Abstract
Background
Ulotaront is a novel psychotropic agent with agonist activity at trace amine-associated receptor 1 (TAAR1) and 5-hydroxytryptamine type 1A (5-HT1A) receptors in phase III clinical development for the treatment of schizophrenia.
Objective
This study aimed to investigate the effect of paroxetine, a strong cytochrome P450 (CYP) 2D6 inhibitor, on ulotaront pharmacokinetics (PK) in healthy volunteers.
Methods
Subjects received a single oral dose of 25 mg ulotaront on Day 1 and an oral dose of 20 mg paroxetine once daily from Days 5 to 10 to achieve steady-state plasma paroxetine levels. On Day 11, subjects received another single oral dose of 25 mg ulotaront, with continued daily oral dosing of 20 mg paroxetine from Days 11 to 14. All 24 subjects were CYP2D6 normal metabolizers.
Results
Coadministration of paroxetine increased ulotaront maximum observed plasma concentration (Cmax) and area under the plasma concentration-time curve from time zero to infinity (AUC∞) by 31% and 72%, respectively, and decreased ulotaront apparent clearance (CL/F) by approximately 42%. While coadministration of paroxetine increased AUC∞ of active but minor metabolite SEP-363854 by 32%, it had no effect on SEP-363854 Cmax, or on SEP-363854 to the ulotaront AUC from time zero to the last quantifiable concentration (AUClast) ratio. Based on the acceptable adverse event profile of ulotaront across previous phase II studies, the increase in ulotaront exposure is unlikely to be clinically meaningful.
Conclusions
Weak drug–drug interactions were observed between ulotaront and the strong CYP2D6 inhibitor paroxetine; however, dose adjustment as a precondition when ulotaront is coadministered with strong CYP2D6 inhibitors or administered to CYP2D6 poor metabolizers should not be necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Strong inhibitors of cytochrome P450 (CYP) 2D6 pheno-convert CYP2D6 normal metabolizers to poor metabolizers and thereby increase ulotaront plasma exposure. |
However, based on an acceptable adverse event profile of ulotaront across previous phase I and II studies, the increase in ulotaront exposure is unlikely to be clinically meaningful. |
Consequently, dose adjustment is not warranted when ulotaront is coadministered with strong CYP2D6 inhibitors or when administered to CYP2D6 poor metabolizers. |
1 Introduction
Ulotaront (SEP-363856) is a trace amine-associated receptor 1 (TAAR1) agonist with 5-hydroxytryptamine type 1A (5-HT1A) receptor agonist activity in phase III clinical development as a novel treatment for patients with schizophrenia [1,2,3,4,5,6,7,8,9,10,11,12]. Unlike other antipsychotic agents, ulotaront does not mediate its effects via blockade or direct binding of D2 or 5-HT2A receptors [1, 2]. Ulotaront has demonstrated broad efficacy in rodent models relating to aspects of schizophrenia, including phencyclidine (PCP)-induced hyperactivity, prepulse inhibition, and PCP-induced deficits in social interaction [1].
In a randomized, double-blind, placebo-controlled, 4-week, phase II clinical trial enrolling 245 patients with an acute exacerbation of schizophrenia, ulotaront demonstrated a statistically significant improvement in change from baseline to Week 4 in the Positive and Negative Syndrome Scale (PANSS) total score compared with placebo [3]. The incidence of adverse events (AEs) was generally similar between ulotaront treatment and the placebo groups, with a difference of 2.5% or less for each event. In addition, in a 26-week, open-label extension study for subjects who completed the initial 4-week, double-blind study, ulotaront was shown to be well tolerated and demonstrated continued effectiveness in the long-term treatment of patients with schizophrenia [4]. Ulotaront was granted Breakthrough Therapy Designation by the US FDA for the treatment of patients with schizophrenia and is currently in phase III clinical trials in this patient population.
A population pharmacokinetic (PK) analysis was conducted [5] using data from seven phase I and two phase II studies, which included 404 subjects (99 were healthy volunteers and 305 were patients with schizophrenia). Ulotaront is absorbed quickly, with a median time to reach maximum observed plasma concentration (tmax) of 2.8 h (90% confidence interval [CI] 1–6.2 h) and cleared quickly from systemic circulation, with a median effective half-life of 7 h (90% CI 4.4–11.4 h) and an accumulation ratio of 1.10 (90% CI 1.02–1.30) after daily dosing to steady-state. Ulotaront exhibits dose proportionality in doses ranging from 10 to 100 mg.
Metabolite SEP-363854 was identified in preclinical and clinical studies [6, 13, 14]. SEP-363854 was identified as an abundant in preclinical studies but as a minor metabolite in humans (< 2% of total). In vitro receptor binding studies indicated that SEP-363854 does bind to TAAR1. Based on the effective half-life for the metabolite (approximately 6 h, unpublished data), there is no concern regarding metabolite accumulation upon repeated daily dosing of ulotaront.
Clinical studies indicate ulotaront is primarily eliminated via hepatic clearance, with renal clearance contributing approximately 15% of the total clearance [13]. Metabolism of ulotaront was evaluated in vitro using recombinant human cytochrome P450 (CYP) and flavin-containing monooxygenase (FMO) enzymes, human liver microsomes, and human hepatocytes. In vitro studies indicate that the metabolism of ulotaront is mainly by CYP2D6, although some metabolism is mediated by other nicotinamide adenine dinucleotide phosphate (NADPH)-dependent and NADPH-independent enzymes. Since ulotaront was relatively stable when incubated with human liver microsomes and human hepatocytes, the contribution of CYP2D6 to ulotaront metabolism could not be accurately assigned from in vitro studies. Population PK analysis of pooled data from CYP2D6 poor metabolizers (PMs; n = 12) and non-PMs (n = 416) show that while the distribution of apparent clearance (CL/F) values in PMs falls within the range of CL/F values for non-PMs, the mean CL/F from PMs was generally lower in comparison with the mean CL/F from non-PMs [5]. The fraction of ulotaront metabolism (fm) via CYP2D6 was estimated to be 0.53 [13], and physiologically based PK (PBPK) simulation predicted that CYP2D6-mediated drug–drug interaction (DDI) potential with ulotaront as the victim of a strong CYP2D6 inhibitor was possible (i.e., AUC ratio > 1.25, unpublished data). Therefore, the impact of CYP2D6 inhibition on ulotaront PK was investigated in a clinical trial.
Paroxetine is a strong inhibitor of CYP2D6 and is commonly used as a standard inhibitor for CYP2D6-mediated DDI studies [15, 16]. Inhibition of CYP2D6 by a strong CYP2D6 inhibitor pheno-converts CYP2D6 extensive metabolizers into PMs [17,18,19,20,21]. Therefore, paroxetine was selected for the clinical DDI study.
This study was designed to determine the effect of CYP2D6 inhibition on the PK of ulotaront. Results of this study would also provide information to accurately assign the contribution of CYP2D6 to ulotaront elimination and to evaluate the impact of CYP2D6 polymorphism on the clinical use of ulotaront in the treatment of schizophrenia.
2 Methods
2.1 Ethics
The clinical study described herein and the informed consent form was approved by an Institutional Review Board (IRB) at the investigational site before enrollment of any subjects into the study and was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and with the ethical principles of the Declaration of Helsinki. This study was conducted at a single site located in Daytona Beach, FL, USA (Covance).
2.2 Subjects
Eligible subjects were males or females aged 18–50 years; a body mass index of 18.0–32.0 kg/m2; normal or intermediate CYP2D6 metabolizers based on genetic testing; and in generally good health based on screening physical examination, medical history, 12-lead electrocardiogram (ECG), and clinical laboratory evaluations. Exclusion criteria included a history or current clinical manifestation of any clinically significant disorder or drug allergies; pregnant or lactating females; history of surgery potentially altering absorption and/or excretion; any prescription medications/products other than nonhormonal intrauterine devices within 14 days prior to check-in; or any medications/products known to alter drug absorption, metabolism, or elimination processes, including St. John’s Wort, within 35 days prior to dosing.
2.3 Study Design
This was a phase I, open-label, fixed sequence study to investigate the effect of paroxetine administration on the PK of ulotaront in healthy male and female subjects. Ulotaront exhibits dose-proportional PK in the dose range of 10–100 mg which covers its therapeutic dose range from 25 to 100 mg, and plasma exposure to ulotaront does not significantly accumulate after repeated daily dosing [3]. Therefore, to allow for the possibility of a significant increase in ulotaront plasma exposure under DDI conditions, single doses of 25 mg were selected for the study, with the first dose to be administered in the absence of paroxetine and the second dose in the presence of paroxetine. Twenty-four subjects were dosed to ensure that 20 subjects completed the study. The number of subjects planned provided 90% power to reject the null hypothesis that the ratio of test mean to reference mean was below 0.80 or above 1.25 for the primary endpoints, assuming that the expected ratio of the mean was within 5%, and the standard deviation (SD) difference (log scale) was 0.212, with one-sided alpha at the 5% level.
Subjects who discontinued the study or withdrew were not replaced. Subjects received a single dose of 25 mg ulotaront on Day 1 followed by paroxetine 20 mg once daily on Days 5–10 to achieve steady-state plasma paroxetine levels. On Day 11, subjects received a second single oral dose of 25 mg ulotaront with an oral dose of 20 mg paroxetine followed by 20 mg paroxetine once daily on Days 12–14. Subjects received ulotaront in the morning after a minimum 8-h overnight fast, and fasted for an additional 4 h post-dose of ulotaront.
2.4 Bioanalytical Methods and Assay Performance
Two validated curve-range bioanalytical methods with a liquid chromatography-tandem mass spectrometry (LC-MS/MS) were used for determination of ulotaront and its N-desmethyl metabolite SEP-363854 concentrations in plasma. The initial bioanalytical method with a lower limit of quantification (LLOQ) of 0.0200 ng/mL and a calibration range from 0.02 to 20 ng/mL for both ulotaront and SEP-363854 (using 200 μL plasma) was validated [6]; an extended curve range from 0.25 to 250 ng/mL (using 100 μL plasma) was then also validated to avoid a large portion of sample dilution needed in supporting clinical studies. During the PK sample measurement for this DDI study, samples were first analyzed by the high curve range method and those samples < 0.25 ng/mL were repeated using the low curve-range method. Thus, the LLOQ for this study was 0.02 ng/mL for both ulotaront and the metabolite. Overall, 19 analytical runs were processed, and the assay precision and accuracy of quality control (QC) samples were found to be 2.6–5.5% coefficient of variation (CV) and − 6.9 to 3.0% relative error (RE) for ulotaront, and 3.1–7.4% CV and − 8.8% to + 0.7% RE for the metabolite SEP-363854, respectively. To further confirm the assay robustness, about 10% of the total analyzed samples were selected for incurred sample reanalysis (ISR) reproducibility evaluation. The ISR results for both analytes had a 98% pass rate (within 20% difference). Both the inter-run precision and accuracy data and the ISR results demonstrated excellent assay performance.
For the period of dosing with paroxetine, plasma paroxetine concentrations were measured by a Covance-owned liquid-liquid extraction coupled with the LC-MS/MS method (paroxetine-d6 as IS) with an LLOQ of 0.0500 ng/mL and a validated curve range of 0.05–50 ng/mL using 100 μL of human plasma. Across 13 sample analysis runs for this study, precision and accuracy of QC samples were 1.6–5.4% CV and − 2.1% to 0.7% RE (i.e. 97.1–100.7% accuracy), respectively. The ISR results from about 10% of the total samples showed 100% meeting the acceptance criterion (within 20% difference), also indicating solid assay reproducibility.
2.5 Pharmacokinetic Analysis
Blood samples for determination of plasma ulotaront and SEP-363854 concentrations were collected on two series starting from Day 1 (to Day 5) and Day 11 (to Day 15) at predose (before study drug administration), as well at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 54, 72, 78, and 96 h post-dose. Day 1 series of 96 h post-dose and Day 11 series were also analyzed for plasma paroxetine concentrations. Trough blood samples from Day 6 to Day 10 at predose (before paroxetine administration) were also collected for determination of plasma paroxetine concentrations.
The PK parameters were determined by noncompartmental methods using Phoenix WinNonlin® version 8.1 (Certara USA, Inc., Princeton, NJ, USA) based on the individual plasma concentration-time data and actual sample collection times. The primary endpoints were area under the plasma concentration-time curve from time zero to infinity (AUC∞) and maximum observed plasma concentration (Cmax) for ulotaront on Day 1 (ulotaront alone) and Day 11 (ulotaront plus paroxetine). All other PK parameters were regarded as secondary or exploratory.
2.6 Pharmacogenomic Assessments
Subjects had a blood sample collected at screening for genetic polymorphism assessment of CYP2D6 using Luminex Platform (xTAG Luminex CYP2D6 IVD assay) by Covance Central Laboratory Services (Indianapolis, IN, USA). This genotyping assay solely detects variants *1(WT),*2, *3, *4, *5, *7, *8, *9, *10, *11, *15, *17, *29, *35, and *41. Definitions of normal metabolizer (NM), intermediate metabolizer, and PM follow Clinical Pharmacogenetics Implementation Consortium [22]. The genotyping and phenotype results for the subjects enrolled in this study are provided in electronic supplementary material [ESM] Table 1.
2.7 Statistical Analysis
For the primary analyses, only subjects with data for the given parameter for both treatments (ulotaront alone and ulotaront and paroxetine) were included. The primary analyses were conducted using a linear mixed-effects model, with the natural log-transformed PK parameter as the dependent variable, treatment as the fixed effect, and subject as a random effect. From this analysis, the point estimates and the corresponding two-sided 90% CIs and the treatment differences between ulotaront plus paroxetine and ulotaront alone, were calculated. These log-transformed results were back-transformed to the original scale by the exponentiation to obtain point estimates and the corresponding two-sided 90% CIs for the ratio of the geometric means of the primary PK parameters between the treatments of ulotaront plus paroxetine and ulotaront alone. All analyses were conducted using SAS version 9.4 or higher (SAS Institute, Inc., Cary, NC, USA).
2.8 Safety Assessments
Safety and tolerability were assessed by clinical laboratory tests, vital signs, ECGs, physical examinations, Columbia-Suicide Severity Rating Scale, and monitoring of AEs. AEs were monitored throughout the study at all visits. Clinical laboratory tests, vital signs, 12-lead ECGs, physical examination, and the Columbia-Suicide Severity Rating Scale were conducted throughout the study period. System organ class and preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA, version 22.0) were used.
2.9 Population Pharmacokinetic Analysis
A population PK analysis [5] was conducted using pooled data from ulotaront phase I and II studies. The ulotaront population PK dataset was comprised of 404 subjects contributing a total of 4149 plasma ulotaront concentrations. Of the 404 subjects, 99 were healthy volunteers and 305 were patients with schizophrenia. For this analysis population, a two-compartment model with first-order absorption adequately described the data. Ulotaront CL/F values for subjects observed in ulotaront clinical studies were determined by the population PK model.
3 Results
3.1 Subject Disposition and Demographics
Twenty-four subjects were enrolled, of whom 23 (95.8%) completed the study. All 24 subjects received a single dose of 25 mg ulotaront on study Day 1 and Day 11. Twenty-three subjects received daily doses of 20 mg paroxetine from study Day 5 through Day 14 (a total of 10 doses). One (4.2%) subject withdrew for family reasons, terminated from the study early (approximately 51 h after the Day 11 dose) and did not receive 20 mg paroxetine on Day 14 (total of 9 doses). This subject still had quantifiable concentrations at the time of termination; however, the contribution of missing data to the overall AUC was considered to be minimal, and no PK parameters were excluded from descriptive statistics.
The mean age of the study population was 36.0 years (range 19–50 years). Nineteen (79.2%) subjects were male and 5 (20.8%) subjects were female. Mean body weight at baseline was 76.10 kg (range 55.7–101.1 kg). Sixteen (66.7%) subjects were White, 7 (29.2%) subjects were Black or African American, and 1 (4.2%) subject was multiracial (Asian, White). Pharmacogenomic results for CYP2D6 indicated all subjects were NMs (refer to ESM Table 1).
3.2 Pharmacokinetics
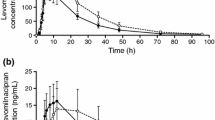
The arithmetic mean (± SD) plasma concentration-time profiles of ulotaront and the minor metabolite SEP-363854 following treatment with ulotaront alone and with paroxetine are presented in linear and semi-logarithmic scales in Fig. 1. Coadministration of ulotaront with paroxetine increased exposure in ulotaront and SEP-363854 when compared with ulotaront administered alone.
Mean plasma concentration-time profiles. a Ulotaront plasma concentration in linear scale; b ulotaront concentration in semi-log scale; c SEP-363854 concentration in linear scale; d SEP-363854 concentration in semi-log scale. Closed and open circles show subjects received ulotaront alone and with paroxetine coadministration, respectively. Error bars represent standard deviation.
A summary of the PK parameters and the associated statistical analyses of ulotaront following treatment with ulotaront alone and with paroxetine are presented in Table 1. Coadministration of paroxetine increased ulotaront geometric mean Cmax from 87.9 to 115 ng/mL, and AUC∞ from 731 to 1259 h*ng/mL compared with ulotaront alone. The geometric mean ratios (90% CI) of ulotaront with paroxetine to ulotaront alone were 131% (126–137%) and 172% (164–181%) for Cmax and AUC∞, respectively. Median tmax value was 2.00 h for both treatments, with similar individual ranges (1.00–4.00 h for ulotaront alone and 1.00–3.00 h for ulotaront with paroxetine). The apparent terminal half-life was slightly longer following treatment of ulotaront with paroxetine compared with ulotaront alone, with respective geometric mean values of 9.80 and 8.95 h, but would still be expected to demonstrate minimal accumulation of ulotaront after dosing to steady state.
Coadministration of paroxetine reduced ulotaront geometric mean CL/F by approximately 42% (Table 1, Fig. 3). In addition, the observed ulotaront CL/F values of the 24 subjects with paroxetine coadministration showed similar distribution to the estimated ulotaront CL/F values of CYP2D6 PMs of the population PK dataset. The estimated ulotaront CL/F values of PMs distribute within the range of non-PMs in the current population PK dataset.
Regarding the active but minor metabolite SEP-363854, a summary of the PK parameters and the associated statistical analyses of SEP-363854 following treatment with SEP-363856 alone and with paroxetine are presented in Table 2. Paroxetine had no effect on metabolite SEP-363854 Cmax and tmax but increased the AUC∞. The geometric mean ratios (90% CI) of SEP-363856 with paroxetine versus without paroxetine were 92.7% (87.3–98.4) and 132% (124–140) for Cmax and AUC∞, respectively. The median tmax value was 3.00 h for both treatments. Coadministration of paroxetine increased the apparent elimination half-life from 5.96 to 9.26 h, but had no effect on the SEP-363854 to ulotaront AUC from time zero to the last quantifiable concentration (AUClast) ratio. The mean ratio was only about 0.02 with or without paroxetine coadministration.
Paroxetine mean plasma trough concentration at steady-state on Day 11 was 19.3 ng/mL, and the mean plasma Cmax and AUC24 on Day 11 were 37.3 ng/mL and 647 h*ng/mL, respectively. The mean plasma paroxetine concentrations at 6 h post-dose on Days 13 and 14 were 45.2 and 44.8 ng/mL, respectively (Fig. 2).
3.3 Safety
Ulotaront was generally well tolerated when administered as a single dose of 25 mg with and without coadministration of 20 mg paroxetine. There were no deaths, serious AEs, or AEs leading to discontinuation during the study. There were no severe AEs, nor were there any clinically meaningful changes in laboratory values, vital sign measurements, or ECG findings.
AEs occurring in two or more subjects overall are summarized in Table 3. Most AEs were mild in severity. Two (8.3%) subjects experienced moderate AEs after receiving ulotaront alone on Day 1. One subject experienced nausea 3 h after receiving 25 mg ulotaront, which was assessed as probably related to study treatment. A second subject experienced presyncope and syncope (vasovagal reaction and vasovagal syncope due to venipuncture, respectively) 3 h after receiving 25 mg ulotaront assessed as not related to study treatment.
4 Discussion
The primary objective of this study was to evaluate the impact of a concomitantly administered strong CYP2D6 inhibitor on ulotaront PK in healthy volunteers with CYP2D6 NMs. Strong CYP2D6 inhibitors are reported to pheno-covert CYP2D6 NMs into PMs [20, 21]. The observed paroxetine concentration profiles in this study were consistent with those reported in the literature and were considered to be sufficient for complete CYP2D6 inhibition [23,24,25] and CYP2D6 pheno-conversion from NMs to PMs [17,18,19].
The geometric mean ratios for Cmax and AUC∞ for ulotaront with coadministered paroxetine compared with ulotaront alone were 131% (90% CI 126–137%) and 172% (90% CI 164–181%), respectively (Table 1). As expected, CYP2D6 inhibition by paroxetine decreased ulotaront CL/F to a level approximating ulotaront CL/F in CYP2D6 PMs (Fig. 3). However, the observed ulotaront CL/F values of the 24 subjects with paroxetine distributed within the range of the estimated ulotaront CL/F values of non-PMs in the current population PK dataset. In addition, the estimated ulotaront CL/F values of PMs overlap the estimated ulotaront CL/F values of non-PMs. These results suggest that dose adjustment is likely not necessary when ulotaront is prescribed with strong CYP2D6 inhibitors or to PMs. Since ulotaront shows dose-proportional PK in the dose range of 10–100 mg based on population PK modeling [5], the results from this DDI study using 25 mg ulotaront can be generalized to include the dose range of 50–100 mg being tested in phase III [26].
Ulotaront CL/F among subjects from phase I and II studies (pooled data). The first and second columns are CL/F values from this study as calculated by non-compartment PK analysis for the CYP2D6 NMs (n = 24) without paroxetine and with paroxetine, respectively. The third and fourth columns are CL/F for CYP2D6 PMs (n = 12) and non-PMs (n = 416) in the current population PK database. CL/F apparent clearance, CYP cytochrome P450, NMs normal metabolizers, PK pharmacokinetics, PMs poor metabolizers
In this study, ulotaront was generally well tolerated in healthy subjects, either when administered alone or in combination with paroxetine. The most commonly reported AEs (dizziness, nausea, somnolence, and orthostatic hypotension) from pooled phase I studies of ulotaront were generally mild, monitorable, and reversible. The common AEs were generally most frequent at times prior to or near tmax, suggesting Cmax as a more relevant exposure metric than AUC for predicting the AE risk (data from previous studies, not reported). Cmax showed a smaller increase in exposure (1.31-fold) than AUC (1.72-fold) in the presence of paroxetine. Therefore, the relatively small increase of ulotaront exposure in the presence of paroxetine is not considered clinically meaningful and is not expected to require dose adjustment, although further investigation will be conducted when the safety results of ongoing phase III studies are available. In a 4-week phase II clinical trial enrolling 245 patients with schizophrenia, the incidence of AEs was generally similar between the ulotaront treatment group and the placebo group, with a difference of < 2.5% for each event [3]. In addition, a 26-week, open-label extension, phase II study demonstrated that ulotaront was well tolerated and effective in the long-term treatment of patients with schizophrenia [4]. Taken together, these results suggest that no dose adjustment of ulotaront is needed when coadministered with strong CYP2D6 inhibitors or when ulotaront is prescribed to CYP2D6 PMs. Dose adjustment for ulotaront should instead be based on patient tolerability if adverse effects are observed.
References
Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, et al. SEP-363856, a novel psychotropic agent with a unique, non-D(2) receptor mechanism of action. J Pharmacol Exp Ther. 2019;371(1):1–14.
Dedic N, Dworak H, Zeni C, Rutigliano G, Howes OD. Therapeutic potential of TAAR1 agonists in schizophrenia: evidence from preclinical models and clinical studies. Int J Mol Sci. 2021;22(24):13185.
Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, et al. A non-D2-receptor-binding drug for the treatment of schizophrenia. N Engl J Med. 2020;382(16):1497–506.
Correll CU, Koblan KS, Hopkins SC, Kent J, Cheng H, Goldman R, et al. Safety and effectiveness of SEP-363856 in schizophrenia: results of a 6-month, open-label extension study. CNS Spectr. 2021;26(2):148–9.
Galluppi GR, Polhamus DG, Fisher JM, Hopkins SC, Koblan KS. Population pharmacokinetic analysis of ulotaront in subjects with schizophrenia. CPT Pharmacometr Syst Pharmacol. 2021;10(10):1245–54.
Chen YL, Shi Y, LaFayette A, Shi L, Koblan KS, Galluppi GR. A sensitive LC-MS/MS method for simultaneous quantification of ulotaront and its N-desmethyl metabolite in human plasma and application to a clinical study. J Pharm Biomed Anal. 2022;207: 114404.
Hopkins SC, Dedic N, Koblan KS. Effect of TAAR1/5-HT(1A) agonist SEP-363856 on REM sleep in humans. Transl Psychiatry. 2021;11(1):228.
Kokkinou M, Irvine EE, Bonsall DR, Natesan S, Wells LA, Smith M, et al. Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: a translational imaging study with ketamine. Mol Psychiatry. 2021;26(6):2562–76.
Begni V, Sanson A, Luoni A, Sensini F, Grayson B, Munni S, et al. Towards novel treatments for schizophrenia: molecular and behavioural signatures of the psychotropic agent SEP-363856. Int J Mol Sci. 2021;22(8):4119.
Hopkins SC, Ogirala A, Worden M, Koblan KS. Depicting safety profile of TAAR1 agonist ulotaront relative to reactions anticipated for a dopamine D2-based pharmacological class in FAERS. Clin Drug Investig. 2021;41(12):1067–73.
Heffernan MLR, Herman LW, Brown S, Jones PG, Shao L, Hewitt MC, et al. Ulotaront: a TAAR1 agonist for the treatment of schizophrenia. ACS Med Chem Lett. 2022;13(1):92–8.
Synan C, Bowen C, Heal DJ, Froger-Colléaux C, Beardsley PM, Dedic N, et al. Ulotaront, a novel TAAR1 agonist with 5-HT1A agonist activity, lacks abuse liability and attenuates cocaine cue-induced relapse in rats. Drug Alcohol Depend. 2022;231: 109261.
Xiao G, Chen YL, Dedic N, Xie L, Koblan KS, Galluppi GR. In vitro ADME and preclinical pharmacokinetics of ulotaront, a TAAR1/5-HT(1A) receptor agonist for the treatment of schizophrenia. Pharm Res. 2022;39(5):837–50.
Chen YL, Tsukada H, Milanovic S, Shi L, Li Y, Mao Y, et al. Comparative bioequivalence of tablet and capsule formulations of ulotaront and the effect of food on the pharmacokinetics of the tablet form in humans. Neurol Ther. 2023;12(3):815–32.
Briciu C, Neag M, Muntean D, Vlase L, Bocsan C, Buzoianu A, et al. A pharmacokinetic drug interaction study between nebivolol and paroxetine in healthy volunteers. J Clin Pharm Ther. 2014;39(5):535–40.
Hemeryck A, Lefebvre RA, De Vriendt C, Belpaire FM. Paroxetine affects metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther. 2000;67(3):283–91.
Laine K, Tybring G, Härtter S, Andersson K, Svensson JO, Widén J, et al. Inhibition of cytochrome P4502D6 activity with paroxetine normalizes the ultrarapid metabolizer phenotype as measured by nortriptyline pharmacokinetics and the debrisoquin test. Clin Pharmacol Ther. 2001;70(4):327–35.
Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9(5):442–73.
Zourková A, Hadasová E. Paroxetine-induced conversion of cytochrome P450 2D6 phenotype and occurence of adverse effects. Gen Physiol Biophys. 2003;22(1):103–13.
Eli Lilly and Company. Atomoxetine [prescribing information]. Indianapolis: Eli Lilly and Company; 2022.
Pharmacia & Upjohn Company LLC. Tolterodine tartrate [prescribing information]. New York: Pharmacia & Upjohn Company LLC; 2012.
Caudle KE, Sangkuhl K, Whirl-Carrillo M, Swen JJ, Haidar CE, Klein TE, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci. 2020;13(1):116–24.
Bahar MA, Kamp J, Borgsteede SD, Hak E, Wilffert B. The impact of CYP2D6 mediated drug-drug interaction: a systematic review on a combination of metoprolol and paroxetine/fluoxetine. Br J Clin Pharmacol. 2018;84(12):2704–15.
Mikami A, Ohtani H, Hori S, Sawada Y. Pharmacokinetic model incorporating mechanism-based inactivation of CYP2D6 can explain both non-linear kinetics and drug interactions of paroxetine. Int J Clin Pharmacol Ther. 2013;51(5):374–82.
Martin DE, Zussman BD, Everitt DE, Benincosa LJ, Etheredge RC, Jorkasky DK. Paroxetine does not affect the cardiac safety and pharmacokinetics of terfenadine in healthy adult men. J Clin Psychopharmacol. 1997;17(6):451–9.
US FDA. Clinical drug interaction studies: cytochrome P450 enzyme- and transporter-mediated drug interactions: guidance for industry. 2020 [cited 1 May 2023]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions.
Acknowledgements
Editorial assistance to prepare this manuscript for publication was provided by Stephen Bublitz, ELS, of PharmaWrite, LLC (Princeton, NJ, USA), and was funded by Sumitomo Pharma America, Inc. (formerly Sunovion Pharmaceuticals Inc.). Sunovion discovered ulotaront in collaboration with PsychoGenics, based in part on a mechanism-independent approach using the in vivo phenotypic SmartCube® platform and associated artificial intelligence algorithms. We thank all the subjects, investigators, and site staff for participating in this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was fully funded by Sunovion Pharmaceuticals Inc.
Conflict of interest
Hironobu Tsukada is an employee of Sumitomo Pharma Co., Ltd. Yu-Luan Chen, Guangqing Xiao, Lisa Lennek, Snezana M. Milanovic, MaryAlice Worden, Yu-Yuan Chiu, Seth C. Hopkins, and Gerald R. Galluppi are employees of Sumitomo Pharma America. Daniel G. Polhamus is an employee of Metrum Research Group and was working under a contract from Sunovion Pharmaceuticals, Inc.
Availability of data and material
Available from the corresponding author on reasonable request.
Ethics approval
The study protocol, including the final version of the subject informed consent form, was approved by the following IRB before enrollment of any subjects into the study: Midlands IRB, 8417 Santa Fe Drive, Suite 100, Overland Park, KS 66212, USA. This study was conducted in accordance with the protocol, International Council for Harmonisation (ICH) Good Clinical Practice, ICH guidelines, and the ethical principles that had their origin in the Declaration of Helsinki. The study was conducted in accordance with applicable local law(s) and regulation(s).
Consent
All subjects signed the informed consent form before any study procedures were performed.
Author contributions
HT, Y-LC, GX, LL, SMM, MAW, Y-YC, SCH, and GRG contributed to the study design, and participated in the conduct of the study and data collection. All authors analyzed the data, wrote the manuscript, and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tsukada, H., Chen, YL., Xiao, G. et al. A Phase I, Open-Label, Fixed Sequence Study to Investigate the Effect of Cytochrome P450 2D6 Inhibition on the Pharmacokinetics of Ulotaront in Healthy Subjects. Clin Pharmacokinet 62, 1755–1763 (2023). https://doi.org/10.1007/s40262-023-01317-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01317-4