Abstract
Background
Sodium zirconium cyclosilicate (SZC) is an approved oral treatment for hyperkalemia that selectively binds potassium (K+) in the gastrointestinal tract and removes K+ from the body through increased fecal excretion. Here, we describe the population pharmacodynamic (PopPD) response of serum K+ concentration in patients with hyperkalemia who are treated with SZC, estimate the impact of patients’ intrinsic and extrinsic factors, and compare predicted serum K+ responses between 5 g alternate daily (QOD) and 2.5 g once daily (QD) maintenance doses.
Methods
PopPD analysis was based on pooled data from seven phase II and III clinical trials for SZC. A semi-mechanistic longitudinal mixed-effects (base) model was used to characterize serum K+ concentration after SZC dosing. Indirect-response, virtual pharmacokinetics–pharmacodynamics (PK–PD) modeling was used to mimic the drug exposure compartment. Full covariate modeling was used to assess covariate impact on the half-maximal effective concentration of drug (EC50), placebo response, and Kout. Models were evaluated using goodness-of-fit plots, relative standard errors, and visual predictive checks, and data were stratified to optimize model performance across subgroups. Covariate effects were evaluated based on the magnitude of change in serum K+ between baseline and end of correction phase dosing (48 h, SZC 10 g three times a day) and maintenance phase dosing (28 days, SZC 10 g QD) using a reference subject.
Results
The analysis data set included 2369 patients and 25,764 serum K+ observations. The mean (standard deviation) patient age was 66.0 (12) years, 61% were male, 68% were White, 34% had congestive heart failure, and 62% had diabetes. Mean (standard deviation) serum K+ at baseline was 5.49 (0.43) mmol/L. Both the base and full covariance models adequately described observed data. In the final model, there was a sigmoid exposure response on Kin, with EC50 of 32.8 g and a Hill coefficient of 1.36. The predicted placebo-adjusted dose–responses of serum K+ change appeared nearly linear in the correction and maintenance phases. No clinically meaningful difference in placebo-adjusted serum K+ change from baseline at 28 days was observed between maintenance regimens of SZC 5 g QOD and 2.5 g QD. A greater SZC treatment response was associated with high serum K+ at baseline, advanced age, lower body weight, lower estimated glomerular filtration rate, and Black/African American and Asian race, compared with the reference patient. The impact of heart failure status and diabetes status was only minor.
Conclusions
The PopPD model of SZC adequately described changes in serum K+ concentration during correction and maintenance phase dosing. A greater treatment response was associated with various covariates, but the impact of each was modest. Overall, these findings suggest that no adjustment in SZC dose is needed for any of the covariates evaluated.
Similar content being viewed by others
PopPD modeling was performed to characterize serum K+ concentrations in sodium zirconium cyclosilicate (SZC)-treated patients who received different dosing regimens, and to evaluate the impact of intrinsic and extrinsic patient factors on treatment response. |
A greater SZC treatment response is associated with high serum K+ at baseline, advanced age, lower body weight, lower estimated glomerular filtration rate, and Black/African American and Asian race, but the impact of each covariate was modest. |
Our results suggest that current SZC dosing regimens do not need adjustment for any patient subgroups studied. |
1 Introduction
Hyperkalemia is a potentially life-threatening electrolyte disorder that is associated with increased risk of adverse cardiorenal outcomes, including fatal cardiac arrhythmias, cardiac arrest, and mortality [1,2,3]. Evidence consistently describes a U-shaped association between serum potassium (K+) concentration and mortality and morbidity, with both low and high K+ levels contributing [3, 4].
Sodium zirconium cyclosilicate (SZC) is an oral, highly selective K+ binder approved for the treatment of hyperkalemia in adults, including adults receiving maintenance hemodialysis. In phase II and III clinical trials, SZC lowered serum K+ levels to within the normal range (3.5–5.0 mmol/L) during the first 48 h of treatment and maintained normokalemia over the longer term (for up to 12 months) [5,6,7,8]. These beneficial effects were consistent across all patient subgroups [i.e., chronic kidney disease, diabetes, heart failure, and concomitant use of renin–angiotensin–aldosterone system inhibitors (RAASi)] [6, 8, 9].
The pharmacodynamic (PD) action of SZC involves selectively capturing K+ ions in exchange for sodium and hydrogen ions in the gastrointestinal tract [10, 11]. SZC reduces the serum K+ concentration and removes K+ from the body through increased fecal excretion, and is not absorbed systemically [10, 11]. The K+-lowering action of SZC is predictable and rapid, leading to a significant reduction of serum K+ within 1 h of administration [9,10,11]. In patients with hyperkalemia participating in a phase III study (ZS-003), SZC demonstrated dose-dependent reductions in serum K+ levels across the 2.5–15 g dose range [10].
Population PD (PopPD) helps to inform the use of drugs in clinical practice and can estimate potential differences in PD in specific populations identified by their intrinsic and extrinsic factors. Here, we describe the PopPD response of serum K+ concentration in patients with hyperkalemia treated with SZC, specifically the longitudinal dose–response of SZC in the correction (0–48 h) and maintenance (> 48 h) phases of treatment, estimate the effects of patients’ intrinsic and extrinsic factors on the serum K+ response, and compare the predicted serum K+ response of SZC 2.5 g once daily (QD) and 5 g alternate daily (QOD) maintenance doses.
2 Methods
2.1 Data
2.1.1 Study Population, Dose, and Samples
This PopPD analysis was based on pooled data from seven phase II and III clinical studies, consisting of 2369 patients with hyperkalemia (screening serum K+ of > 5.0 mmol/L) with a total of 25,764 serum K+ observations. A summary of the studies included in this analysis is provided in Supplementary Table 1.
2.1.2 Analytical Method
Analysis of serum K+ concentration was performed at a central laboratory. No data were recorded with serum K+ assays below the limit of quantitation.
2.2 Model Development
2.2.1 Base Model
A semi-mechanistic longitudinal mixed-effects model was used to describe serum K+ concentration after SZC dosing (Fig. 1). An indirect-response, virtual pharmacokinetics–PD (PK–PD) modeling approach was used to mimic the drug exposure compartment without any measurements (none were available as the drug is not absorbed). The virtual SZC exposure in this compartment is driven by the amount and frequency of SZC doses and cleared by a first-order elimination rate parameter typically used in standard PK models. Variation in serum K+ concentration is governed by a zero-order constant for input or production response (Kin) and a dissociation rate constant (Kout). A sigmoid maximum effect (Emax) exposure–response inhibition function applied to the input rate (Kin) represents the serum K+-lowering effect of SZC.
The SZC virtual exposure was scaled to a target of 1 (mean 24 h exposure) for steady state 1 g QD treatment. As a result, the half-maximal effective concentration (EC50) parameter of the sigmoid Emax exposure–response inhibition function also represents dose at half-maximal effect (ED50) for the QD regimen. Model equations are shown in the Supplementary Appendix.
2.2.2 Covariate Model Development
The influence of prespecified baseline covariates on EC50, the placebo response, and Kout were explored using full covariate modeling. The covariates assessed were based on physiological plausibility and prior knowledge: age, body weight, estimated glomerular filtration rate (eGFR), serum K+, race, sex, congestive heart failure (CHF) status, and diabetes status. Continuous covariates were evaluated using one functional form or a combination of functional forms as appropriate, and dichotomous and categorical variables were evaluated as a proportional shift using NONMEM software (version 7.3.0; ICON Development Solutions, Ellicott City, MD, USA). The equations are shown in the Supplementary Appendix.
Race was investigated as a categorical variable. Each race category other than White was identified by its own unique variable that was set to 1 or 0. Each race variable was tested as a covariate on prespecified parameters relative to White race being the reference.
The use of RAASi was not considered for covariate analysis because RAASi medications have been shown to have little impact on the serum K+-lowering performance of SZC, and the collection of RAASi medication data were not consistent throughout the pooled studies to enable modeling.
2.2.3 Model Evaluation
Model evaluation included graphical analysis of goodness-of-fit (GOF) plots, relative standard errors, and visual predictive checks (VPCs) (Supplementary Appendix). Stratification by dose, visit, or covariates was used as appropriate to ensure that models would perform adequately across important subgroups of the data.
The adequacy of the model was evaluated using a simulation-based VPC method (for absolute serum K+) and empirical Bayes-estimated individual random effects (Supplementary Appendix). Predictions for the population average dose–response were computed by sampling virtual patients (500 replicates, each with 1000 patients with replacement) from the database of actual patients with baseline covariates observed in the modeled studies (Supplementary Appendix).
Simulation with between-patient variability and parameter uncertainty was used to provide confidence intervals (CIs) for treatment effect predictions. Parameter vectors were simulated using a multivariate normal distribution with the mean set to the estimated value of each model parameter and using the estimated variance–covariance matrix.
2.3 Inference of the Covariate Effects on SZC Serum K+ Response
The effects of covariates were evaluated based on their capacity to alter the predicted change from baseline in serum K+ relative to the reference patient at the end of correction phase dosing [48 h, SZC 10 g three times a week (TID)] and maintenance phase dosing (28 days, SZC 10 g QD). The effect of each covariate was compared versus a reference patient, based on prevalent values of categorical covariates and a clinically meaningful value (near the population median) for continuous covariates. The reference patient is a nondiabetic, non-CHF, White male, 65 years of age, with eGFR of 45 mL/min/1.73 m2, body weight of 85 kg, and baseline serum K+ of 5.4 mmol/L. Predictions with respect to covariates were presented as forest plots showing the change from baseline as a point estimate with 95% CI for the correction and maintenance phase regimens.
2.4 Software Details
The software package NONMEM version 7.3.0 was used for this analysis. Model fitting was performed in a Linux environment (CentOS 7; Red Hat Software, Raleigh, NC, USA) with GFortran FORTRAN Compiler version 5.2 (GNU Compiler Collection; Free Software Foundation, Boston, MA, USA). nonmem2R version 0.2.1 (CRAN.R-project.org/package=nonmem2R), PsN version 4.4.8. (sourceforge.net/projects/psn/), and R version 3.5.1 (R-project; http://www.r-project.org) were used for the exploratory analysis, executing NONMEM runs and postprocessing of NONMEM output, for example, to assess GOF. mrgsolve version 0.8.12 (CRAN.R-project.org/package=mrgsolve) was used for simulation-based illustration of the impact of covariates using stochastic simulations.
3 Results
3.1 Dataset
All patients included in the intention-to-treat (ITT) analysis set (ITT correction phase) were assessed (n = 2369), with a total of 25,764 serum K+ observations. Serum K+ concentrations measured more than 240 h (10 days) after the last intake of experimental drug (active or placebo) were not included in the analysis (12 observations). Three serum K+ observations were considered to be outliers [extreme values based on the standard deviation (SD) of serum K+ for each patient] and were excluded from the analysis. For the longer clinical studies (ZS-005 and D9482C00001), the observations beyond 28 days from the start of maintenance dosing were excluded.
3.2 Study Population
Patients’ baseline demographics and characteristics are presented in Table 1. Overall, the mean (SD) patient age was 66.0 (12) years, 61% were male, 68% were White, 34% had CHF, and 62% had diabetes. Mean (SD) serum K+ at baseline was 5.49 (0.43) mmol/L.
Between-study differences in patient race resulted in differences in baseline clinical characteristics. Body weight and rates of CHF were lower in the three studies that predominantly or exclusively enrolled Asian patients (D9480C00002, D9482C00001, and D9482C00002; Table 1).
The distributions of continuous and categorical covariates in the SZC dataset are shown in Supplementary Fig. S1. No continuous covariates were sufficiently correlated to cause concern for model estimation, and no continuous characteristics appeared to covary with categorical groups (except for the effect of Asian race as described above).
3.3 SZC PopPD Model
3.3.1 Base Model
A semi-mechanistic longitudinal mixed-effects model was used to describe serum K+ concentration after SZC dosing. Parameter estimates and their precision for the base model are presented in Table 2.
Shrinkage in ETA (the discrepancy of an individual parameter from the typical population value, a distribution of mean of 0 and variance OMEGA; 18.9% Kin and 35.2% EC50) and EPS (random variability associated with individual observations, a distribution with a mean of 0 and variance SIGMA; 5.1%) were reasonable.
Inspection of model diagnostics showed that the model described the data adequately. GOF plots showed no bias in predictions across serum K+ value, time, or dose in the stable regimen studies or in those using maintenance dose titration (Supplementary Figs. S2 and S3).
3.3.2 Full Covariance Model
Parameter estimates of the final covariate model are presented in Table 2. The sigmoid Emax exposure response was characterized by a high value for the EC50 (32.8 g) and a Hill coefficient of 1.36.
Inspection of model diagnostics showed good agreement between the observed data and the model. Specifically, GOF plots showed no bias in predictions across serum K+ value, time, or dose in the stable regimen studies or in those using maintenance dose titration (Supplementary Figs. S4 and S5). The full model empirical Bayesian estimates of the random effect on Kin (ETA1) and EC50 (ETA2) were consistent across the categorical and continuous covariates studied, including correction and maintenance phase dose (Supplementary Fig. S6), and shrinkage in ETA (16.4% Kin and 46.5% EC50) and EPS (4.7%) remained reasonable.
The model was deemed acceptable for use in dose–response simulation and the empirical Bayesian estimates appropriate for covariate impact analysis. VPCs are shown in Supplementary Figs. S7 and S8.
From the base model to the full covariate model, most parameters remained similar or decreased (between patient and residual variance); however, the estimate of EC50 increased from 15.3 to 32.8 g and the estimate of Emax increased from 43.4 to 63.3%.
3.4 Dose–Response Simulation
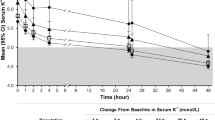
The predicted placebo-adjusted dose–response of serum K+ changes from baseline at 48 h and 4 weeks are shown in Fig. 2. In the correction phase, the predicted placebo-adjusted dose–response of serum K+ change from baseline after 48 h appeared nearly linear. In the maintenance phase, the predicted placebo-adjusted dose–response for a regimen of SZC 10 g TID dosing for 48 h followed by 0–15 g QD for 4 weeks appeared nearly linear at 28 days.
Predicted dose–response in the SZC correction and maintenance phases. CFB change from baseline, CI confidence interval, K+ potassium, QD once daily, SZC sodium zirconium cyclosilicate, TID three times a day. Graphs: Predicted population average (line) with 95% CI (shaded region). Table: Correction phase predictions were calculated using a TID regimen and the maintenance phase predictions were calculated based on a regimen of SZC 10 g TID for 48 h, followed by SZC 10 g QD for 28 days. The CI was determined by simulating 500 virtual trials of 1000 patients each, calculating the mean population dose–response and distribution, from which the 2.5 and 97.5% quantiles were determined
Dosing regimens in the correction and maintenance phase had similar predicted serum K+-lowering behavior based on predicted placebo-adjusted change from baseline (Fig. 2). No clinically meaningful difference in placebo-adjusted serum K+ change from baseline at 28 days was observed between maintenance regimens of SZC 5 g QOD and 2.5 g QD [− 0.19 (95% CI − 0.23 to − 0.14) versus − 0.17 (95% CI − 0.21 to − 0.13), respectively]. Simulated individual trajectories and median serum K+ over time after 48 h with 10 g TID followed by 4 weeks of 0–15 g QD are shown in Fig. 3.
Simulated serum K+ time course after 48 h of SZC 10 g TID dosing followed by 4 weeks of dosing with placebo (0 g), or SZC 5, 10, or 15 g QD. K+ potassium, QD once daily, SZC sodium zirconium cyclosilicate, TID three times a day. Individual data are shown in yellow (subset of 100 patients). Median (red solid line), 25 and 75% quantiles (red dashed lines), and 5 and 95% quantiles (red dotted lines) are based on 1000 simulated patients, with model uncertainty and residual variability
3.5 Effects of Covariates on SZC Serum K+ Response
The full covariate modeling approach considered the following covariates: five on EC50: baseline serum K+, age, body weight, eGFR, and race; four on placebo (Kin) response: baseline serum K+, eGFR, heart failure, and diabetes; and one on serum K+ dynamics (Kout): sex.
The impact of covariates on the predicted 48 h and 28 day serum K+ placebo-adjusted treatment response (change from baseline) relative to the reference patient is displayed in Fig. 4. Greater SZC treatment response is associated with high baseline serum K+, more advanced age, lower body weight, lower eGFR, and Black/African American and Asian race, compared with the reference patient.
Predicted serum K+ change from baseline for selected covariates for the correction phase (48 h 10 g TID) and maintenance phase (48 h 10 g TID followed by 4 weeks 10 g QD). CHF congestive heart failure, eGFR estimated glomerular filtration rate, K+ potassium, QD once daily, SZC sodium zirconium cyclosilicate, TID three times a day. The vertical black line in each panel represents the placebo-adjusted treatment response of SZC 10 g for the reference patient: a nondiabetic, non-CHF, White male, 65 years of age, with eGFR of 45 mL/min/1.73 m2, body weight of 85 kg, and baseline serum K+ of 5.4 mmol/L. Dashed lines in each panel display the SZC 5 and 15 g placebo-adjusted treatment response in the reference patient
The placebo-adjusted predicted serum K+ changes from baseline of SZC 10 g TID for the 10 and 90% quantile of baseline serum K+, age, and body weight were all within the range defined by the SZC 5 and 15 g response of the typical patient (− 0.21 to − 0.76 mmol/L). A stronger impact was observed for eGFR with SZC treatment response, predicted to be − 0.37 mmol/L and − 0.71 mmol/L for patients with eGFR of 88 mL/min/1.73 m2 and 17 mL/min/1.73 m2, respectively, compared with −0.48 mmol/L for the reference patient with eGFR of 45 mL/min/1.73 m2. Point estimates for Black/African American race (− 0.69 mmol/L) and Asian race (− 0.55 mmol/L) were likewise estimated within the 5–15 g range of the reference patient response.
The impact of heart failure status and diabetes status was very minor, with a predicted placebo-adjusted change from baseline of − 0.48 and − 0.49 mmol/L, respectively, compared with − 0.48 mmol/L for the reference patient who had neither medical condition. Taken together, the predicted treatment responses suggest that no SZC dose adjustment is needed for the intrinsic and extrinsic covariates considered.
4 Discussion
This pooled PopPD analysis was based on 25,764 serum K+ samples from 2369 patients across seven trials, and is the first to characterize the effects of prespecified covariates on SZC PD. A virtual modeling approach was used to mimic drug exposure effect without having any measurements of drug exposure (none were available, as the drug is not absorbed). The serum K+ over time was described by an indirect response model where K+ is added to and eliminated from a compartment corresponding to serum K+ measurements. The impact of SZC exposure was modeled as inhibition of the K+ production rate, consistent with the SZC mechanism of action to bind K+ and lower its absorption and appearance in circulation. Inspection of model diagnostics showed that the model adequately described the data. Overall, the findings from the PopPD analysis show that there is no need for clinical dose adjustment of SZC based on the patient covariates considered, and that the SZC label dosing is appropriate.
The full covariate model was deemed appropriate for use in dose–response simulation and covariate impact analysis. From the base model to the full covariate model, the estimate of EC50 increased from 15.3 to 32.8 g and the estimate of Emax increased from 43.4 to 63.3%. Black/African American and Asian patients comprised 9 and 21% of the analysis pool, respectively, and the EC50 for each was lower than that for White patients. In the base model, the EC50 is an estimate for the entire analysis population, while the EC50 in the full covariate model is that of the prevalent White patients. For these reasons, the full covariate model EC50 is expected to be higher than that of the base model. A further contributing factor may be that EC50 and Emax are correlated (due to the dose range over which data exist) and, therefore, despite Emax and EC50 increasing together, the net result leads to no change in the drug effect when comparing the inhibition of Kin at the same SZC dose.
The sigmoid Emax exposure–response implemented in the model was characterized by a high value for the EC50 (32.8 g) and a Hill coefficient of 1.36, and the predicted dose–response appeared nearly linear within the range of SZC doses studied (0–15 g). To assess doses below 5 g QD, both 2.5 g QD and 5 g QOD (using the same 5 g sachets of SZC) were evaluated. Dose–response simulations showed no clinically meaningful difference between maintenance regimens of 5 g QOD and 2.5 g QD in the pooled patient population studied, indicating that either regimen may be used in clinical practice to maintain serum K+.
Evaluation of covariates in the final model revealed that greater treatment response (i.e., serum K+ change from baseline) was associated with high serum K+ at baseline, more advanced age, lower body weight, and lower eGFR. Furthermore, a greater treatment response was predicted for Black/African American and Asian patients compared with White patients but remained within the range of SZC response defined by 5 and 15 g lower and higher doses, respectively. Diabetes (versus nondiabetic) and heart failure (versus no heart failure) did not clinically impact the serum K+ dose–response of SZC. The modest quantitative impact of each covariate relative to the reference dose range (5–15 g) and the overall results of this analysis support the conclusion that no dose adjustment is needed for SZC based on any of the tested covariates.
Serum K+ change from baseline is a PD endpoint; therefore, traditional concepts of PK bioequivalence are not necessarily appropriate for assessing the importance of categorical and continuous covariate relationships. Instead of a prescribed range, such as 80–125% maximum concentration (Cmax) or area under the concentration-time curve (AUC), the impact of each covariate relative to the reference patient was simulated virtually. Virtual simulations have been used in previous models to help determine the mean response to an intervention and also increase confidence that prospective simulations of response to novel therapeutics will reflect the interpatient variability seen in clinical practice [12, 13]. Furthermore, a K+ homeostasis mathematical model in patients who were hyperkalemic was previously developed, which used a virtual population to describe K+ distribution in the gastrointestinal tract, blood, and extracellular and intracellular spaces of tissue, the renal clearance of K+, and the K+–SZC binding and excretion in the gastrointestinal tract.
5 Strengths and Limitations of the Analysis
The strengths of this study were that PopPD modeling was performed using pooled data from seven clinical trials, which together spanned a broad patient population. In addition, the dataset was representative of the patients for whom SZC is used in clinical practice.
However, there were limitations. Some intrinsic variables were generated by virtual PopPD modeling, as SZC is not absorbed systemically [10, 11] and so no detailed SZC exposure data were available. The PK–PD relationship of drugs acting in the gastrointestinal tract can be complex due to the variability of the intestinal environment. As SZC can be taken with or without food and because there are no expected effects of other medicinal products on the pharmacologic action of SZC [10, 11], oral absorption factors were not expected to impact the PopPD modeling. However, differences in dietary K+ intake and gastrointestinal pH may impact the effectiveness of SZC. In addition, there were limitations of observability for certain physiological parameters, and the PopPD model relied on a number of assumptions, for example, that the disease state did not change or progress over the 28 days of SZC maintenance therapy.
6 Conclusions
The PopPD model of SZC adequately described the changes in serum K+ concentration during correction and maintenance phase dosing in the seven clinical studies. Greater treatment response was associated with high serum K+ at baseline, more advanced age, lower body weight, lower eGFR, and Black/African American and Asian race; however, the impact of each covariate was modest. The overall results of this analysis support the conclusion that no dose adjustment is needed for SZC based on any of the tested covariates.
References
Nakhoul GN, Huang H, Arrigain S, Jolly SE, Schold JD, Nally JV Jr, et al. Serum potassium, end-stage renal disease and mortality in chronic kdney disease. Am J Nephrol. 2015;41(6):456–63.
Campese VM, Adenuga G. Electrophysiological and clinical consequences of hyperkalemia. Kidney Int Suppl. 2016;6(1):16–9.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–21.
Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39(17):1535–42.
Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–31.
Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–33.
Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–11.
Spinowitz BS, Fishbane S, Pergola PE, Roger SD, Lerma EV, Butler J, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;7(14):798–809.
Packham DK, Kosiborod M. Pharmacodynamics and pharmacokinetics of sodium zirconium cyclosilicate [ZS-9] in the treatment of hyperkalemia. Expert Opin Drug Metab Toxicol. 2016;12(5):567–73.
AstraZeneca Pharmaceuticals LP. LOKELMA. Highlights of prescribing information. 2022 [cited 2023 June]. Available from: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/6de8f71b-d3af-4f76-9600-907c98616be6/6de8f71b-d3af-4f76-9600-907c98616be6_viewable_rendition__v.pdf.
AstraZeneca AB. LOKELMA. Summary of product characteristics. 2023 [cited 2023 June]. Available from: https://www.ema.europa.eu/en/documents/product-information/lokelma-epar-product-information_en.pdf.
Zhou Y, Hu P, Huang Y, Nuoer S, Song K, Wang H, et al. Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative HR7056 in Chinese healthy subjects. Front Pharmacol. 2018;9:1316.
Allen RJ, Rieger TR, Musante CJ. Efficient generation and selection of virtual populations in quantitative systems pharmacology models. CPT Pharmacometrics Syst Pharmacol. 2016;5(3):140–6.
Acknowledgements
Medical writing support was provided by Shaun W. Foley, BSc (Hons), and editorial support was provided by Jess Galbraith, BSc, of Core (a division of Prime, London, UK), supported by AstraZeneca according to Good Publication Practice guidelines (Link).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
AstraZeneca sponsored the underlying clinical studies and this analysis.
Conflict of interest
RCP, MÅ, DWB, MN: are employees of, and hold stock in, AstraZeneca.
Availability of data and material
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca’s data sharing policy, described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. AstraZeneca Group of Companies allows researchers to submit a request to access anonymized patient level clinical data, aggregate clinical or genomics data (when available), and anonymized clinical study reports through the Vivli web-based data request platform.
Ethics approval
All studies were conducted in accordance with the ethical principles set forth in the Declaration of Helsinki, the International Conference on Harmonisation guideline regarding Good Clinical Practice (E6 Consolidated Guidance, April 1996), and the ethical requirements referred to in the European Union Directive 2001/20/EC. The institutional review boards or ethics committees of the study sites approved the study protocols. Informed consent was obtained from all individual participants included in the studies.
Consent to participate
Informed consent was obtained from all individual participants included in the studies.
Consent for publication
Not applicable.
Code availability
The representative NONMEM control stream can be made available by contacting the corresponding author.
Author contributions
Study conception and design: RCP, MÅ, DWB, MN. Material preparation: RCP, MÅ. Data collection and statistical analysis: RCP, MÅ. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Penland, R.C., Åstrand, M., Boulton, D.W. et al. Population Pharmacodynamic Dose–Response Analysis of Serum Potassium Following Dosing with Sodium Zirconium Cyclosilicate. Clin Pharmacokinet 63, 551–560 (2024). https://doi.org/10.1007/s40262-024-01360-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-024-01360-9