Abstract
Background and Objectives
Isavuconazole is a broad-spectrum antifungal agent for the management of invasive fungal disease. Optimised drug exposure is critical for patient outcomes, specifically in the critically ill population. Solid information on isavuconazole pharmacokinetics including protein binding in patients in the intensive care unit is scarce. We aimed to describe the total and unbound isavuconazole pharmacokinetics and subsequently propose a dosage optimisation strategy.
Methods
A prospective multi-centre study in adult intensive care unit patients receiving isavuconazole was performed. Blood samples were collected on eight timepoints over one dosing interval between days 3–7 of treatment and optionally on one timepoint after discontinuation. Total and unbound isavuconazole pharmacokinetics were analysed by means of population pharmacokinetic modelling using NONMEM. The final model was used to perform simulations to assess exposure described by the area under the concentration–time curve and propose an adaptive dosing approach.
Results
Population pharmacokinetics of total and unbound isavuconazole were best described by an allometrically scaled two-compartment model with a saturable protein-binding model and interindividual variability on clearance and the maximum binding capacity. The median (range) isavuconazole unbound fraction was 1.65% (0.83–3.25%). After standard dosing, only 35.8% of simulated patients reached a total isavuconazole area under the concentration–time curve > 60 mg·h/L at day 14. The proposed adaptive dosing strategy resulted in an increase to 62.3% of patients at adequate steady-state exposure.
Conclusions
In critically ill patients, total isavuconazole exposure is reduced and protein binding is highly variable. We proposed an adaptive dosing approach to enhance early treatment optimisation in this high-risk population.
Clinical Trial Registration
ClinicalTrials.gov identifier: NCT04777058.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We investigated the population pharmacokinetics of total and unbound isavuconazole in an intensive care unit population. Our findings show low total isavuconazole exposure, warranting dosage optimisation. To aid this, we developed an adaptive dosing strategy. |
Isavuconazole protein binding was highly variable in our critically ill population. Our findings on unbound drug concentrations provide complementary information to the current understanding of isavuconazole pharmacokinetics. |
1 Introduction
Isavuconazole is a broad-spectrum triazole antifungal agent registered for the treatment of invasive aspergillosis and mucormycosis [1, 2]. In the intensive care unit (ICU), the reported mortality rates for these invasive fungal diseases have been substantially high for decades [3, 4]. Over the past years, we have even seen a surge in viral infection-associated pulmonary aspergillosis requiring antifungal treatment [5, 6]. Triazoles are the cornerstone for treatment of invasive aspergillosis, with isavuconazole recommended as a first-line treatment option. The registered dosing regimen consists of a loading dose of isavuconazole 200 mg three times daily on days 1 and 2, followed by a maintenance dose of isavuconazole 200 mg once daily [1]. Compared with other triazoles, isavuconazole shows less drug–drug interactions and apparent fewer side effects [7]. Unfortunately, knowledge on the behaviour of this drug in the critically ill population is limited.
It is commonly recognised that critically ill patients demonstrate altered and highly variable pharmacokinetics, challenging adequate drug exposure to ensure optimal clinical efficacy and minimal toxicity [8]. Organ dysfunction, capillary leakage, and fluid redistribution in the ICU population are likely to impact isavuconazole pharmacokinetics, as it is an extensively distributed drug with an elimination half-life of 100–130 h in the general population [8, 9]. Isavuconazole is a highly protein-bound drug, mainly to plasma albumin, with reported protein binding of 99.2–99.4% in healthy volunteers [9]. To date, information on isavuconazole protein binding in critically ill patients is absent. In the ICU population, changes in protein binding are common and the occurrence of hypoalbuminemia is frequent [10]. At high concentrations, bilirubin and urea can be physiological inhibitors of drug protein binding [11]. As only the unbound fraction of a drug is available for metabolism, clearance, and distribution to the target site, changes in protein binding may cause changes in pharmacokinetics that require subsequent adjustments in dosing. The current one-dose-fits-all dosing strategy may, therefore, not be suitable for the ICU population.
International guidelines recommend to perform therapeutic drug monitoring (TDM) of isavuconazole under selected clinical conditions. In the absence of a definite target concentration, the average trough concentration (Cmin) of 2–4 mg/L in individuals who responded to therapy in the phase II/III clinical studies is used [12]. Further to this, area under the concentration–time curves (AUCs) of 60 and 233 mg·h/L are considered as these reflect the 25th percentile of patients responding to treatment and the minimum exposure observed in patients with increased toxicity in these studies, respectively [13, 14]. In a retrospective evaluation of total isavuconazole plasma concentrations in an ICU population, the aforementioned threshold was frequently not attained, warranting additional information on isavuconazole pharmacokinetics in this group of patients [15]. Population pharmacokinetic models may aid in the process of model informed precision dosing to improve exposure.
Only with thorough knowledge on the pharmacokinetics of isavuconazole in the critically ill patient population is individualised dosing possible. Therefore, we aim to describe total and unbound isavuconazole pharmacokinetics through population pharmacokinetic modelling following a prospective observational study in critically ill patients and subsequently propose dosage optimisation strategies.
2 Methods
2.1 Study Design and Population
We performed a prospective multi-centre pharmacokinetic study on the intensive care units of the Radboud University Medical Center (Radboudumc) in Nijmegen, the Netherlands and the University Hospitals Leuven in Leuven, Belgium. This study was conducted according to the ethical guidelines of the Declaration of Helsinki and carried out in accordance with the applicable rules concerning the review of research ethics committees and informed consent in the Netherlands (EC identifier: 2021-7327) and Belgium (EC identifier: S65556). All patients or legal representatives were informed about the details of this study and provided informed consent. The study was registered at ClinicalTrials.gov (identifier: NCT04777058).
All adult patients admitted to the ICU who received isavuconazole intravenously as part of their treatment and were managed with a central venous catheter and/or arterial line for blood sampling were eligible for inclusion. Isavuconazole dose and duration of therapy were determined at the discretion of the treating physician. Dosage adjustments following TDM were allowed. Between days 3 and 7 after initiation of intravenous isavuconazole therapy, samples were collected on a single occasion at t = 0 (pre-dose), and t = 0.5, 1, 2, 4, 6, 8 and 12 h after the end of the infusion. An optional additional sample was collected 96–300 h after discontinuation of isavuconazole therapy.
We collected demographic, microbiological and biochemical data from the electronic patient record, including age, sex, total body weight, height, serum urea, serum bilirubin, Acute Physiology And Chronic Health Evaluation II (APACHE II) score and Sequential Organ Failure Assessment (SOFA) score at ICU admission, indication for isavuconazole therapy, identified pathogen, duration of ICU admission and drug dose history. Serum albumin and serum total protein levels were centrally quantitated for one occasion per individual per study day.
2.2 Study and Sampling Design Justification
We performed a stochastic simulation and estimation analysis of 500 virtual pharmacokinetic studies to evaluate the design of this study a priori using a previously developed pharmacokinetic model [16]. We used a real-life database from the ICU department of the Radboudumc with demographic data of 11,472 patients. The abovementioned study design with a sample size of 20 patients was tested with and without the assumption that in half of patients the optional sample after discontinuation could be collected. Both scenarios resulted in an accurate and precise estimation of pharmacokinetic parameters reflected by a relative bias and imprecision of ≤ 15%.
2.3 Bio-Analytical Method
After collection, all samples were centrifuged at 1900g for 5 min at room temperature and stored locally as plasma at − 40 °C or − 80 °C until analysis. Total and unbound isavuconazole concentrations in plasma were centrally quantified using a fully validated, liquid chromatography-tandem mass spectrometry assay. The unbound isavuconazole fraction was obtained by ultrafiltration at 37 °C (1650g for 20 min) using an Amicon® 30K Ultra Centrifugal filter. The assay was validated in the dynamic range of 0.05–10.0 mg/L and 0.001–0.5 mg/L for total and unbound isavuconazole concentrations, respectively. The accuracy range was 95.20–100.22% and 97.80–101.50% for total and unbound isavuconazole concentrations, respectively. Within-day and between-day precision for total isavuconazole concentrations varied between 1.35–3.04% and 0.00–2.44%, respectively. For unbound isavuconazole concentrations, within-day and between-day precision varied between 3.73–8.29% and 7.06–12.41%, respectively.
2.4 Pharmacokinetic Analysis
We performed a population pharmacokinetic analysis of total and unbound isavuconazole concentrations by means of non-linear mixed-effects modelling using the NONMEM® software package (version 7.5.1) with Pirana (version 2.9.9.) as an interface for NONMEM, Perl-Speaks-NONMEM, Xpose and R [17]. The model was parameterised on the unbound concentrations. We considered one-, two- and three-compartment models and allometrically scaled all clearance and volume parameters a priori to a standardised total body weight of 70 kg with allometric exponents of 0.75 and 1 for clearance and volume parameters, respectively. The inter- and intra-individual variability were initially assumed to be log-normally distributed, unless the distribution of etas showed another distribution as visualised by density plots. We used the first-order conditional estimation method throughout model development, with the interaction option in case of a proportional residual error model. Residual variability on total and unbound concentrations was evaluated using additive, proportional, and combined additive and proportional models with and without correlation.
We based the structural model selection and covariate analysis on physiological plausibility, diagnostic goodness-of-fit plots, a successful covariance step and parameter correlation assessment. Additionally, nested models were statistically compared using the change in objective function value. A decrease in objective function value of at least 3.84 for chi-squared distribution with one degree of freedom for a significance level of p < 0.05 relative to the comparative model was considered superior. Non-nested models were compared using the Akaike Information Criterion, where models with a lower Akaike Information Criterion were considered superior. We assessed parameter precision using the Sampling Importance Resampling procedure [18].
For subjects receiving oral isavuconazole administrations prior to intravenous administrations and subsequent study inclusion, we included the absorption rate constant (ka) and oral bioavailability (F) in the model as fixed values based on a previously developed model [19]. Values were fixed because no pharmacokinetic samples were obtained during these pre-study oral administrations. A sensitivity analysis was performed, by running models with values for ka and F that were considered at the maximum of realistic, confirming that this not heavily affected predictions for this individual nor the model in general. For two subjects, information regarding the timing of the infusion on the day of pharmacokinetic sampling was not adequately documented. Therefore, the variability in the duration of infusion was estimated for these individuals and then fixed in subsequent models.
We tested three different protein-binding models to describe the relationship between total and unbound concentrations: a specific (saturable), a non-specific (linear), and a combined specific and non-specific protein-binding model [20, 21]. Details on the protein-binding analysis are provided in the Electronic Supplementary Material. In the covariate analysis, serum albumin, total protein, urea and total bilirubin levels were tested as continuous covariates on the protein-binding parameters.
2.5 Evaluation of Unbound Concentration Prediction
To assess whether the developed model is suitable to predict unbound concentrations from total concentrations, we performed an evaluation analysis using the final model. Empirical Bayes estimates were determined with the final dataset without observations for unbound concentrations to retrieve individual predicted total concentrations. We then calculated the model derived unbound concentrations from the individual predicted total concentrations using the final model.
Additionally, we calculated unbound concentrations from measured total concentrations under the assumption of a constant fixed unbound fraction of 1%, as has been considered in all pharmacokinetic and pharmacodynamic studies with isavuconazole to date [9]. The predictive performance of both methods compared to the actual measured unbound concentrations was determined by evaluation of the mean prediction error (MPE) and the normalised root mean squared error (NRMSE), normalised to the mean of the measured unbound concentrations [22]. We calculated confidence intervals for MPE and NRMSE as proposed by Sheiner and Beal, and Faber, respectively [22, 23]. For both parameters, a value < 20% was considered acceptable.
2.6 Dosage Optimisation
The final population pharmacokinetic model was used to perform Monte Carlo simulations for 5000 virtual patients receiving the standard isavuconazole dose of three times 200 mg per day for 2 days, followed by 200 mg once daily. Unbound and total isavuconazole Cmin and AUC were simulated at days 3, 7 and 14 of therapy reflecting the end of loading doses, the early-phase TDM sample assessment and steady state, respectively. We compared our findings to the currently recommended threshold total isavuconazole Cmin of 2 mg/L and total isavuconazole AUC of 60 mg·h/L [12, 14].
Additionally, we developed an adaptive exposure-driven dosing strategy with the aim to select patients at risk for inadequate steady-state exposure early during treatment. We defined inadequate exposure as a total AUC at steady state below 60 mg·h/L or above 233 mg·h/L, corresponding to the median exposure observed in patients with successful treatment outcome in the SECURE trial [12,13,14]. Additional Monte Carlo simulations were performed similar to described above, but now with assessment of the total isavuconazole Cmin at 24 h after first dosing and the total isavuconazole AUC at steady state (day 14). All simulated trough concentrations at 24 h were plotted against AUC at day 14. Based on these results, we divided patients in groups with adequate and inadequate exposure at day 14. For the group with inadequate exposure at day 14, we adjusted dosages based on their Cmin at 24 h. The selection of the Cmin threshold that prompted dose adjustments was informed by the 95th percentile value of observed Cmin at 24 h in the inadequate exposure group. Dose adjustments were implemented starting from the first maintenance dose onwards under the assumption that results for Cmin are not available within 1 day, i.e. before all loading doses are administered. We then re-assessed the steady-state exposure after dose adjustments to determine the effect of our strategy.
3 Results
Twenty patients were included in the study. One patient with icteric plasma samples was excluded from the analysis, following the observation that extensive bilirubin levels (> 500 µmol/L) were interfering with the quantitation of the isavuconazole unbound concentration (data not shown). This resulted in 19 patients with 152 unbound and 152 total isavuconazole concentrations eligible for analysis. Patient characteristics of those included in the analysis are presented in Table 1. None of the patients was managed by means of extracorporeal membrane oxygenation during ICU admission. Seven individuals received renal replacement therapy as continuous veno-venous hemofiltration or intermittent haemodialysis at the day of sample collection. All patients received the standard isavuconazole dose of 200 mg three times daily for 2 days, followed by a once-daily dose of 200 mg, except for three patients. One patient received 1 day of loading doses instead of 2 days followed by standard maintenance dosing, one patient received one dose of 100 mg instead of 200 mg on day 3 after which therapy was continued with 200 mg again, and one patient received eight 200-mg doses of isavuconazole orally, followed by intravenous administrations in an increased dosage of 300 mg daily from day 6 of therapy onwards. For the latter patient, the pharmacokinetic sampling was performed on day 5 of intravenous isavuconazole therapy and day 10 of total isavuconazole therapy. The median (range) total therapy duration until the first sample collection was 5 (3–10) days. Examination of the patients’ drug history retrieved no drugs with clinically relevant drug–drug interactions for any patient. Measured unbound and total concentrations ranged from 0.012 to 0.131 mg/L and from 0.701 to 7.232 mg/L, respectively. All measured concentrations were above the lower limit of quantification. The median (range) observed unbound fraction of isavuconazole was 1.65% (0.83–3.25%). Isavuconazole unbound fraction versus unbound concentrations are presented in Fig. 1. Isavuconazole unbound fraction increases non-linearly with the unbound concentration.
3.1 Population Pharmacokinetics
Population pharmacokinetics of unbound and total isavuconazole were best described by a linear two-compartmental model with saturable protein binding. The final model included interindividual variability on clearance and maximum binding capacity (Bmax) and a proportional residual error model for both total and unbound concentrations. Box-cox transformation of the interindividual variability on clearance significantly improved the fit of the model. Protein binding visually showed to be saturable and was best described by a protein-binding model with specific binding. As it was not possible to estimate kd separately from Bmax, reflected by high correlation between thetas, kd was fixed to a value of 0.0806 mg/L from a previous study on isavuconazole protein binding in non-critically ill patients (submitted for publication; data on file Radboudumc). It was assumed that for one drug, kd is not likely to vary substantially between patient populations. No significant covariates were identified. Table 2 summarises the parameter estimates with 95% confidence intervals (CIs) of the final model parametrised on unbound concentrations. The final model control stream with final parameter estimates is available via the ESM.
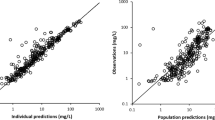
The observed data were overall adequately described by the final model, as illustrated by the goodness-of-fit plots (Fig. S1) and the prediction-corrected visual predictive check plots (Fig. 2). The homogenously distributed conditional weighted residuals over time and predicted concentrations (mainly) within the − 2 to 2 range indicate that there was no major model misspecification for both the unbound and total isavuconazole data. The slight underprediction shown in the observed versus population predicted total concentration plot is mainly driven by a few observations deviating from the line of unity at higher concentrations, indicating that there is still some unexplained variability remaining in the current model.
Prediction-corrected visual predictive check of the final model for unbound and total isavuconazole concentration data for the first 24 h after the dose presented on a linear scale. The observed concentrations are shown as circles. The median and 5th and 95th percentiles of the observed data are shown as solid lines, dashed lines and dashed lines, respectively. Grey shaded areas represent the confidence intervals of the median (dark grey) and 5th and 95th percentiles (light grey) of simulated concentrations (n = 1000) based on the original dataset
3.2 Evaluation of Unbound Concentration Prediction
Results of the evaluation analysis are presented in Fig. 3 and Fig. S2 of the ESM. As our final model included a specific protein-binding model, the model-derived unbound concentrations were calculated using Eq. (1).
where Cunbound is the protein unbound isavuconazole concentration, kd is the equilibrium dissociation constant, Bmax is the maximum binding capacity, and Ctotal is the total isavuconazole concentration.
The model-based method to derive unbound concentrations from total concentrations using our final model was more accurate [MPE (95% CI) 10.5% (2.3–18.6)] than the method assuming a constant unbound fraction of 1% [MPE (95% CI) − 75.5% (-78.5 – -72.4)]. This is also reflected by data points being closer to the line of unity in Fig. S2 of the ESM. The model-based method and calculation method were comparably imprecise, as NRMSE values just slightly differed: NRMSE (95% CI) 57.2% (50.7–63.6) and 57.3% (50.8–63.7), respectively.
3.3 Dosage Optimisation
Figure 4 shows the simulated median concentration–time curve with a 95% CI based on 5000 virtual patients receiving standard isavuconazole dosing until day 7 of therapy. The median population does not reach a total isavuconazole Cmin above 2 mg/L on or after day 3 of therapy following standard dosing. This is also reflected by the simulated Cmin and AUC at days 3, 7 and 14 of therapy shown in Figs. S3 and S4 of the ESM. Of the simulated patients, 64.2% did not reach the total AUC above 60 mg·h/L at day 14.
Median (solid line) simulated concentration–time curve with 90% (dark shaded area) and 95% (light shaded area) confidence intervals based on a simulation of 5000 virtual critically ill patients receiving standard intravenous isavuconazole dosing for 7 days. The dashed horizontal line represents the threshold total isavuconazole trough concentration of 2 mg/L currently recommended by international guidelines [12]
Figure 5 shows the results of our adaptive dosing strategy. Dosing adjustments were made as follows: when Cmin at 24 h after the start of therapy was below 1.0 mg/L, between 1.0 and 1.5 mg/L, or above 5.0 mg/L, daily maintenance doses were adjusted to 400 mg, 300 mg and 100 mg, respectively. Our strategy resulted in an increase of patients at adequate exposure on steady state from 35.8 to 62.3% compared with standard dosing.
Box-and-whisker plots of simulated total isavuconazole area under the concentration–time curve at steady state (AUCss) following standard dosing (left) and dosing according our adaptive exposure-informed dosing strategy (right). Boxes represent the median and 25th and 75th percentiles, whiskers represent the range of maximum and minimum values within 1.5 × the interquartile range. The dashed horizontal line represents the total isavuconazole area under the concentration–time curve of 60 mg·h/L
4 Discussion
We developed a population pharmacokinetic model of total and unbound isavuconazole in an ICU population showing low total exposure and a high variability in protein binding. Our findings warrant dosage optimisation in the critically ill population. To aid this, we developed an adaptive dosing strategy. Moreover, our findings on unbound drug concentrations provide complementary information to the current understanding of isavuconazole pharmacokinetics.
Low isavuconazole exposure in critically ill patients has been previously reported in several retrospective analyses [15, 24]. Only a single analysis describes the population pharmacokinetics of isavuconazole in patients with COVID-19-associated pulmonary aspergillosis admitted to the ICU based on retrospectively collected through concentration data [24]. The authors described the pharmacokinetics using a one-compartment model, which differs from our model structure. This difference may be explained by their trial design using TDM data only. The inclusion of concentrations over a wider time range allowed us to describe the concentration–time course of isavuconazole more precisely. Both the latter study and our study found significant pharmacokinetic variability between patients. In our model, variability on clearance was much larger. This may be the consequence of a more heterogeneous group of patients in our study as inclusion was not limited to patients with COVID-19-associated pulmonary aspergillosis only. Because of the differences in parametrisation, other final parameter estimates could not be compared to our findings. The lower exposure to isavuconazole seen in critically ill patients may potentially explain observations in previous studies. We could hypothesise that results in the ACTIVE study, where isavuconazole was not found to be non-inferior to caspofungin for the primary treatment of invasive candidiasis, could be explained by suboptimal exposure of isavuconazole [25]. This hypothesis can unfortunately not be substantiated as isavuconazole pharmacokinetics were not assessed in this study.
Our results should be evaluated in light of the high variability in isavuconazole protein binding seen in our critically ill population. Consequently, unbound, i.e. pharmacologically active, concentrations differ greatly from the assumed unbound concentrations calculated form a constant unbound fraction of 1%. We described isavuconazole protein binding by means of a capacity limited binding model. In this, we were unable to separately estimate Bmax and kd with our data and therefore fixed kd to findings of a previous study (submitted for publication; data on file Radboudumc). Our study design evaluation prior to conduct of the study did not account for the assessment of protein binding. The estimate for Bmax in our study was lower than seen in a non-critically ill paediatric patient population in the previous study, which is probably caused by the lower albumin levels in our patients. Although albumin is the main binding protein for isavuconazole [9], we were unable to identify the serum albumin level as a covariate for any of the protein-binding parameters. A possible explanation may be that our albumin data were not rich enough. As all pharmacokinetic samples were obtained on 1 day for the majority of the patients, only one albumin level per individual was available for the covariate analysis. This also applied to the other covariates we tested on protein binding. The remaining unexplained variability in our final model, as seen in the goodness-of-fit plots, may be the consequence of the above. Our study was not designed for the purpose of covariate identification. Other covariates, in addition to total body weight, were not included in the final model. Additional studies are needed to identify factors accounting for isavuconazole pharmacokinetic variability in critically ill patients, for example that of extracorporeal membrane oxygenation on isavuconazole clearance.
The five-fold range in unbound fractions of isavuconazole advocates for measuring unbound plasma concentrations using a yet-to-establish unbound pharmacokinetic/pharmacodynamic target. Caution should be exercised when interpreting unbound isavuconazole concentrations in the presence of bilirubin levels exceeding 500 µmol/L, although not often observed in clinical practice. In the absence of a validated assay to quantitate unbound concentrations, pharmacometric models as developed in this study could potentially aid in estimating the unbound concentration from the measured total concentration. Our findings show that with our model we are able to predict unbound isavuconazole concentrations with minimal bias, but with imprecision values exceeding ± 20%. For the method of calculating unbound concentrations assuming an unbound fraction of 1%, as deployed thus far, both bias and imprecision were greater than ± 20%. Although not ideal, our model-derived unbound concentration prediction is a big step forward compared with the currently practiced calculated unbound concentration. Future research may focus on developing a model with reduced imprecision to predict unbound isavuconazole concentrations.
Until an unbound pharmacokinetic/pharmacodynamic target is available, current recommendations to measure total isavuconazole exposure should be followed. In such a setting, and specifically in the critically ill patient, early target attainment is desired. For this purpose, we provided an adaptive dosing approach with exposure assessment already after 24 hours of treatment. Additional TDM sampling may further increase the already highly improved percentage of patients reaching adequate isavuconazole exposure. It may be argued that because isavuconazole has shown minimal toxicity, an alternate approach with a higher empirical dose for all patients could be envisioned. In light of the current high costs for isavuconazole, we consider it more cost-effective to adjust doses only for those patients at risk for inadequate exposure. With the current strategy, only one individual had a simulated AUC at day 14 above 233 mg·h/L prompting dose reduction. Recently, a concentration of 5 mg/L, similar to an AUC of 120 mg·h/L, was associated with gastrointestinal toxicity [26]. We considered implementing this in our strategy. However, the approach where individuals with an anticipated steady-state AUC above 120 mg·h/L received a reduced maintenance dose did not result in a higher percentage of patients reaching the desired exposure. Additionally, we argue that the retrospective nature and the relatively small number of patients included in the previous toxicodynamic analysis may be insufficient to base such a toxicity threshold on. The proposed adaptive algorithm in the current study may be directly implemented in practice, although ideally the benefit of this approach is validated in a future study.
5 Conclusions
This study described the unbound and total pharmacokinetics of isavuconazole in critically ill patients showing highly variable protein binding and decreased total isavuconazole exposure. In these patients, increased dosing may be necessary. In the absence of unbound pharmacokinetic/pharmacodynamic targets, we proposed a pragmatic and easily implementable adaptive dosing approach to enhance early target attainment in a vulnerable population.
References
European Medicines Agency (EMA). Summary of product characteristics: Cresemba. https://www.ema.europa.eu/en/documents/product-information/cresemba-epar-product-information_en.pdf. Accessed 4 Jul 2023.
US Food and Drug Administration. Astellas Pharma Inc. CRESEMBA (isavuconazonium sulfate); 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf. Accessed 4 Jul 2023.
Prattes J, Wauters J, Giacobbe DR, Salmanton-Garcia J, Maertens J, Bourgeois M, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2022;28(4):580–7.
Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–92.
Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43(5):577–84.
Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA): from immunology to treatment. J Fungi (Basel). 2020;6(2):91.
Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–9.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509.
European Medicines Agency. Cresemba (isavuconazole). Assessment report; 2015. https://www.ema.europa.eu/en/documents/assessment-report/cresemba-epar-public-assessment-report_en.pdf. Accessed 5 Jan 2023.
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50(2):99–110.
Tillement JP, Lhoste F, Giudicelli JF. Diseases and drug protein binding. Clin Pharmacokinet. 1978;3(2):144–54.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl. 1):e1–8.
Arrieta AC, Neely M, Day JC, Rheingold SR, Sue PK, Muller WJ, et al. Safety, tolerability, and population pharmacokinetics of intravenous and oral isavuconazonium sulfate in pediatric patients. Antimicrob Agents Chemother. 2021;65(8): e0029021.
Desai AV, Kovanda LL, Hope WW, Andes D, Mouton JW, Kowalski DL, et al. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother. 2017;61(12): e01034-17.
Hohl R, Bertram R, Kinzig M, Haarmeyer GS, Baumgartel M, Geise A, et al. Isavuconazole therapeutic drug monitoring in critically ill ICU patients: a monocentric retrospective analysis. Mycoses. 2022;65(7):747–52.
Desai A, Kovanda L, Kowalski D, Lu Q, Townsend R, Bonate PL. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to aspergillus and other filamentous fungi. Antimicrob Agents Chemother. 2016;60(9):5483–91.
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacom Syst Pharmacol. 2013;26(2): e50.
Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43(6):583–96.
Kovanda LL, Marty FM, Maertens J, Desai AV, Lademacher C, Engelhardt M, et al. Impact of mucositis on absorption and systemic drug exposure of isavuconazole. Antimicrob Agents Chemother. 2017;61(6): e00101-17.
Toutain PL, Bousquet-Melou A. Free drug fraction vs free drug concentration: a matter of frequent confusion. J Vet Pharmacol Ther. 2002;25(6):460–3.
Taira Z, Terada H. Specific and non-specific ligand binding to serum albumin. Biochem Pharmacol. 1985;34(11):1999–2005.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12.
Faber NKM. Estimating the uncertainty in estimates of root mean square error of prediction: application to determining the size of an adequate test set in multivariate calibration. Chemom Intell Lab. 1999;49(1):79–89.
Perez L, Corne P, Pasquier G, Konecki C, Sadek M, Le Bihan C, et al. Population pharmacokinetics of isavuconazole in critical care patients with COVID-19-associated pulmonary aspergillosis and Monte Carlo simulations of high off-label doses. J Fungi (Basel). 2023;9(2):211.
Kullberg BJ, Viscoli C, Pappas PG, Vazquez J, Ostrosky-Zeichner L, Rotstein C, et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive Candida infections: the ACTIVE trial. Clin Infect Dis. 2019;68(12):1981–9.
Furfaro E, Signori A, Di Grazia C, Dominietto A, Raiola AM, Aquino S, et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother. 2019;74(8):2341–6.
Acknowledgements
The authors thank the patients for participating in the study, the analytical staff for analysing the samples and the nursing staff of the departments of intensive care for their help in conducting this study. The authors thank Minou van Seyen, Emilie Gieling and Dylan de Lange for their contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Internal funds were provided by the Radboud University Medical Center.
Conflict of Interest
Joost Wauters received speakers’ fees, travel grants and investigator-initiated grants from Pfizer, MSD and Gilead, all outside the scope of this study. Paul E. Verweij reports grants or contracts from Gilead Sciences and F2G, and participation on a data safety monitoring board or advisory board for Pfizer, MundiPharma, Gilead Sciences and F2G, outside the submitted work. All payments were made to the institute. Roger J.M. Brüggemann has served as consultant to Astellas Pharma, Inc., F2G, Amplyx, Gilead Sciences, Merck Sharp & Dohme Corp., MundiPharma and Pfizer, Inc., and received unrestricted research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharp & Dohme Corp. and Pfizer outside this study. All contracts and payments were through the institute. Yves Debaveye reports travel support from Pfizer; participation in advisory boards of Pfizer and MSD, outside the submitted work. Anouk M.E. Jansen, Beatrijs Mertens, Isabel Spriet, Jeroen Schouten and Rob Ter Heine have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The study was approved by the Ethics Committees of the Radboud University Medical Center, the Netherlands (EC identifier: 2021-7327) and the University Hospitals Leuven, Belgium (EC identifier: S65556).
Consent to Participate
Informed consent was obtained from all individual participants or their legal representatives.
Consent for Publication
Not applicable.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request and following legal approval. The final model control stream is available in the supplementary information.
Code Availability
Not applicable.
Author Contributions
AJ, RB and RtH designed the study; AJ, BM, IS, JS, JW and YD performed the data collection; AJ performed the analysis with RB and RtH; AJ interpreted results with RB and RtH; AJ drafted the manuscript; and all authors critically reviewed the manuscript and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jansen, A.M.E., Mertens, B., Spriet, I. et al. Population Pharmacokinetics of Total and Unbound Isavuconazole in Critically Ill Patients: Implications for Adaptive Dosing Strategies. Clin Pharmacokinet 62, 1701–1711 (2023). https://doi.org/10.1007/s40262-023-01305-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01305-8