Abstract
Background and Objective
Thioguanine (TG), azathioprine (AZA), and mercaptopurine (MP) are thiopurine prodrugs commonly used to treat diseases, such as leukemia and inflammatory bowel disease (IBD). 6-thioguanine nucleotides (6-TGNs) have been commonly used for monitoring treatment. High levels of 6-TGNs in red blood cells (RBCs) have been associated with leukopenia, the cutoff levels that predict this side effect remain uncertain. Thiopurines are metabolized and incorporated into leukocyte DNA. Measuring levels of DNA-incorporated thioguanine (DNA-TG) may be a more suitable method for predicting clinical response and toxicities such as leukopenia. Unfortunately, most methodologies to assay 6-TGNs are unable to identify the impact of NUDT15 variants, effecting mostly ethnic populations (e.g., Chinese, Indian, Malay, Japanese, and Hispanics). DNA-TG tackles this problem by directly measuring thioguanine in the DNA, which can be influenced by both TPMT and NUDT15 variants. While RBC 6-TGN concentrations have traditionally been used to optimize thiopurine therapy due to their ease and affordability of measurement, recent developments in liquid chromatography–tandem mass spectrometry (LC–MS/MS) techniques have made measuring DNA-TG concentrations in lymphocytes accurate, reproducible, and affordable. The objective of this systematic review was to assess the current evidence of DNA-TG levels as marker for thiopurine therapy, especially with regards to NUDT15 variants.
Methods
A systematic review and meta-analysis were performed on the current evidence for DNA-TG as a marker for monitoring thiopurine therapy, including methods for measurement and the illustrative relationship between DNA-TG and various gene variants (such as TPMT, NUDT15, ITPA, NT5C2, and MRP4). PubMed and Embase were systematically searched up to April 2024 for published studies, using the keyword “DNA-TG” with MeSH terms and synonyms. The electronic search strategy was augmented by a manual examination of references cited in articles, recent reviews, editorials, and meta-analyses. A meta-analysis was performed using R studio 4.1.3. to investigate the difference between the coefficients (Fisher’s z-transformed correlation coefficient) of DNA-TG and 6-TGNs levels. A meta-analysis was performed using RevMan version 5.4 to investigate the difference in DNA-TG levels between patients with or without leukopenia using randomized effect size model. The risk of bias was assessed using the Newcastle–Ottowa quality assessment scale.
Results
In this systematic review, 21 studies were included that measured DNA-TG levels in white blood cells for either patients with ALL (n = 16) or IBD (n = 5). In our meta-analysis, the overall mean difference between patients with leukopenia (ALL + IBD) versus no leukopenia was 134.15 fmol TG/µg DNA [95% confidence interval (CI) (83.78–184.35), P < 0.00001; heterogeneity chi squared of 5.62, I2 of 47%]. There was a significant difference in DNA-TG levels for patients with IBD with and without leukopenia [161.76 fmol TG/µg DNA; 95% CI (126.23–197.29), P < 0.00001; heterogeneity chi squared of 0.20, I2 of 0%]. No significant difference was found in DNA-TG level between patients with ALL with or without leukopenia (57.71 fmol TG/µg DNA [95% CI (− 22.93 to 138.35), P < 0.80]). DNA-TG monitoring was found to be a promising method for predicting relapse rates in patients with ALL, and DNA-TG levels are likely a better predictor for leukopenia in patients with IBD than RBC 6-TGNs levels. DNA-TG levels have been shown to correlate with various gene variants (TPMT, NUDT15, ITPA, and MRP4) in various studies, points to its potential as a more informative marker for guiding thiopurine therapy across diverse genetic backgrounds.
Conclusions
This systematic review strongly supports the further investigation of DNA-TG as a marker for monitoring thiopurine therapy. Its correlation with treatment outcomes, such as relapse-free survival in ALL and the risk of leukopenia in IBD, underscores its role in enhancing personalized treatment approaches. DNA-TG effectively identifies NUDT15 variants and predicts late leukopenia in patients with IBD, regardless of their NUDT15 variant status. The recommended threshold for late leukopenia prediction in patients with IBD with DNA-TG is suggested to be between 320 and 340 fmol/µg DNA. More clinical research on DNA-TG implementation is mandatory to improve patient care and to improve inclusivity in thiopurine treatment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Thiopurines are drugs used for the treatment of various diseases, and traditionally erythrocyte 6-TGN levels are used to optimize therapy. |
However, most methodologies to assay 6-TGNs are unable to identify the impact of NUDT15 variants, affecting mostly ethnic populations (e.g., Asians and Hispanics). |
DNA-TG levels, which are directly measured in the DNA and, thus, accounts for the effect of NUDT15 variants, is a promising new inclusive method for monitoring thiopurine-treated patients. |
DNA-TG levels were valuable for predicting relapse-free survival in acute lymphocytic leukemia and predicting late leukopenia in patients with IBD. More clinical evaluations are needed to assess the implementation of DNA-TG as an inclusive marker for thiopurine therapy. |
1 Introduction
Thioguanine (TG), azathioprine (AZA), and mercaptopurine (MP) are thiopurine prodrugs used to treat diseases as varied as acute lymphocytic leukemia (ALL) and inflammatory bowel disease (IBD) [1, 2]. The metabolism of thiopurine drugs is complex and involves the conversion to different metabolites (Fig. 1) [3]. The efficacy and safety is impacted by the relative distribution of formed metabolites, of which the 6-thioguanine nucleotides (6-TGNs) are considered the active metabolites, and metabolites such as 6-methylmercaptopurine (6-MMP) are associated with hepatotoxicity and leukopenia [4, 5]. 6-TGNs have been shown to affect lymphocyte proliferation by two mechanisms of action by: (1) substitution of RAC1 (ras-related C3 botulinum toxin substrate 1)-GTP second messenger to form RAC1-thioGTP [6] to so alter proliferation and apoptosis of activated lymphocytes and (2) by incorporation into DNA (DNA-TG) and RNA [1], TG inhibits the function of several enzymes involved in DNA replication and repair [7]. Furthermore, DNA-TG also induces DNA damage such as single strand-breaks, DNA-protein cross-links and chromatid exchanges [8,9,10]. In current practice, erythrocyte (RBC) 6-TGNs are commonly used as a marker for thiopurine-related toxicities, but their utility is questionable [11, 12], as they are measured in RBCs instead of white blood cells (WBCs), which are the target cells of thiopurine therapies [13]. DNA-TG, on the other hand, is measured in WBCs and could, therefore, be a more accurate marker [12]. Furthermore, when 6-TGNs are measured, the assay typically removes the phosphate groups and, thus, actually measures 6-thioinosine monophosphate (TGMP), thioguanosine diphosphate (TGDP), and thioguanosine triphosphate (TGTP), collectively. Most methodologies to assay 6-TGNs are unable to identify the impact of NUDT15 variants, affecting mostly ethnic populations (e.g., Asians and Hispanics) where NUDT15 variants are more common [14]. DNA-TG tackles this problem by directly measuring thioguanine in the DNA, which can be influenced by both TPMT and NUDT15 variants. DNA-TG testing could offer a more inclusive, sensitive, and considerate approach to TDM of thiopurines. DNA-TG includes all patients, also ethnic populations, with NUDT15 variants (e.g., Chinese, Indian, Malay, Japanese, and Hispanics) [14,15,16,17] in contrast to 6-TGN measurements which are more relevant for populations with wild-type metabolism or TPMT variants (e.g., more prevalent in European and African ancestry) [16, 18].
Mechanism of thiopurine metabolism [3]. TG thioguanine, AZA azathioprine, MP mercaptopurine, Me methyl, HGPRT hypoxanthine guanine phosphoribosyltransferase, TPMT thiopurine methyltransferase, NUDT15 nudix hydrolase 15, ITPase inosine triphosphatase, XO xanthine oxidase, IMPDH1 inosine monophosphate dehydrogenase1, GST glutathione S-transferase, NT5C2 5′-nucleotidase cytosolic II, GMPS guanosine monophosphate synthetase, TITP thioinosine triphosphate, MeTITP methyl-thioinosine triphosphate, TIMP thioinosine monophosphate, TGMP thioguanosine monophosphate, TGDP thioguanosine diphosphate, TGTP thioguanosine triphosphate, TGNs TGMP, TGDP and TGTP (light green), TGR thioguanosine ribose, MPR 6-mercaptopurine riboside, TdGDP 6-thio-deoxy-guanosine diphosphate, TdGTP 6-thio-deoxy-guanosine triphosphate, TGMP thioguanosine monophosphate, RAC1 ras-related C3 botulinum toxin substrate 1, ABCC4 multidrug resistance-associated protein 4, TU thiouric acid, TX thioxanthine. Created with BioRender.com
A meta-analysis showed that the pooled prevalence of NUDT15 *1/*3 variant was 18% in Chinese patients, while the prevalence of NUDT15 *3/*3 variants was 1%. In Japanese patients, prevalence was 20% and 3%, for NUDT15 *1/*3 and *3/*3 variant, respectively [19].
However, recent data suggests that NUDT15 variants are also identified in European and African ancestry, suggesting a need for inclusive therapeutic drug monitoring approaches [20, 21]. Previous methods for measuring DNA-TG were technically difficult and required a large sample volume [22], but recent assays using liquid chromatography–tandem mass spectrometry (LC–MS/MS) techniques utilize smaller samples and have improved accuracy and reliability [23, 24]. In this systematic review and meta-analysis, the latest evidence for DNA-TG as a marker of therapeutic drug monitoring in thiopurine-treated patients is reviewed.
2 Methods
2.1 Search Criteria and Study Selection
PubMed and Embase were systematically searched up to April 2024 for published studies, using the keyword “DNA-TG” with MeSH terms and synonyms. This electronic search strategy was augmented by a manual examination of references cited in articles, recent reviews, editorials, and meta-analyses. No restrictions were imposed on the language, study period, or sample size. Studies were included that measured DNA-TG in human samples. Data outcomes that were specifically sought for were DNA-TG levels, 6-TGN levels, 6-MMP levels, leukopenia, remission rates of IBD, relapse free-survival rates of ALL, adverse events, and reporting of gene variants within thiopurine metabolism (e.g., TPMT, NUDT15, ITPA, and MRP4). The search and data collection were performed by one reviewer.
2.2 Data Extraction, Data Synthesis, and Quality Assessment
From the included studies the following data were extracted: author’s name, year of publication, number of patients, disease, the thiopurine prodrug used in the individual studies, other reported drugs, metabolites measured, matrix in which the metabolite was measured, and tested genes. Furthermore, the analytical methods for measuring DNA-TG and 6-TGNs and their levels were noted. A meta-analysis was performed using R studio 4.1.3. to investigate the difference between the coefficients (Fisher’s z-transformed correlation coefficient) of DNA-TG and 6-TGNs levels (Fig. 2). A meta-analysis was also performed using RevMan version 5.4 to investigate the difference in DNA-TG levels between patients with or without leukopenia using randomized effect size model (Fig. 3). An overview of all included studies is provided in Table 1. This study was reported according to the PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration [25]. This systematic review was not registered before it was conducted, and also, no protocol was prepared. The risk of bias was assessed using the Newcastle–Ottowa quality assessment scale (NOS). [26]
Meta-analysis of correlation coefficients of the correlation between DNA-TG and RBC TGN levels. The Fisher’s z-transformed correlation coefficient was used for meta-analysis to combine the results of several studies that have reported correlation coefficients. In a fixed effects model, a Fisher’s z-transformed correlation coefficient of 0.59 (95% CI 0.54–0.64) was obtained. This suggests a moderate-to-strong correlation between DNA-TG and RBC TGN levels. I2 (total heterogeneity) of 93.2%, test for heterogeneity Q(df) = 58.89, P < 0.0001
Forest plot of DNA-TG levels of thiopurine-treated patients (ALL + IBD) with leukopenia compared with thiopurine-treated patients without leukopenia. The overall mean difference between (ALL + IBD) patients with leukopenia versus no leukopenia was 134.15 fmol TG/µg DNA [95% CI (83.78–184.35), P < 0.00001]. Heterogeneity chi squared of 5.62, I2 = 47%. There was a significant difference in DNA-TG levels for patients with IBD with and without leukopenia [161.76 fmol TG/µg DNA [95% CI (126.23–197.29), P < 0.00001]. Heterogeneity chi squared of 0.20, I2 = 0%. No significant difference was found in DNA-TG level between patients with ALL with or without leukopenia (57.71 fmol TG/µg DNA [95% CI (−22.93 to 138.35), P < 0.80])
3 Results
3.1 Study Selection
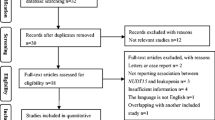
In this systematic review, 21 studies were included that measured DNA-TG levels in WBC for either patients with ALL (n = 16) or IBD (n = 5). Furthermore, the studies, which investigated different methods for measuring DNA-TG, are discussed here below in greater detail. A flowchart of the included studies can be found in Fig. 4.
PRISMA flowchart of included studies in this systematic review [25]
3.2 Analytical Methods for Measuring DNA-TG and 6-TGNs
In the late 1970s, initial attempts were made to measure DNA-TG. Tidd and Dedhar (1978) [27] used a high-performance liquid chromatography (HPLC) technique based on DNA hydrolysis and quantitation of a fluorescent oxidation product of 6-TG by anion-exchange HPLC in leukocytes. However, this method was not suitable for therapeutic drug monitoring (TDM) due to its low sensitivity and large quantities of pure DNA required for analysis. From the early 1990s to the late 2000s, various studies were published that involved quantification of TG in the DNA of leukocytes [28,29,30,31,32]. In all these studies, DNA was isolated from peripheral blood and digested using nucleases and phosphatases, resulting in free deoxynucleosides which could be detected using HPLC with UV absorption or fluorescence detection. In 2012, Jacobsen et al. [24] published a novel method to quantify DNA-TG using liquid chromatography-tandem mass spectrometry (LC–MS/MS). Coulthard et al. [33] also described a method of measuring DNA-TG levels in thiopurine-treated patients with IBD. The difference in these latter studies was that the method of Jacobsen, but not Coulthard, required derivatization before analysis, necessitating standard dTG-incorporated DNA to control for variation in the derivatization reaction. In 2021, Choi et al. [23] published a modified assay to measure DNA-TG levels in leukocytes by LC–MS/MS, which they tested in patients with ALL. The advantage of their method is that it required—and was validated with—only small volumes of DNA (1 µg), making it potentially suitable for routine use of DNA-TG measurements in clinical practice.
Currently, measurement of RBC 6-TGNs is the most widely used thiopurine therapeutic drug monitoring. The assays of Lennard et al. [34] and Dervieux [35] are the most commonly utilized in practice, with the Dervieux [35] assay being widely adopted due to its slightly lower coefficient of variation and greater simplicity. Results and therapeutic ranges from the two assays cannot be directly compared, because RBC 6-TGN levels with the Dervieux [35] assay are 2.6-fold higher than those obtained by the Lennard assay [36]. The analytical methods used in the studies covered by this review are provided in Table 2 [23, 24, 33]. A meta-analysis of WBC DNA-TG/RBC 6-TGN correlation coefficients where provided, using the Fisher’s z-transformed correlation coefficient for meta-analysis was performed (and is shown in Fig. 2). In a fixed effects model, the overall Fisher’s z-transformed correlation coefficient was 0.59 [95% confidence interval (CI) 0.54–0.64], consistent with a moderate to strong correlation between WBC DNA-TG and RBC 6-TGN levels.
3.3 DNA-TG in Relationship to Efficacy and Safety
3.3.1 Relapse-Free Survival in Acute Lymphoblastic Leukemia
Nielsen et al. [37] investigated the association of DNA-TG, RBC 6-TGNs, and other metabolites with relapse-free survival within the NOPHO ALL2008 trial for patients with acute lymphoblastic leukemia (ALL). The treatment protocol consisted of maintenance therapy with MP 75 mg/m2 once per day and methotrexate 20 mg/m2 once per week. It was found that relapse-free survival was significantly associated with DNA-TG concentrations [adjusted HR (HRa) 0.81 per 100 fmol/µg DNA increase; 95% CI 0.67–0.98]. Hence, relapse-free survival in patients with ALL increased with increasing DNA-TG concentrations. In comparison, relapse-free survival was not associated with RBC 6-TGN (adjusted HR 0.96 per 100 nmol/mmol hemoglobin increase, 95% CI 0.80–1.27). In an attempt to further optimize the abovementioned maintenance therapy, Larsen et al. [38] added incremental low doses of TG to the treatment protocol (TEAM protocol, starting TG dose 2.5 mg/m2/day to maximum dose 12.5 mg/m2/day) [39]. It was found that addition of TG to the treatment protocol led to higher levels of DNA-TG compared with historical patients who received only standard therapy of methotrexate and MP. Unsurprisingly, higher 6-TGN levels were found in patients in whom TG was added to the treatment protocol, but addition of TG did not lead to increased myelotoxicity or hepatotoxicity. The superiority of adding TG to conventional antileukemic 6MP/methotrexate maintenance therapy in pediatric ALL is currently being tested in an international randomized clinical trial [40]. The association between DNA-TG concentrations and the relapse rate in acute lymphoblastic leukemia (ALL) was also studied by Toksvang et al. [41] in an individual participant data meta-analysis (IPD-MA). In their IPD-MA, they included six trials that involved 1901 pediatric and young adult patients with ALL treated with MP (start dose, 75 mg/m2/day). The median DNA-TG level was 415 fmol/μg [interquartile range (IQR) 282–587, n = 1609] and did not differ between males and females, age groups, patients with white blood cell count < 50 versus ≥ 50 × 109/L at diagnosis. The median follow-up time was 6.0 years (IQR 4.1–8.4, n = 1910). In the pooled data-analysis, the relapse-specific HRa was 0.94 per 100 fmol/μg increase in DNA-TG (95% CI 0.88–1.00, n = 1910). In patients with end-of-induction minimal residual disease (EOI MRD)-positive patients (n = 839), the HRa’s were 0.87 (95% CI 0.78–0.97) and 0.90 (95% CI 0.82–0.99) per 100 fmol/μg increase in DNA-TG for relapse and any event (relapse, second cancer, or death). DNA-TG levels were not associated with relapse or any event in EOI MRD-negative patients. Hence, this IPD-MA showed that DNA-TG is inversely associated with the relapse rate in patients with ALL, especially in EOI MRD-positive patients with ALL. As the authors mentioned, the latter is particularly encouraging as EOI MRD is a very important prognostic marker for event-free survival in pediatric and young adult patients with ALL. This also supports the investigation and utility of clinical monitoring of DNA-TG concentrations in patients with ALL.
3.3.2 Leukopenia in Patients with ALL
Leukopenia has been described in various studies using DNA-TG levels in patients with ALL. Ju et al. [42] reported that 8 out of 63 patients who underwent MP maintenance therapy for ALL developed leukopenia (WBC < 1500/μL) at the time of DNA-TG sampling. Four patients had NUDT15 variants (two *1/*2, one *1/*5, and one *1/*6 genotype), while no patients had TPMT variants. In their study, the DNA-TG levels ranged from 27.8 to 504.8 fmol TG/μg DNA. Furthermore, no correlation was found between DNA-TG (P = 0.093) or 6-TGN (P = 0.425) levels with WBC counts. Fan et al. [43] reported that patients with NUDT15 variants (c.36_37insGGAGTC, c.352G > A, c.415C > T) experienced significantly higher episodes of leukopenia during therapy. Patients with intermediate or low NUDT15 activity showed a higher risk of developing leukopenia compared with wild-type activity (odds ratio of 6.00, P = 0.009). However, no statistically significant correlation was observed between DNA-TG levels and lowest WBC and lowest absolute neutrophil count. Although, a significantly higher DNA-TG/MP dose ratio was found in patients who experienced one or more leukopenia episodes (P = 0.025, n = 16). In our meta-analysis, no significant difference (mean difference is 57.7 (95% CI − 22.93 to 138.35, P = 0.16) was found between DNA-TG levels of patients with ALL who developed leukopenia compared with those who did not develop leukopenia. The explanation why no association between DNA-TG and leukopenia was found in patients with ALL might be due to various reasons such as use of concomitant methotrexate [43], no correction for thiopurine dosage, or NUDT15/TPMT/other variants, and lack of 6-MMP measurements.
3.3.3 DNA-TG Measurements Predict Late Leukopenia in Inflammatory Bowel Disease
Two studies were performed that compared DNA-TG and 6-TGN measurements in predicting leukopenia in patients with IBD [44]. The first study was performed by Zhu et al. [44] who used DNA-TG and 6-TGN measurements at time of late leukopenia or after 8 weeks after stable dose was obtained and investigated whether these measurements were able to predict late leukopenia (developed after 2 months) during a prospective follow-up of patients. It was found that patients with IBD who developed late leukopenia (> 2 months) had significantly higher DNA-TG levels compared with patients who did not develop late leukopenia (423.3, IQR 361.1–577.4 versus 270.0; IQR 188.1–392.4 fmol/µg DNA). However, no significant differences in RBC 6-TGN concentrations were found between patients with IBD who developed late leukopenia compared with patients with IBD who did not develop late leukopenia.
With respect to DNA-TG, these investigators used a cutoff level of 340.1 fmol/µg DNA for the prediction of late leukopenia (sensitivity 83.7% and specificity 63.4%; positive predictive value 27.1% and negative predictive value 96.0%). Interestingly, they also compared the abovementioned DNA-TG cutoff level with a 6-TGN cutoff level (≤ 355 pmol/8 × 108 RBC) from literature based on patients from Asian descent [45]. The results showed that of IBD patients that developed leukopenia who were deemed to have “normal” 6-TGN-levels, 56.5% of them had “high” DNA-TG levels. DNA-TG was also associated with late leukopenia in NUDT15 variants and NUDT15 nonvariant patients with IBD. Conversely, 6-TGNs were only associated with leukopenia in patients with IBD with NUDT15 nonvariants but not in patients with NUDT15 variants. This shows that DNA-TG does predict late leukopenia not only in NUDT15 nonvariants but also in NUDT15 variant patients highlighting the inclusive potential of DNA-TG measurements for patients with different ethnicities. Yang et al. [46] has also performed a study investigating early proactive monitoring of DNA-TG levels in patients with IBD. Late leukopenia was defined as a leukocyte count < 3.5 × 109/L over 2 months. In total, late leucopenia was observed in 15.6% (17/109) of NUDT15/TPMT wild-type and 64.1% (25/39) of intermediate metabolizers. DNA-TG levels were significantly higher in patients who developed leukopenia compared with those who did not develop leukopenia [394.9 ± 137.4 (median ± IQR, n = 42) versus 225.3 ± 139.6 (median ± IQR, n = 106) fmol/µg DNA; P = 4.9 × 10−13]. In TPMT/NUDT15 wild-type patients, the DNA-TG levels were also significantly higher in patients with leukopenia compared with those who did not [401.1 ± 222.9 (median ± IQR, n = 17) versus 221.1 ± 140.8 (median ± IQR, n = 92) fmol/µg DNA; P = 4.9 × 10−9). Furthermore, DNA-TG levels were also higher in variant patients who developed leukopenia compared with who did not develop leukopenia [382.5 ± 143.1 (median ± IQR, n = 25) versus 240.5 ± 248.3 (median ± IQR, n = 14) fmol/µg DNA; P = 0.024; 3 for TPMT*3C, 21 for NUDT15*3, 2 for NUDT15*6, and 14 for NUDT15*2]. With regards to 6-TGNs, in the entire cohort 6-TGN levels were significantly higher in patients who developed leukopenia compared with those who did not [322.4 ± 210.6 (median ± IQR, n = 42) versus 247.5 ± 182.5 (median ± IQR, n = 106) pmol/8 × 108 RBCs; P = 0.021). In wild-type patients, a similar result was found, in which 6-TGNs were higher in patients who developed leukopenia [333.7 ± 270.7 (median ± IQR, n = 17) versus 247.5 ± 162.9 (median ± IQR, n = 92) pmol/8 × 108 RBCs; P = 0.018]. However, similarly to the report of Zhu et al. [44], 6-TGNs were not able to predict leukopenia in patients with TPMT/NUDT15 variants [310.0 ± 180.6 (median ± IQR, n = 25) versus 249.9 ± 303.9 (median ± IQR, n = 14) pmol/8 × 108 RBCs; P = 0.55]. Again, this suggests that 6-TGNs are unable to predict leukopenia in variant-type patients, hindering the utility in especially patients with Asian and Hispanic ethnicities [16]. We have also performed a meta-analysis, which showed that DNA-TG is able to predict late leukopenia in patients with IBD. The DNA-TG levels were significantly higher in patients with leukopenia compared with those without leukopenia (mean difference 161.8, 95% CI 126.2–197.3, P < 0.00001).
3.3.4 Sinusoidal Obstruction Syndrome
Past discussions have highlighted concerns about the acute liver toxicity associated with thiopurine therapy, particularly for TG, especially in relation to conditions such as nodular regenerative hyperplasia and sinusoidal obstruction syndrome (SOS) [47,48,49]. Toksvang et al. [50] performed a subanalysis in the UKALL97 trial (11 cases with SOS) by measuring DNA-TG concentrations. In this trial, in which patients with ALL were randomized to either MP (n = 80) or TG (n = 52), no difference was found in DNA-TG levels between patients who received MP or TG. Furthermore, there was no difference in DNA-TG levels between patients who developed SOS compared with patients who did not develop SOS (HR 0.91 per 100 fmol/µg DNA-TG increase, 95% CI 0.76–1.09), using a Cox-regression analysis of relapse, death, second cancer, and SOS. This suggests that higher levels of DNA-TG are not directly related to SOS. They also investigated platelet counts as a derivative marker for hepatic angiopathy. In an analysis of the NOPHO ALL2008 study, no association was found between the median platelet count and median DNA-TG level during maintenance therapy [37]. Also, in the previously mentioned study by Larsen et al. [38], which added incremental low dosages of TG to MP and methotrexate (TEAM protocol) [39], no differences were found in platelet count compared with conventional protocol (NOPHO ALL2008). Hence, these studies suggest that higher levels DNA-TG are not directly related to SOS, which is consistent with the primary pathogenesis of SOS being due to a toxic effect of thioguanosine triphosphate on the sinusoidal endothelial cells rather than the circulating immune cells. [51]
3.3.5 Osteonecrosis
Osteonecrosis is a treatment-related toxicity in patients with ALL who are treated with MP/MTX maintenance therapy [52]. Toksvang et al. [53] prospectively registered osteonecrosis in 1234 patients with ALL who were treated with ALL2008 protocol (MP/MTX). After a median follow-up of 5.6 years, the cumulative incidence of osteonecrosis in their study was 2.7%. The median DNA-TG levels in the entire cohort were 384 fmol/μg DNA (IQR 211–367). Neither RBC 6-TGNs or DNA-TG were significantly associated with the risk of osteonecrosis in Cox models stratified by three age groups and adjusted for sex.
3.4 Relationship to Gene Variants of Thiopurine Metabolism
Numerous studies have examined the relationship between DNA-TG, thiopurine dosage, and RBC 6-TGN levels [23, 24, 37, 53,54,55], specifically focusing on gene variants involved in thiopurine metabolism such as TPMT, NUDT15, ITPA, NT5C2 and MRP4. This is noteworthy, as it may reveal DNA-TG’s potential to detect gene variants. Should this be the case, DNA-TG levels could potentially serve as a screening method to identify gene variants without the need for preliminary genetic testing. The significance of DNA-TG in relation to these genetic variants is detailed below.
3.4.1 TPMT
TPMT is an important enzyme in the metabolism pathways of thiopurines (Fig. 1). Raised levels of 6-TGNs are caused by a loss-of-function in the TPMT enzyme, which leads to an increased risk of developing leukopenia [56, 57]. The determination of TPMT genotype/phenotype can help guide physicians for dose adjustments of thiopurines, DNA-TG might be used to identify TPMT variant patients who did not have TPMT determination or may predict TPMT variant-related toxicities. Gerbek et al. [54] found significantly higher median DNA-TG levels in patients with ALL with TPMT low-activity variants compared with patients with wild-type TPMT (549 versus 364 fmol/µg, P = 0.001). Patients with TPMT-low variants also had higher RBC 6-TGN levels (491 versus 232 nmol/mmol Hb, P = 0.001), and DNA-TG levels positively correlated (rs = 0.37, P < 0.001) with RBC 6-TGN levels. Nielsen et al. [58] also reported that DNA-TG levels were higher for patients who were heterozygous for TPMT variants (760.9 fmol/µg DNA, IQR 568.7–890.3) compared with TPMT wild-type patients (492.7 fmol/µg DNA, IQR 382.1–634.6; P < 0.001). Patients carrying wild-type TPMT had higher TPMT activity compared with TPMT heterozygous genotypes [TPMT levels from maintenance therapy evaluated in patients who remained in remission at follow-up, N = 752, 16.6 (range 7.6−28.9) versus 9.6 (range 7.2−16.1) U/mL RBC; P < 0.001] but did not differ in their risk of leukemic relapse [58]. Ju et al. [42] measured DNA-TG levels in patients with ALL (n = 72) who were tested for TPMT and NUDT15 genotypes. No significant difference was found between TPMT variants (n = 3, *1/*3) and TPMT wild type in DNA-TG levels. No patients (n = 3) with a TPMT variant (*1/*3) developed leukopenia (WBC < 1500 WBC/μL). In their study, a significant positive correlation was found between MP intensity and DNA-TG concentration (P < 0.001). Furthermore, Zhu et al. [44] found that patients with IBD with TPMT*3C variants had higher levels of DNA-TG and RBC 6-TGNs compared with TPMT nonvariants, but no association was found between DNA-TG levels in TPMT*3C variants and leukopenia. However, in their study, the numbers of patients with TPMT variants was low.
3.4.2 ITPA
ITPA plays an important role in the hydrolysis of TITP to TIMP (Fig. 1), and the deficiency in ITPA leads to accumulation of TITP, which subsequently leads to toxicity [59]. A common single nucleotide polymorphism (SNP) in the ITPA gene is 94C > A, which was found to be associated with neutropenia in pediatric patients with ALL [60, 61]. Furthermore, from literature, patients with IBD with this ITPA variant had higher levels of 6-TGNs compared with patients with wild-type ITPA. In the same study, this ITPA genotype was associated with clinical effectiveness [62]. The effects of ITPA variants on DNA-TG levels were studied by Gerbek et al. [54] They reported significantly higher DNA-TG levels in patients with a low-activity ITPA variant compared with patients with wild-type ITPA [465 (IQR 419–558) versus 387 (IQR 287–448) fmol/µg DNA TG; P = 0.04). However, no differences were found between RBC 6-TGN levels (median 305 versus 260 nmol/mmol Hb, P = 0.086), RBC MethylMP levels (median 20,866 versus 15,902 nmol/mmol Hb, P = 0.12). In patients with low activity ITPA less TITP is reconverted to TIMP, resulting in higher levels of MeTITP. The authors suggested that this would lead to increased pools of methylated thiopurine metabolites, which might inhibit de novo purine synthesis and, thus, lead to lower levels of endogenous purines to compete with 6-TGN to be incorporated into DNA.
3.4.3 NUDT15
NUDT15 is a purine-specific nudix hydrolase that involves the hydrolysis of nucleosides-diphosphates [63]. NUDT15 is involved in the conversion of 6-TGTP and deoxy-TGTP metabolites to TGMP and deoxy-TGMP (Fig. 1) [64]. Deficiency in NUDT15 leads to relatively higher TGTP formation, which hypothetically promotes the formation of DNA-TG and inhibition of Rac1 [65]. Thus, NUDT15 may potentially increases the risk of developing leukopenia in patients with “normal” total 6-TGN levels, due to more abundant TGTP. Furthermore, a meta-analysis found that the risk of thiopurine-induced leukopenia was increased in 1136 NUDT15 R139C variant carriers compared with 4987 wild-type patients [odds ratio (OR) 6.9, 95% CI 5.2–9.1). [57] Thus, genotyping of NUDT15 seems clinically relevant, but the role of RBC 6-TGNs as a predictor for NUDT15-related leukopenia has not been clarified. However, it is anticipated that measurements of 6-TGN, which cannot detect NUDT15 variants, will also be unable to predict leukopenia. DNA-TG could present a compelling alternative to 6-TGN measurements since it targets the end-metabolite and is influenced by NUDT15 variants. Ju et al. [42] reported that the DNA-TG/MP dose ratio was significantly higher for patients carrying NUDT15 variants compared with patients with wild-type NUDT15 (P < 0.001). Four patients with NUDT15 variants (two *1/*2, one *1/*5, one *1/*6 genotype) developed leukopenia. The DNA-TG concentrations during these leukopenia episodes ranged from 27.8 to 504.8 fmol TG/μg DNA. Patients with NUDT15 variants had DNA-TG values of 235.9 (*1/*5), 68.7 (*1/*2), 62.0 (*1/*2), and 27.8 (*1/*6) (fmol TG/μg DNA). White blood cell count did not show a significant correlation with DNA-TG level (P = 0.093) or RBC 6-TGN (P = 0.425). Furthermore, Moriyama et al. [55] performed a study which compared RBC 6-TGN and DNA-TG levels in patients with ALL with NUDT15 variants. The mean RBC 6-TGN and DNA-TG levels were 134.1 pmol/4 × 108 RBC (range 0.46–315.5 pmol/4 × 108 RBC) and 442.8 fmol/μg DNA (range 78.1–1054.0 fmol/μg DNA). Patients with the low-activity NUDT15 diplotypes had the highest DNA-TG/dosage ratio, followed by the intermediate-activity diplotype group, and lowest in patients with wild-type NUDT15 (P = 4.0 × 10−9). A DNA-TG/6-TGN-ratio was used in their study and found that this ratio was significantly higher in NUDT15 deficient patients compared with NUDT15 wild-type patients (P = 3.6 × 10−9).
Choi et al. [23] validated an analytical assay that measured DNA-TG in Korean patients with ALL. They reported a moderate correlation between DNA-TG levels and RBC 6-TGNs in 257 measurements (ρ = 0.405, P < 0.0001), a moderate correlation between DNA-TG level and MP dose (ρ = 0.417, P < 0.0001). Furthermore, a higher DNA-TG/6-TGN-ratio was found in patients who were NUDT15 intermediate metabolizers (*1/*2, *1/*3) and indeterminate NUDT15 variants (*1/*5, *1/*6) compared with NUDT15 wild type. They also reported a linear relationship between DNA-TG and 6-TGN levels in NUDT15 intermediate metabolizers (R2 = 0.641, P < 0.001) and indeterminate genotypes (R2 = 0.859, P < 0.001).
Toyonaga et al. [66] studied the effect of DNA-TG incorporation in IBD-patient derived leukocytes. In total, 54 patients with IBD were included, who received a median MP dose of 0.33 mg/kg/day. Thirteen patients were heterozygous and two were homozygous for the NUDT15 variant allele. No difference was observed in RBC 6-TGN levels between NUDT15 variants and nonvariant patients (646.8 versus 585.2 pmol/8 × 108 RBC per mg/kg/day of MP, P = 0.566), while DNA-TG levels were different between NUDT15 variant and nonvariant patients [nonvariants versus heterozygous variants versus homozygous variants, 295.3 versus 663.0 versus 4418.0 mol/106 moles dA per mg/kg/day of MP (P = 0.004)]. This is consistent with RBC 6-TGN being a measurement of total 6-TGN (thioguanosine mono-, di-, and triphosphates), whereas DNA-TG measurement reflects mainly thioguanosine triphosphate [67]. The ratio of DNA-TG/RBC 6-TGN was significantly higher in NUDT15 variants compared with nonvariants (P = 0.017). Furthermore, the authors showed that DNA-TG levels negatively correlated with peripheral lymphocyte count (r = −0.31, P = 0.012). Although, RBC 6-TGNs did not correlate with peripheral lymphocyte counts (r = − 0.12, P = 0.17). The authors also incubated patient-derived CD4+ T lymphocytes with exogenous TG. The level of DNA-incorporated TG was significantly higher in NUDT15 homozygous variants than in nonvariants (median DNA-TGlymphocytes at 72 h; nonvariants versus heterozygous variants versus homozygous variants, 311.0 versus 554.7 versus 1070.0 fmol/μg DNA, P < 0.01). Also, the proliferation of CD4+ T cells was significantly more inhibited in NUDT15 homozygous variants compared with nonvariants (0.1 μM 6-TG; nonvariants versus heterozygous variants vsersus homozygous variants, 78.6% versus 65.2% versus 59.4%, P < 0.05). This is consistent with more DNA-TG accumulation in patients with NUDT15 variants, which significantly affects the proliferation of CD4+ T cells.
3.4.4 MRP4
MRP4 is encoded by the ABCC4 gene and is a ATP-binding cassette transporter, which is responsible for the transport of monophosphorylated nucleosides [68]. Deficiencies in MRP4 function may lead to higher levels of 6-TGNs intracellularly, and thus, there might be a higher risk of developing leukopenia. Ban et al. [69] reported higher RBC 6-TGN levels and an increased risk of developing leukopenia in patients with MRP4 variants compared with patients with wild-type MRP4. Only one study investigated the effects of MRP4 variants on the levels of DNA-TG. It was found that patients with ABCC4 c.2128G > A variants demonstrated a significantly higher DNA-TG to MP dose ratio (P = 0.031, n = 16) and a higher DNA-TG to MP dose ratio was associated with higher risk of developing leukopenia (P = 0.025) [43]. This suggests that if there is a deficiency in the function of ABCC4, more DNA-TG can accumulate within the leukocyte and that there might be more risk of developing leukopenia.
3.4.5 NT5C2
NT5C2, which stands for 5′-nucleotidase, cytosolic II, is an enzyme that plays a crucial role in the metabolism of thiopurines. NT5C2 dephosphorylates the thiolated nucleotides TIMP and TGMP. The thiopurine nucleosides that are formed are transported out of the cell, and thus, the natural function of NT5C2 is to regulate intracellular levels of purine monophosphates, but in the context of thiopurine therapy, it also reduces the amount of 6-TGN available for
DNA incorporation (Fig. 1) [68, 70]. However, the acquisition of somatic gain-of-function NT5C2 mutation leads to resistance to thiopurine therapy [71, 72]. Tulstrup et al. [73] studied the effects of NT5C2 germline variants on thiopurine metabolism in ALL. Patients were treated according to the NOPHO ALL2008 trial protocol. In their study, the following DNA-TG values were obtained for NT5C2 rs72846714 GG (n = 153), AG (n = 25), and AA (n = 2) genotypes: NT5C2GG: 509.3 fmol/µmol DNA (IQR 405.3–661.4), NT5C2GA: 538.3 fmol/µmol DNA (IQR 399.9–688.9), and NT5C2AA: 497.4 fmol/µmol DNA (IQR 318.4–654.7). The corresponding values for 6-TGNs were 245 nmol/mmol hemoglobin (IQR 186.9–324.6), 206.8 nmol/mmol hemoglobin (IQR 161–270.1), and 192.5 nmol/mmol hemoglobin (IQR 116.2–219.4), for the GG, AG, and AA genotypes. The ratios of DNA-TG/6-TGNs were 2.0 (IQR 1.5–2.6), 2.5 (IQR 1.7–3.2), and 2.9 (IQR 2.1–3.9), respectively. The association between rs72846714 genotypes and DNA-TG/6-TGN-ratio was most likely caused by differences in 6-TGNs, while the effect on DNA-TG was less pronounced. Increased NT5C2 expression by the rs72846714-A allele results in a depletion of not only intracellular 6-TGN but also guanine nucleotides (GN), resulting in an increased 6-TGN/GN ratio and increased DNA incorporation of 6-TGN despite lower absolute concentrations. In their study, rs72846714-A genotype was not significantly associated with relapse-specific hazard [per-allele hazard ratio 0.85 (95% confidence interval 0.50–1.43], P = 0.53), on-therapy relapse-specific hazard, or the risk of any events.
4 Discussion
The metabolism of thiopurine prodrugs is complex and has a significant impact on their efficacy and safety in treating diseases like ALL and IBD. In this systematic review, we focused on the utility of DNA-TG measurements as a marker for therapeutic drug monitoring of thiopurines. The review findings shed light on the potential of DNA-TG in optimizing treatment strategies to enhance patient outcomes. Current literature suggests that leukocyte DNA-TG levels are more accurate in the prediction of leukopenia in patients with IBD and relapse in patients with ALL than RBC-6-TGN levels. Furthermore, DNA-TG levels were found to be affected by genetic variants of thiopurine metabolism such as NUDT15, TPMT, MRP4, NT5C2, and ITPA. This is particularly relevant to identify variants in patients using thiopurines.
4.1 DNA-TG is a More Inclusive Measurement for Predicting Leukopenia in IBD
As previously discussed, the majority of methods for measuring 6-TGNs fail to detect the influence of NUDT15 mutations. This issue particularly impacts Asian patients, who are more likely to carry NUDT15 variants and, therefore, are at a higher risk of adverse events like thiopurine-induced leukopenia [74, 75]. Our meta-analysis has showed that in patients with IBD, early proactive DNA-TG monitoring predicts leukopenia [44]. DNA-TG was able to predict leukopenia in wild-type patients and patients with TPMT/NUDT15 variants. In both IBD studies, 6-TGNs were able to predict leukopenia in wild-type patients but was not able to predict leukopenia in patients with variants [44]. Maillard et al. [16] showed that in a diverse group of participants from worldwide clinical trials for ALL, individuals with variants in both TPMT and NUDT15 genes were predominantly of Hispanic (68%) and Asian (23%) backgrounds. They also demonstrated that mice carrying NUDT15 mutations and those with mutations in both TPMT and NUDT15 had elevated DNA-TG levels when compared with both wild-type mice and those with only TPMT mutations. Additionally, it was observed that mice with either NUDT15 mutations or both TPMT and NUDT15 mutations exhibited significantly reduced neutrophil counts compared with mice with TPMT mutations and wild-type mice, at an equivalent dosage of 20 mg/kg. Furthermore, Toyonaga et al. [66] reported that patients with IBD with NUDT15 variants had significantly higher DNA-TG levels compared with nonvariants, while patients with NUDT15 variants did not have significantly higher 6-TGN levels compared with nonvariants.
All those findings highlight the potential of DNA-TG as a promising inclusive marker for toxicity in thiopurine-treated patients, especially those with NUDT15 variants.
4.2 Limitations of This Review
This systematic review and meta-analysis presents some limitations. First, the studies analyzed included patients with ALL and IBD, who were subjected to varied treatment protocols, including different dosages and concurrent medications such as methotrexate. Additionally, the reporting of outcomes across these studies was heterogeneous, making it challenging to extract relevant data, such as the mean or median levels of DNA-TG and 6-TGN. The unavailability of complete datasets further complicated the assessment of the impact of dosage adjustments and other confounding factors on DNA-TG/6-TGN levels. While the studies reviewed indicate that DNA-TG can predict late leukopenia in patients with IBD, they, along with others included in this review, did not account for 6-MMP values. This is a significant limitation, given the association of these metabolites with leukopenia [5]. Another limitation is the variety of methodologies applied to measure DNA-TG and 6-TGN levels, which poses challenges to the comparability of the studies. Despite these limitations, it is noteworthy that our importat finding—that DNA-TG predicts late leukopenia in patients with IBD—was derived using consistent methodologies for measuring DNA-TG and 6-TGN levels [44, 46].
4.3 Future Outlook of DNA-TG Measurements
Currently, most methods used to measure 6-TGN levels in clinical practice do not detect NUDT15 variants and appear unable to predict leukopenia in patients with IBD. Based on the results of this review, it seems that not only can DNA-TG better predict leukopenia, it is also more inclusive for ethnic populations with NUDT15 variants. Advances in tandem mass spectrometry have enabled the measurement of DNA-TG levels from small volumes of blood, with the cost of each test ranging from approximately $50 to $100. Given that the costs are comparable with those of 6-TGNs, it is plausible that in the near future DNA-TG could be a routine laboratory marker. Nevertheless, all the studies reviewed here overlook one issue: the importance of 6-MMP, a metabolite associated with hepatotoxicity and myelotoxicity [5, 76]. Addressing this issue could involve either combining RBC 6-TGN/6-MMP measurements with WBC DNA-TG measurements or exploring methods to measure WBC 6-MMP directly. Furthermore, other markers for thiopurine therapy, such as the measurement of individual nucleotides 6-TGMP, 6-TGDP and 6-TGTP [77], or RAC1/Pstat3 expression in lymphocytes, are used as a pharmacodynamics marker [78]. Future research should focus on how to further implement DNA-TG measurements in regular practice in ALL and IBD patient care.
5 Conclusions
This systematic review provides compelling evidence for the clinical utility of DNA-TG as a marker for therapeutic drug monitoring of thiopurines. Its associations with treatment outcomes, including relapse-free survival in ALL and leukopenia risk in IBD, highlight its potential to improve individualized treatment strategies. DNA-TG is a more inclusive and sensitive to identify NUDT15 variants and to predict in late leukopenia in both NUD15 variant and non-NUDT15 variant patients with IBD. The suggested cutoff value for predicting late leukopenia in patients with IBD using DNA-TG should be between 320 and 340 fmol/µg DNA. More clinical research on DNA-TG implementation is mandatory to improve patient care and to improve inclusivity in thiopurine treatment.
References
Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8(1):24–36. https://doi.org/10.1038/nrc2292.
Bayoumy AB, Simsek M, Seinen ML, Mulder CJJ, Ansari A, Peters GJ, De Boer NK. The continuous rediscovery and the benefit-risk ratio of thioguanine, a comprehensive review. Expert Opin Drug Metab Toxicol. 2020;16(2):111–23. https://doi.org/10.1080/17425255.2020.1719996.
Zaza G, Cheok M, Krynetskaia N, et al. Thiopurine pathway. Pharmacogenet Genomics. 2010;20(9):573–4. https://doi.org/10.1097/FPC.0b013e328334338f.
Kreijne JE, Seinen ML, Wilhelm AJ, Bouma G, Mulder CJ, van Bodegraven AA, de Boer NK. Routinely established skewed thiopurine metabolism leads to a strikingly high rate of early therapeutic failure in patients with inflammatory bowel disease. Ther Drug Monit. 2015;37(6):797–804. https://doi.org/10.1097/ftd.0000000000000213.
Meijer B, Kreijne JE, van Moorsel SAW, et al. 6-methylmercaptopurine-induced leukocytopenia during thiopurine therapy in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2017;32(6):1183–90. https://doi.org/10.1111/jgh.13656.
de Boer NKH, Peyrin-Biroulet L, Jharap B, et al. Thiopurines in inflammatory bowel disease: new findings and perspectives. J Crohns Colitis. 2018;12(5):610–20. https://doi.org/10.1093/ecco-jcc/jjx181.
Toksvang LN, Lee SHR, Yang JJ, Schmiegelow K. Maintenance therapy for acute lymphoblastic leukemia: basic science and clinical translations. Leukemia. 2022;36(7):1749–58. https://doi.org/10.1038/s41375-022-01591-4.
Inamochi H, Higashigawa M, Shimono Y, et al. Delayed cytotoxicity of 6-mercaptopurine is compatible with mitotic death caused by DNA damage due to incorporation of 6-thioguanine into DNA as 6-thioguanine nucleotide. J Exp Clin Cancer Res. 1999;18(3):417–24.
Christie NT, Drake S, Meyn RE, Nelson JA. 6-Thioguanine-induced DNA damage as a determinant of cytotoxicity in cultured Chinese hamster ovary cells. Cancer Res. 1984;44(9):3665–71.
You C, Dai X, Yuan B, Wang Y. Effects of 6-thioguanine and S6-methylthioguanine on transcription in vitro and in human cells. J Biol Chem. 2012;287(49):40915–23. https://doi.org/10.1074/jbc.M112.418681.
Mateen B, Patel M, Akobeng A, Gordon M, Hayee BH. Systematic review: the effectiveness of 6-thioguanine nucleotide-based dose optimisation of thiopurines in the treatment of inflammatory bowel disease. Wellcome Open Res. 2023;8:60. https://doi.org/10.12688/wellcomeopenres.18846.1.
Duley JA, Florin TH. Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides. Ther Drug Monit. 2005;27(5):647–54. https://doi.org/10.1097/01.ftd.0000169061.52715.3e.
Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;79–80:153–70. https://doi.org/10.1093/bmb/ldl020.
Khoo XH, Wong SY, Ibrahim NRW, et al. Nudix hydroxylase 15 mutations strongly predict thiopurine-induced leukopenia across different Asian ethnicities: implications for screening in a diverse population. Front Med (Lausanne). 2022;9: 880937. https://doi.org/10.3389/fmed.2022.880937.
Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33(11):1235–42. https://doi.org/10.1200/jco.2014.59.4671.
Maillard M, Nishii R, Yang W, et al. Additive effects of TPMT and NUDT15 on thiopurine toxicity in children with acute lymphoblastic leukemia across multiethnic populations. J Natl Cancer Inst. 2024. https://doi.org/10.1093/jnci/djae004.
Jena A, Jha DK, Kumar MP, et al. Prevalence of polymorphisms in thiopurine metabolism and association with adverse outcomes: a South Asian region-specific systematic review and meta-analysis. Expert Rev Clin Pharmacol. 2021;14(4):491–501. https://doi.org/10.1080/17512433.2021.1900729.
Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64(8):753–67. https://doi.org/10.1007/s00228-008-0478-6.
Yu N, Sriranganathan D, Walker GJ, et al. Prevalence of NUDT15 genetic variants and incidence of thiopurine-induced leukopenia in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2023;17(12):1920–30. https://doi.org/10.1093/ecco-jcc/jjad107.
Moriyama T, Yang Y-L, Nishii R, et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood. 2017;130(10):1209–12. https://doi.org/10.1182/blood-2017-05-782383.
Walker GJ, Harrison JW, Heap GA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019;321(8):773–85. https://doi.org/10.1001/jama.2019.0709.
Lancaster DL, Patel N, Lennard L, Lilleyman JS. Leucocyte versus erythrocyte thioguanine nucleotide concentrations in children taking thiopurines for acute lymphoblastic leukaemia. Cancer Chemother Pharmacol. 2002;50(1):33–6. https://doi.org/10.1007/s00280-002-0442-6.
Choi R, Chun MR, Park J, et al. Quantification of thioguanine in DNA using liquid chromatography-tandem mass spectrometry for routine thiopurine drug monitoring in patients with pediatric acute lymphoblastic leukemia. Ann Lab Med. 2021;41(2):145–54. https://doi.org/10.3343/alm.2021.41.2.145.
Jacobsen JH, Schmiegelow K, Nersting J. Liquid chromatography-tandem mass spectrometry quantification of 6-thioguanine in DNA using endogenous guanine as internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;881–882:115–8. https://doi.org/10.1016/j.jchromb.2011.11.032.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. https://doi.org/10.1136/bmj.b2700.
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000.
Tidd DM, Dedhar S. Specific and sensitive combined high-performance liquid chromatographic-flow fluorometric assay for intracellular 6-thioguanine nucleotides metabolites of 6-mercaptopurine and 6-thioguanine. J Chromatogr. 1978;145(2):237–46. https://doi.org/10.1016/s0378-4347(00)81344-4.
Warren DJ, Slørdal L. A high-performance liquid chromatographic method for the determination of 6-thioguanine residues in DNA using precolumn derivatization and fluorescence detection. Anal Biochem. 1993;215(2):278–83. https://doi.org/10.1006/abio.1993.1587.
Warren DJ, Andersen A, Slørdal L. Quantitation of 6-thioguanine residues in peripheral blood leukocyte DNA obtained from patients receiving 6-mercaptopurine-based maintenance therapy. Cancer Res. 1995;55(8):1670–4.
Erdmann GR, Steury JC, Carleton BC, Stafford RJ, Bostrom BC, Canafax DM. Reversed-phase high-performance liquid chromatographic approach to determine total lymphocyte concentrations of 6-thioguanine, methylmercaptopurine and methylthioguanine in humans. J Chromatogr. 1991;571(1–2):149–56. https://doi.org/10.1016/0378-4347(91)80441-e.
Olesen KM, Hansen SH, Sidenius U, Schmiegelow K. Determination of leukocyte DNA 6-thioguanine nucleotide levels by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;864(1–2):149–55. https://doi.org/10.1016/j.jchromb.2008.02.007.
Hedeland RL, Hvidt K, Nersting J, Rosthøj S, Dalhoff K, Lausen B, Schmiegelow K. DNA incorporation of 6-thioguanine nucleotides during maintenance therapy of childhood acute lymphoblastic leukaemia and non-Hodgkin lymphoma. Cancer Chemother Pharmacol. 2010;66(3):485–91. https://doi.org/10.1007/s00280-009-1184-5.
Coulthard SA, Berry P, McGarrity S, Ansari A, Redfern CPF. Liquid chromatography-mass spectrometry for measuring deoxythioguanosine in DNA from thiopurine-treated patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1028:175–80. https://doi.org/10.1016/j.jchromb.2016.06.017.
Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992;583(1):83–90. https://doi.org/10.1016/0378-4347(92)80347-s.
Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998;44(3):551–5.
Shipkova M, Armstrong VW, Wieland E, Oellerich M. Differences in nucleotide hydrolysis contribute to the differences between erythrocyte 6-thioguanine nucleotide concentrations determined by two widely used methods. Clin Chem. 2003;49(2):260–8. https://doi.org/10.1373/49.2.260.
Nielsen SN, Grell K, Nersting J, et al. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol. 2017;18(4):515–24. https://doi.org/10.1016/s1470-2045(17)30154-7.
Larsen RH, Utke Rank C, Grell K, et al. Increments in DNA-thioguanine level during thiopurine-enhanced maintenance therapy of acute lymphoblastic leukemia. Haematologica. 2021;106(11):2824–33. https://doi.org/10.3324/haematol.2020.278166.
Toksvang LN, Als-Nielsen B, Bacon C, et al. Thiopurine Enhanced ALL Maintenance (TEAM): study protocol for a randomized study to evaluate the improvement in disease-free survival by adding very low dose 6-thioguanine to 6-mercaptopurine/methotrexate-based maintenance therapy in pediatric and adult patients (0-45 years) with newly diagnosed B-cell precursor or T-cell acute lymphoblastic leukemia treated according to the intermediate risk-high group of the ALLTogether1 protocol. BMC Cancer. 2022;22(1):483. https://doi.org/10.1186/s12885-022-09522-3.
M. Heyman. A Treatment Study Protocol of the ALLTogether Consortium for infants, children and young adults (0-45 years of age) with newly diagnosed acute lymphoblastic leukaemia (ALL): a pilot study. https://www.clinicaltrials.gov/study/NCT04307576.
Toksvang LN, Grell K, Nersting J, et al. DNA-thioguanine concentration and relapse risk in children and young adults with acute lymphoblastic leukemia: an IPD meta-analysis. Leukemia. 2022;36(1):33–41. https://doi.org/10.1038/s41375-021-01182-9.
Ju HY, Lee JW, Cho HW, et al. DNA-thioguanine nucleotide as a treatment marker in acute lymphoblastic leukemia patients with NUDT15 variant genotypes. PLoS ONE. 2021;16(1): e0245667. https://doi.org/10.1371/journal.pone.0245667.
Fan POL, Leung KT, Chan KYY, et al. ABCC4, ITPA, NUDT15, TPMT and their interaction as genetic predictors of 6-mercaptopurine intolerance in chinese patients with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2022;39(3):254–66. https://doi.org/10.1080/08880018.2021.1973628.
Zhu X, Chao K, Yang T, et al. DNA-thioguanine nucleotides as a marker for thiopurine induced late leukopenia after dose optimizing by NUDT15 C415T in Chinese patients with IBD. Clin Pharmacol Ther. 2022. https://doi.org/10.1002/cpt.2730.
Feng R, Guo J, Zhang SH, et al. Low 6-thioguanine nucleotide level: effective in maintaining remission in Chinese patients with Crohn’s disease. J Gastroenterol Hepatol. 2019;34(4):679–85. https://doi.org/10.1111/jgh.14465.
Yang T, Chao K, Zhu X, et al. Early proactive monitoring of DNA-thioguanine in patients with Crohn’s disease predicts thiopurine-induced late leucopenia in NUDT15/TPMT normal metabolizers. World J Gastroenterol. 2024;30(12):1751–63.
Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11(5):787–97. https://doi.org/10.1002/hep.1840110512.
Stanulla M, Schaeffeler E, Möricke A, et al. Hepatic sinusoidal obstruction syndrome and short-term application of 6-thioguanine in pediatric acute lymphoblastic leukemia. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01203-7.
Ferlitsch A, Teml A, Reinisch W, et al. 6-thioguanine associated nodular regenerative hyperplasia in patients with inflammatory bowel disease may induce portal hypertension. Am J Gastroenterol. 2007;102(11):2495–503. https://doi.org/10.1111/j.1572-0241.2007.01530.x.
Toksvang LN, Grell K, Nielsen SN, et al. DNA-TG and risk of sinusoidal obstruction syndrome in childhood acute lymphoblastic leukemia. Leukemia. 2022;36(2):555–7. https://doi.org/10.1038/s41375-021-01420-0.
Oancea I, Png CW, Das I, et al. A novel mouse model of veno-occlusive disease provides strategies to prevent thioguanine-induced hepatic toxicity. Gut. 2013;62(4):594–605. https://doi.org/10.1136/gutjnl-2012-302274.
Kunstreich M, Kummer S, Laws HJ, Borkhardt A, Kuhlen M. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica. 2016;101(11):1295–305. https://doi.org/10.3324/haematol.2016.147595.
Toksvang LN, Andrés-Jensen L, Rank CU, et al. Maintenance therapy and risk of osteonecrosis in children and young adults with acute lymphoblastic leukemia: a NOPHO ALL2008 sub-study. Cancer Chemother Pharmacol. 2021;88(5):911–7. https://doi.org/10.1007/s00280-021-04316-z.
Gerbek T, Ebbesen M, Nersting J, Frandsen TL, Appell ML, Schmiegelow K. Role of TPMT and ITPA variants in mercaptopurine disposition. Cancer Chemother Pharmacol. 2018;81(3):579–86. https://doi.org/10.1007/s00280-018-3525-8.
Moriyama T, Nishii R, Lin TN, et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017;27(6):236–9. https://doi.org/10.1097/fpc.0000000000000282.
Liu YP, Xu HQ, Li M, Yang X, Yu S, Fu WL, Huang Q. Association between thiopurine S-methyltransferase polymorphisms and azathioprine-induced adverse drug reactions in patients with autoimmune diseases: a meta-analysis. PLoS ONE. 2015;10(12): e0144234. https://doi.org/10.1371/journal.pone.0144234.
van Gennep S, Konte K, Meijer B, Heymans MW, D’Haens GR, Lowenberg M, de Boer NKH. Systematic review with meta-analysis: risk factors for thiopurine-induced leukopenia in IBD. Aliment Pharmacol Ther. 2019;50(5):484–506. https://doi.org/10.1111/apt.15403.
Nielsen SN, Toksvang LN, Grell K, et al. No association between relapse hazard and thiopurine methyltransferase geno- or phenotypes in non-high risk acute lymphoblastic leukemia: a NOPHO ALL2008 sub-study. Cancer Chemother Pharmacol. 2021;88(2):271–9. https://doi.org/10.1007/s00280-021-04281-7.
Marinaki AM, Ansari A, Duley JA, et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. 2004;14(3):181–7.
Barba E, Kontou PI, Michalopoulos I, Bagos PG, Braliou GG. Association of ITPA gene polymorphisms with adverse effects of AZA/6-MP administration: a systematic review and meta-analysis. Pharmacogenomics J. 2022;22(1):39–54. https://doi.org/10.1038/s41397-021-00255-3.
Lee Y, Jang EJ, Yoon HY, Yee J, Gwak HS. Effect of ITPA polymorphism on adverse drug reactions of 6-mercaptopurine in pediatric patients with acute lymphoblastic leukemia: a systematic review and meta-analysis. Pharmaceuticals (Basel). 2022. https://doi.org/10.3390/ph15040416.
Luo X, Yan S, Jin L, Zhu H, Zhang X, Ge W. Inosine triphosphate pyrophosphatase and NUDT15 are good predictors of clinical outcomes in thiopurine-treated Chinese patients with inflammatory bowel disease. Ther Drug Monit. 2022;44(3):391–5. https://doi.org/10.1097/ftd.0000000000000965.
Gad H, Koolmeister T, Jemth AS, et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature. 2014;508(7495):215–21. https://doi.org/10.1038/nature13181.
Valerie NC, Hagenkort A, Page BD, et al. NUDT15 hydrolyzes 6-thio-deoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 2016;76(18):5501–11. https://doi.org/10.1158/0008-5472.Can-16-0584.
Meijer B, Mulder CJ, de Boer NK. NUDT15: a novel player in thiopurine metabolism. J Gastrointest Liver Dis JGLD. 2016;25(2):261–2. https://doi.org/10.15403/jgld.2014.1121.252.nud.
Toyonaga T, Kobayashi T, Kuronuma S, et al. Increased DNA-incorporated thiopurine metabolite as a possible mechanism for leukocytopenia through cell apoptosis in inflammatory bowel disease patients with NUDT15 mutation. J Gastroenterol. 2021;56(11):999–1007. https://doi.org/10.1007/s00535-021-01820-0.
Coulthard SA, McGarrity S, Sahota K, Berry P, Redfern CPF. Three faces of mercaptopurine cytotoxicity in vitro: methylation, nucleotide homeostasis, and deoxythioguanosine in DNA. Drug Metab Dispos. 2018;46(8):1191–9. https://doi.org/10.1124/dmd.118.081844.
Wielinga PR, Reid G, Challa EE, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62(6):1321–31. https://doi.org/10.1124/mol.62.6.1321.
Ban H, Andoh A, Imaeda H, et al. The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2010;45(10):1014–21. https://doi.org/10.1007/s00535-010-0248-y.
Moriyama T, Liu S, Li J, et al. Mechanisms of NT5C2-mediated thiopurine resistance in acute lymphoblastic leukemia. Mol Cancer Ther. 2019;18(10):1887–95. https://doi.org/10.1158/1535-7163.Mct-18-1112.
Tzoneva G, Perez-Garcia A, Carpenter Z, et al. Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat Med. 2013;19(3):368–71. https://doi.org/10.1038/nm.3078.
Meyer JA, Wang J, Hogan LE, et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet. 2013;45(3):290–4. https://doi.org/10.1038/ng.2558.
Tulstrup M, Grosjean M, Nielsen SN, et al. NT5C2 germline variants alter thiopurine metabolism and are associated with acquired NT5C2 relapse mutations in childhood acute lymphoblastic leukaemia. Leukemia. 2018;32(12):2527–35. https://doi.org/10.1038/s41375-018-0245-3.
Kakuta Y, Kinouchi Y, Shimosegawa T. Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: prospects for clinical application of NUDT15 genotyping. J Gastroenterol. 2018;53(2):172–80. https://doi.org/10.1007/s00535-017-1416-0.
Lee KM, Kim YS, Seo GS, Kim TO, Yang SK. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015;13(3):193–207. https://doi.org/10.5217/ir.2015.13.3.193.
Seidman EG, Amre D. High 6-MMP/6-TGN ratios predict non-response and hepatotoxicity to thiopurine therapy in Crohn’s disease. Off J Am Coll Gastroenterol ACG. 2005;100:839.
Neurath MF, Kiesslich R, Teichgräber U, et al. 6-thioguanosine diphosphate and triphosphate levels in red blood cells and response to azathioprine therapy in Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3(10):1007–14. https://doi.org/10.1016/s1542-3565(05)00697-x.
Deben DS, van Adrichem AJ, Drent R, et al. Rac1/pSTAT3 expression: a pharmacodynamic marker panel as a first step toward optimization of thiopurine therapy in inflammatory bowel disease patients. Cytometry A. 2022;101(2):167–76. https://doi.org/10.1002/cyto.a.24506.
Larsen RH, Hjalgrim LL, Degn M, Nersting J, Als-Nielsen B, Grell K, Schmiegelow K. Dynamics of leucocyte DNA thioguanine nucleotide levels during maintenance therapy of childhood acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2021;88(1):53–60. https://doi.org/10.1007/s00280-020-04219-5.
Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48(4):367–73. https://doi.org/10.1038/ng.3508.
Ebbesen MS, Nersting J, Jacobsen JH, et al. Incorporation of 6-thioguanine nucleotides into DNA during maintenance therapy of childhood acute lymphoblastic leukemia-the influence of thiopurine methyltransferase genotypes. J Clin Pharmacol. 2013;53(6):670–4. https://doi.org/10.1002/jcph.81.
Cuffari C, Li DY, Mahoney J, Barnes Y, Bayless TM. Peripheral blood mononuclear cell DNA 6-thioguanine metabolite levels correlate with decreased interferon-gamma production in patients with Crohn’s disease on AZA therapy. Dig Dis Sci. 2004;49(1):133–7. https://doi.org/10.1023/b:ddas.0000011614.88494.ee.
Lennard L, Maddocks JL. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983;35(1):15–8. https://doi.org/10.1111/j.2042-7158.1983.tb04255.x.
Bruunshuus I, Schmiegelow K. Analysis of 6-mercaptopurine, 6-thioguanine nucleotides, and 6-thiouric acid in biological fluids by high-performance liquid chromatography. Scand J Clin Lab Invest. 1989;49(8):779–84. https://doi.org/10.3109/00365518909091557.
Acknowledgements
We thank Dr Toksvang (University Hospital Rigshospitalet) and Dr Coulthard (retired) for their input during the creation of this review. We thank professor Chi-Kong Li (Chinese University of Hong Kong) for providing raw data from their study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work is part of the Danish nation-wide research program Childhood Oncology Network Targeting Research, Organisation and Life expectancy (CONTROL) and supported by the Danish Cancer Society (R-257-A14720) and the Danish Childhood Cancer Foundation (2019-5934 and 2020-5769).
Conflict of interest
A.B. has no conflict of interests and received no funding for the work. N.d.B. has served as a speaker for AbbVie and MSD and has served as consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr. Falk, TEVA Pharma BV, MLDS and Takeda. All outside the submitted work. C.J.J.M. has served as consultant for HLW Pharma BV, the Netherlands and Douglas Pharma New Zealand, otherwise no conficts of interest. T.H.J.F. is a director of an Australian startup company ProdrugXtend Pty Ltd, which owns a PCT relating to thiopurines. A.R.A. has served as consultant and speaker for Dr. Falk. He has received unrestricted research grants from Janssen Cilag and MSD. K.S. is a speaker and/or Advisory Board Honoraria from Illumina (2021), Jazz Pharmaceuticals (2020, 2021, 2023), and Servier (2020, 2021, 2023); speaker fee from Amgen (2020, 2021) and Medscape (2020, 2021); Educational grants from Servier (2020, 2021, 2023); research grant from Novo Nordisk Foundation (2020,2022). Stocks in Novo Nordisk.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code availability
Not applicable.
Author contributions
Systematic search, data excretion, data synthesis, and quality assessment: Bayoumy. Meta-analysis calculations: Bayoumy. Figure and table creation: Bayoumy. Manuscript writing and reviewing: Bayoumy, Ansari, De Boer, Mulder, Florin, and Schmiegelow.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bayoumy, A.B., Ansari, A.R., Mulder, C.J.J. et al. Innovating Thiopurine Therapeutic Drug Monitoring: A Systematic Review and Meta-Analysis on DNA-Thioguanine Nucleotides (DNA-TG) as an Inclusive Biomarker in Thiopurine Therapy. Clin Pharmacokinet 63, 1089–1109 (2024). https://doi.org/10.1007/s40262-024-01393-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-024-01393-0