total sleep time, and poor sleep quality [47, 48]. The mechanisms by which this occurs are not well understood, and the impacts of comorbidities and ADHD medication on sleep remain unclear [47, 48]. Clinical guidelines provide recommendations for the management of sleep disturbance [49].
Sleep impairments, including insomnia, have been recorded as TEAEs in multiple clinical trials assessing the use of stimulants to treat ADHD, indicating that stimulant therapy may be the cause of sleep problems in some patients [50]. However, in a randomized, double-blind, placebo-controlled study in children, neither once-daily OROS-MPH nor transdermal MPH appeared to cause sleep problems or to exacerbate existing sleep impairments [51]. In addition, results from a 6-week, open-label study in 24 children with ADHD indicated that OROS-MPH treatment did not impair sleep and may even improve some aspects of sleep [52]. Kooij et al. [53] reported improved sleep quality in a small sample of adults with ADHD (N = 8) following 3 weeks of open-label stimulant therapy. Similarly, another study, which included 34 adults with ADHD, found that open-label treatment with MPH had beneficial effects on sleep compared with no treatment [54]. Insomnia was reported as a TEAE in 11–19 % of patients of all ages receiving LDX in short-term, randomized, placebo-controlled, parallel-group trials, compared with 0–5 % of patients receiving placebo (Table 1). In longer-term extension studies, the proportions of patients (12–20 %) receiving LDX who reported insomnia were similar to those observed in the short-term trials (Table 4).
In study 303 in adults (N = 420), mean global scores for the self-rated Pittsburgh Sleep Quality Index (PSQI) indicated that sleep quality at baseline was generally poor but did not differ between the treatment groups (LDX 5.8, placebo 6.3, p = 0.19). By week 4, least squares mean change from baseline in PSQI global score (where a decrease indicates an improvement in sleep quality) suggested that LDX was not associated with an overall worsening of sleep quality compared with placebo (LDX −0.8, placebo –0.5, p = 0.33), but was associated with improvement in the daytime functioning component compared with placebo (p = 0.0001) [55]. A post hoc analysis of this study examining categorical changes in PSQI found that similar proportions of adults receiving placebo and LDX shifted from good sleep (PSQI ≤5) at baseline to poor sleep (PSQI >5) at endpoint (8.2 and 7.7 %, respectively), while 8.2 % of the placebo group and 20.9 % of the LDX group had better sleep at endpoint than at baseline (p = 0.03, LDX vs. placebo) [56]. Thus, while reports of sleep-related TEAEs are elevated in patients receiving LDX compared with placebo, these findings are not reflected in impaired sleep quality in adults with ADHD as measured by the PSQI [56].
Polysomnography and actigraphy parameters were examined in 24 children (aged 6–12 years) with ADHD before and after treatment with LDX in a randomized, placebo-controlled, double-blind, parallel-group study [57]. There was no statistically significant increase in latency to persistent sleep in patients treated with LDX compared with the placebo group. Furthermore, there were no significant differences between LDX and placebo in actigraphy and secondary polysomnography measures. However, the number of awakenings after sleep onset significantly decreased from 7.9 at baseline to 3.3 at week 7 in the LDX treatment group (p < 0.0001 compared with baseline). However, owing to the small sample size and exploratory nature of this pilot study, these results should be interpreted with caution.
Overall, the impact of stimulants on sleep in patients with ADHD is unclear. The heterogeneity of observations across studies may reflect differences in the class of drug, formulation, and dose-scheduling protocols [49]. Intuitively, a stimulant with a duration of action lasting into the evening following a single morning dose might be expected to be associated with sleep-related TEAEs yet, paradoxically, patients receiving shorter-acting formulations may experience sleep disturbances due to a rebound effect in the evening after their medication wears off [49].
4.3 Abuse Potential
Like other stimulants, LDX is a controlled substance with the potential for non-medical use (NMU) and diversion [21]. Several pharmacokinetic and physicochemical characteristics of LDX may lower the potential for abuse, misuse, or diversion compared with immediate-release stimulant formulations. First, in common with all long-acting stimulants, once-daily dosing makes parental supervision easier to enforce [5]. Second, the maximum plasma concentration of d-AMP is reached approximately 3.5 h after a single dose of LDX in children with ADHD, with an elimination half-life ranging from 8.61 to 8.90 h [16]. The ‘high’ associated with stimulants is dependent on a rapid rise in stimulant concentration and the resultant increase in monoamine receptor occupancy [58]. Accordingly, the absence of an early sharp rise and spike in systemic d-AMP concentrations following LDX administration may result in a lower abuse potential compared with immediate-release AMP formulations [59]. Third, the requirement for LDX to be converted to d-AMP via rate-limited hydrolysis in the blood means that opening LDX capsules, or dissolving the contents in water, will not yield the active ingredient d-AMP for direct administration [59]. Finally, a randomized, crossover study in healthy men suggested that switching between oral and intranasal routes of administration of LDX does not markedly modify d-AMP plasma concentration–time profiles [60].
Drug-liking scores for LDX were assessed in two phase I studies in adult volunteers with a history of stimulant abuse. These studies found that drug-liking scores for oral (100 mg) and intravenous (25 and 50 mg) LDX were not significantly different from placebo and were lower than those for equivalent doses of immediate-release d-AMP [59, 61]. The lower drug-liking of LDX compared with d-AMP at equivalent doses are presumably due to the delayed pharmacodynamic properties of the former that result from the prodrug nature. At the supra-therapeutic oral dose of 150 mg, the drug-liking score for LDX was similar to that of 40 mg d-AMP, despite a 50 % greater AMP free-base content in the former compared with the latter, and drug-disliking scores were higher [59]. While these results are suggestive of a lower potential for the abuse of LDX than d-AMP, it should be noted that the studies enrolled small numbers of individuals who received LDX for short periods of time under controlled conditions.
Large-scale, post-marketing data relevant to the abuse-liability of LDX are beginning to emerge. An internet survey of 10,000 US adults (aged 18–49 years) reported lifetime NMU of pain medications, sedatives/tranquilizers, sleep medications, and prescription stimulants to be 24.6, 15.6, 9.9, and 8.1 %, respectively. Within prescription stimulants, product-specific rates of NMU (per 100,000 prescriptions dispensed) were generally low but highest for immediate-release formulations (Ritalin®, 1.62; Adderall®, 1.61) compared with longer-acting preparations (Adderall XR® 0.62, Concerta® 0.19, LDX 0.13) [62]. The most commonly reported motivation for stimulant NMU in this study were ‘increasing alertness’ (33–61 %) and ‘enhancing academic or work performance’ (39–57 %) rather than ‘getting high’ (20–30 %) [62]. A second evaluation of the NMU of prescription ADHD stimulants among adults was based on 147,816 assessments from the National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO) system. NMU, over the previous 30 days, of prescription stimulants (1.29 %) was lower than for opioids (19.79 %) and sedatives (10.62 %). Again, NMU of stimulant products was low: Ritalin®, 0.16; Adderall® 0.62; Adderall XR®, 0.42; Concerta®, 0.08; LDX 0.12) [63]. A cross-sectional, population-based US survey, which included 443,041 respondents from the 2002–2009 National Survey on Drug Use and Health, found that lifetime NMU of prescription ADHD stimulants was reported by 3.4 % of respondents aged 12 years or older, most of whom had already been engaged in the abuse of an illicit drug or NMU of another prescription drug [64]. In addition, data from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS®) System, a US national surveillance system that monitors the abuse, misuse, and diversion of prescription controlled substances, indicated that RADARS System Poison Center call rates and RADARS System Drug Diversion rates for prescription stimulants were low and that rates for extended-release AMP formulations, including LDX, were similar to those for extended-release MPH (from third quarter of 2007 to second quarter of 2011) [65].
4.4 Cardiovascular Safety
Case reports of sudden death in stimulant-treated patients, combined with the sympathomimetic properties of this class of drug, led European and North American treatment guidelines to recommend that clinicians be aware of any cardiovascular risks that may affect a patient’s suitability for ADHD medication [1, 2, 6, 66]. Thus, prescribing information for LDX warns of the risk of serious cardiovascular reactions, including sudden death, and recommends that its use is avoided in patients with cardiac abnormalities, cardiomyopathy, serious heart arrhythmia, or coronary artery disease [21]. Furthermore, the checking of fingers and toes for circulation problems (peripheral vasculopathy, including Raynaud’s phenomenon) has recently become a requirement for patients receiving stimulants, including LDX, for the treatment of ADHD [21]. However, most large-scale epidemiological studies and randomized, controlled trials have failed to substantiate concerns of elevated cardiovascular risk of ADHD medications [67–69]. Of a series of five retrospective, administrative claims-based US studies in children and adolescents, the two smallest studies did report a slightly increased risk of emergency department visits attributed to cardiac symptoms such as tachycardia or palpitations, but the three largest studies, each comprising more than a million patients, found no association between stimulants and composite endpoints of sudden cardiac death, myocardial infarction, stroke, and ventricular arrhythmia [68]. Similarly, a retrospective study that examined the UK General Practice Research Database found no increased risk of sudden death associated with ADHD medications (stimulants or ATX) in a population of 18,637 aged 2–21 years [70]. Although background rates of serious cardiovascular events in children and adolescents are small [68], evidence of an increased risk of serious cardiac events in adult patients receiving ADHD medications is also limited. A retrospective US study of healthcare records of 443,198 adults aged 25–64 years (150,359 of whom received ADHD medications) found no evidence of sudden cardiac death, myocardial infarction, or stroke associated with the use of ADHD medication compared with no use [71]. Finally, a recent retrospective study of Medicaid and commercial US databases of 43,999 adult (≥18 years of age) new MPH users and 175,955 matched non-users found a small increased risk of sudden death or ventricular arrhythmia (but not stroke, myocardial infarction, or combined stroke/myocardial infarction) among MPH users, although the lack of a dose-response effect argued against a causal relationship [72].
Cardiovascular-related serious TEAEs and discontinuations, and ECG abnormalities, were rare in clinical trials of LDX. In short-term double-blind, randomized, controlled, phase III trials in patients with ADHD of all ages, LDX was associated with modest increases in systolic and diastolic BP and pulse rate [23–27]. Outlier data reported for study 317 in children and adolescents indicated that 15.0 % of patients receiving LDX had a pulse rate ≥100 bpm compared with 24.2 % of patients receiving ATX at some point during the study. In this study, similar proportions of children receiving LDX and ATX experienced systolic BP (SBP) ≥120 mmHg (LDX 12.8 %, ATX 11.2 %) or diastolic BP (DBP) >80 mmHg (LDX 11.7 %, ATX 13.3 %), and similar proportions of adolescents experienced SBP ≥130 mmHg (LDX 6.1 %, ATX 8.8 %) or DBP >80 mmHg (LDX 21.2 %, ATX 17.6 %). There were no cases of QTcF interval ≥450 ms [27]. In study 305 in adolescents, 3.0 % of patients receiving LDX had a heart rate ≥100 bpm at endpoint compared with none receiving placebo. No participants had a QTcF interval of ≥480 ms [26]. Post hoc analyses of cardiovascular parameters in adults (study 303) found that the proportions of patients who experienced a pulse rate of ≥100 bpm during treatment with LDX ranged from 3.3 % for the 70-mg dose to 8.5 % for the 50-mg dose; no patients in the placebo group exceeded this threshold [31]. There were no clinically meaningful ECG abnormalities [23]. Modest increases in cardiovascular vital signs were also reported during the crossover phase of a placebo-controlled classroom study in children (aged 6–12) with ADHD in both LDX and placebo groups [18]. Maximum mean (SD) increases in pulse rate, SBP, and DBP (9.9 [9.8] bpm, 4.2 [9.2] mmHg, and 4.7 (8.5) mmHg, respectively) were all observed in the LDX 70-mg group. Finally, a small (N = 28), 4- to 5-week, single-blind, modified laboratory school study in children (aged 6–12 years) with ADHD reported one case each of tachycardia, BP >95th percentile of normal range (both occurred once only), and a prolongation of QTc (461 ms, which resolved at medication discontinuation and did not reappear at the resumption of treatment) in patients receiving LDX [73].
In the short-term, randomized, double-blind trial in children (study 301), ECG voltage criteria for ventricular hypertrophy led to discontinuation of at least 1 % of patients receiving LDX [21], although subsequent analysis of these data suggested that minor variations in ECG interpretation contributed to these discontinuations (data on file). To assess the impact of LDX treatment on cardiovascular and cardiopulmonary structure and function using comprehensive provocative physiological testing, a prospective open-label study was conducted in 15 adults with ADHD [74]. Participants were treated with LDX for up to 6 months and underwent transthoracic echocardiography and cardiopulmonary exercise testing. This study found no clinically meaningful changes in cardiac structure and function, or in metabolic and ventilatory variables at maximum exertion. However, the authors acknowledged that, while their results are generally reassuring, these findings were limited by the small sample size and uncontrolled nature of the study design.
5 Caveats of Reported Lisdexamfetamine Dimesylate (LDX) Safety Outcomes
The interpretation of safety and tolerability data from the LDX clinical trial program requires that several limitations be considered. First, the relatively small number of patients enrolled in clinical trials of relatively short duration means that rare TEAEs, or TEAEs that emerge only after extended treatment, are unlikely to be detected. Second, it is important to note that individuals with comorbid psychiatric disorders, extremes of weight, or major neurological and cardiovascular conditions were excluded from the clinical trials, and all patients were in generally good health. Third, is the tendency for long-term studies in particular to self-select for responders. Thus, it is unlikely that these phase III clinical trials of LDX reflect the full spectrum of patients seen in clinical practice. Finally, it should be acknowledged that all of the clinical trials described were sponsored by the manufacturer of LDX.
Post-marketing surveillance can provide additional information regarding drug safety in clinical practice and TEAEs reported in patients treated with LDX during the post-marketing period [21]. However, these data rely on voluntary reporting of TEAEs from a population of uncertain size, making it difficult to estimate the frequency of events or to establish a causal relationship to drug exposure reliably [21]. The EU-based, Attention Deficit Drugs Use Chronic Effects (ADDUCE) Consortium has been established, at the request of the European Medicines Agency and with European Union FP7 funding, in response to the lack of knowledge regarding the long-term effects of stimulants [75]. Initially focusing on MPH treatment, the ADDUCE project plans to perform a series of pharmacovigilance investigations into the long-term effects of stimulants on growth, the neurological system, psychiatric states, and the cardiovascular system, and it is hoped that new research tools developed during this process can then be applied to other ADHD medications, including LDX. With the exception of LDX misuse mentioned earlier, published large-scale, post-marketing data on LDX are currently limited. However, a company-sponsored phase IV, open-label study (study 404) is underway and will provide information regarding the safety profile of LDX in children and adolescents with ADHD over a 2-year treatment period.
6 Conclusions
Results from clinical trials of LDX indicate that this once-daily, long-acting prodrug stimulant has a safety and tolerability profile similar to that of other stimulants. The TEAEs reported most commonly in children, adolescents, and adults include decreased appetite and insomnia. Most TEAEs are mild to moderate in severity. Due to the sympathomimetic effects of LDX, small mean increases in blood pressure and pulse rate can occur. These changes alone would not be expected to have short-term consequences, but all patients receiving LDX should be monitored for larger changes in blood pressure and pulse rate, and LDX should not be used in patients with serious cardiac problems. As a result of its prodrug formulation, there is low intra- and inter-patient variability in the systemic exposure to d-AMP, which may help facilitate LDX dose optimization. The prodrug formulation of LDX may also lead to reduced abuse potential of LDX compared with immediate-release d-AMP. Overall, the choice of medication for patients with ADHD should be based on the benefit–risk ratio for each individual.
References
Banaschewski T, Coghill D, Santosh P, et al. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15(8):476–95.
Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA). Canadian ADHD practice guidelines. 3rd ed. Toronto: CADDRA;2011:http://www.caddra.ca/cms4/pdfs/caddraGuidelines2011.pdf. Accessed 18 June 2013.
Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–22.
Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. 2010;10:67.
Graham J, Banaschewski T, Buitelaar J, et al. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17–37.
National Institute for Health and Clinical Excellence. Diagnosis and management of ADHD in children, young people and adults. London: National Clinical Practice Guideline Number 72; 2009. http://www.nice.org.uk/nicemedia/live/12061/42060/42060.pdf. Accessed 18 June 2013.
Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. P T. 2009;34(12):678–94.
Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19(4):353–64.
Hodgkins P, Shaw M, McCarthy S, Sallee FR. The pharmacology and clinical outcomes of amphetamines to treat ADHD: does composition matter? CNS Drugs. 2012;26(3):245–68.
Goodman DW. Lisdexamfetamine dimesylate (vyvanse), a prodrug stimulant for attention-deficit/hyperactivity disorder. P T. 2010;35(5):273–87.
Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–27.
Ermer JC, Adeyi BA, Pucci ML. Pharmacokinetic variability of long-acting stimulants in the treatment of children and adults with attention-deficit hyperactivity disorder. CNS Drugs. 2010;24(12):1009–25.
Haffey MB, Buckwalter M, Zhang P, et al. Effects of omeprazole on the pharmacokinetic profiles of lisdexamfetamine dimesylate and extended-release mixed amphetamine salts in adults. Postgrad Med. 2009;121(5):11–9.
Krishnan S, Zhang Y. Relative bioavailability of lisdexamfetamine 70-mg capsules in fasted and fed healthy adult volunteers and in solution: a single-dose, crossover pharmacokinetic study. J Clin Pharmacol. 2008;48(3):293–302.
Ermer J, Homolka R, Martin P, Buckwalter M, Purkayastha J, Roesch B. Lisdexamfetamine dimesylate: linear dose-proportionality, low intersubject and intrasubject variability, and safety in an open-label single-dose pharmacokinetic study in healthy adult volunteers. J Clin Pharmacol. 2010;50(9):1001–10.
Boellner SW, Stark JG, Krishnan S, Zhang Y. Pharmacokinetics of lisdexamfetamine dimesylate and its active metabolite, d-amphetamine, with increasing oral doses of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: a single-dose, randomized, open-label, crossover study. Clin Ther. 2010;32(2):252–64.
Najib J. Lisdexamfetamine in the treatment of adolescents and children with attention-deficit/hyperactivity disorder. Adolesc Health Med Ther. 2012;3:51.
Wigal SB, Kollins SH, Childress AC, Squires L. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;3(1):17.
Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav Brain Funct. 2010;6:34.
Soutullo C, Banaschewski T, Lecendreux M, et al. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743–51.
Shire US Inc. Vyvanse® (lisdexamfetamine dimesylate) US prescribing information. Revised June 2013. http://pi.shirecontent.com/PI/PDFs/Vyvanse_USA_ENG.pdf. Accessed 4 Nov 2013.
Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213–37.
Adler LA, Goodman DW, Kollins SH, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–73.
Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29(3):450–63.
Coghill DR, Banaschewski T, Lecendreux M, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;. doi:10.1016/j.euroneuro.2012.11.012.
Findling RL, Childress AC, Cutler AJ, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(4):395–405.
Dittmann RW, Cardo E, Nagy P, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind. Phase IIIb study. CNS Drugs. 2013;27(12):1081–92.
Adler LA, Dirks B, Deas PF, et al. Lisdexamfetamine dimesylate in adults with attention-deficit/ hyperactivity disorder who report clinically significant impairment in executive function: results from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(7):694–702.
Findling RL, Childress AC, Krishnan S, McGough JJ. Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectr. 2008;13(7):614–20.
Weisler R, Young J, Mattingly G, Gao J, Squires L, Adler L. Long-term safety and effectiveness of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. CNS Spectr. 2009;14(10):573–85.
Adler LA, Weisler RH, Goodman DW, Hamdani M, Niebler GE. Short-term effects of lisdexamfetamine dimesylate on cardiovascular parameters in a 4-week clinical trial in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2009;70(12):1652–61.
Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007;62(9):970–6.
DuPaul GJ, Weyandt LL, Rossi JS, et al. Double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in college students with ADHD. J Atten Disord. 2012;16(3):202–20.
Findling RL, Ginsberg LD, Jain R, Gao J. Effectiveness, safety, and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: an open-label, dose-optimization study. J Child Adolesc Psychopharmacol. 2009;19(6):649–62.
Wigal SB, Wong AA, Jun A, Stehli A, Steinberg-Epstein R, Lerner MA. Adverse events in medication treatment-naive children with attention-deficit/hyperactivity disorder: results from a small, controlled trial of lisdexamfetamine dimesylate. J Child Adolesc Psychopharmacol. 2012;22(2):149–56.
Coghill D, Banaschewski T, Lecendreux M, et al. Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: randomized withdrawal design. Poster presented at the EUNETHYDIS 2nd international ADHD conference, Barcelona; 2012. http://www.shirecongressposters.com/247852. Accessed 18 June 2013.
Findling RL, Cutler AJ, Saylor K, et al. A long-term open-label safety and effectiveness trial of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(1):11–21.
Brams M, Weisler R, Findling RL, et al. Maintenance of efficacy of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: randomized withdrawal design. J Clin Psychiatry. 2012;73(7):977–83.
Spiller HA, Griffith JR, Anderson DL, Weber JA, Aleguas A. Poison centers detect an unexpectedly frequent number of adverse drug reactions to lisdexamfetamine. Ann Pharmacother. 2008;42(7):1142–3.
Brahm NC, Hamilton DR. Alopecia following initiation of lisdexamfetamine in a pediatric patient. Prim Care Companion J Clin Psychiatry. 2009;11(6):365.
Hood B, Nowicki MJ. Eosinophilic hepatitis in an adolescent during lisdexamfetamine dimesylate treatment for ADHD. Pediatrics. 2010;125(6):e1510–3.
Ford JB, Albertson TE, Owen KP, Sutter ME, McKinney WB. Acute, sustained chorea in children after supratherapeutic dosing of amphetamine-derived medications. Pediatr Neurol. 2012;47(3):216–8.
Akingbola OA, Singh D. Dexmedetomidine to treat lisdexamfetamine overdose and serotonin toxidrome in a 6-year-old girl. Am J Crit Care. 2012;21(6):456–9.
Swanson JM, Elliott GR, Greenhill LL, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1015–27.
Faraone SV, Spencer TJ, Kollins SH, Glatt SJ. Effects of lisdexamfetamine dimesylate treatment for ADHD on growth. J Am Acad Child Adolesc Psychiatry. 2010;49(1):24–32.
Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994–1009.
Spruyt K, Gozal D. Sleep disturbances in children with attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2011;11(4):565–77.
Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48(9):894–908.
Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54(3):227–46.
Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509–17.
Faraone SV, Glatt SJ, Bukstein OG, Lopez FA, Arnold LE, Findling RL. Effects of once-daily oral and transdermal methylphenidate on sleep behavior of children with ADHD. J Atten Disord. 2009;12(4):308–15.
Kim HW, Yoon IY, Cho SC, et al. The effect of OROS methylphenidate on the sleep of children with attention-deficit/hyperactivity disorder. Int Clin Psychopharmacol. 2010;25(2):107–15.
Kooij JJ, Middelkoop HA, van Gils K, Buitelaar JK. The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: an open-label case-control study. J Clin Psychiatry. 2001;62(12):952–6.
Sobanski E, Schredl M, Kettler N, Alm B. Sleep in adults with attention deficit hyperactivity disorder (ADHD) before and during treatment with methylphenidate: a controlled polysomnographic study. Sleep. 2008;31(3):375–81.
Adler LA, Goodman D, Weisler R, Hamdani M, Roth T. Effect of lisdexamfetamine dimesylate on sleep in adults with attention-deficit/hyperactivity disorder. Behav Brain Funct. 2009;5:34.
Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72(7):903–8.
Giblin JM, Strobel AL. Effect of lisdexamfetamine dimesylate on sleep in children with ADHD. J Atten Disord. 2011;15(6):491–8.
Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160(11):1909–18.
Jasinski DR, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol. 2009;23(4):419–27.
Ermer JC, Dennis K, Haffey MB, et al. Intranasal versus oral administration of lisdexamfetamine dimesylate: a randomized, open-label, two-period, crossover, single-dose, single-centre pharmacokinetic study in healthy adult men. Clin Drug Investig. 2011;31(6):357–70.
Jasinski DR, Krishnan S. Human pharmacology of intravenous lisdexamfetamine dimesylate: abuse liability in adult stimulant abusers. J Psychopharmacol. 2009;23(4):410–8.
Cassidy TA, Varughese S, Russo L, Budman SH, Eaton TA, Butler SFB. Nonmedical use and diversion of ADHD stimulants among U.S. adults ages 18–49: a national internet survey. J Atten Disord. 2012.
Cassidy TA, McNaughton EC, Varughese S, Russo L, Zulueta M, Butler SF. Nonmedical use of prescription ADHD stimulant medications among adults in a substance abuse treatment population: early findings from the NAVIPPRO surveillance system. J Atten Disord. 2013.
Sweeney CT, Sembower MA, Ertischek MD, Shiffman S, Schnoll SH. Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. J Addict Dis. 2013;32(1):1–10.
Sembower MA, Ertischek MD, Buchholtz C, Dasgupta N, Schnoll SH. Surveillance of diversion and nonmedical use of extended-release prescription amphetamine and oral methylphenidate in the United States. J Addict Dis. 2013;32(1):26–38.
Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921.
Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15–30.
Winterstein AG. Cardiovascular safety of stimulants in children: findings from recent population-based cohort studies. Curr Psychiatry Rep. 2013;15(8):379.
Westover AN, Halm EA. Do prescription stimulants increase the risk of adverse cardiovascular events? A systematic review. BMC Cardiovasc Disord. 2012;12(1):41.
McCarthy S, Cranswick N, Potts L, Taylor E, Wong IC. Mortality associated with attention-deficit hyperactivity disorder (ADHD) drug treatment: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Saf. 2009;32(11):1089–96.
Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673–83.
Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178–85.
Wigal SB, Jun A, Wong AA, Stehli A, Steinberg-Epstein R, Lerner MA. Does prior exposure to stimulants in children with ADHD impact cardiovascular parameters from lisdexamfetamine dimesylate? Postgrad Med. 2010;122(5):27–34.
Hammerness P, Zusman R, Systrom D, et al. A cardiopulmonary study of lisdexamfetamine in adults with attention-deficit/hyperactivity disorder. World J Biol Psychiatry. 2012;14(4):299–306.
Consortium TA. http://adhd-adduce.org/page/view/2/Home. Accessed 18 June 2013.
Acknowledgments
The authors thank Drs Elizabeth Gandhi and Eric Southam of Oxford PharmaGenesis™ who provided editorial support funded by Shire, including collating the comments of the authors and editing the manuscript for submission.
Disclosures
LDX is manufactured and marketed by Shire. B Caballero, S Sorooshian, and R Civil are employees of Shire and own stock/stock options. DR Coghill has received compensation for serving as a consultant or speaker; or has, or the institutions he works for have, received research support or royalties from the following companies or organizations: Flynn Pharma, Janssen-Cilag, Lilly, Medice, Novartis, Otsuka, Oxford University Press, Pfizer, Schering-Plough, Shire, UCB, Vifor Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Coghill, D.R., Caballero, B., Sorooshian, S. et al. A Systematic Review of the Safety of Lisdexamfetamine Dimesylate. CNS Drugs 28, 497–511 (2014). https://doi.org/10.1007/s40263-014-0166-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0166-2