Abstract
Background
With the progress of therapeutic drug monitoring (TDM) technology and the development of evidence-based medicine, many guidelines were developed and implemented in recent decades.
Objective
The aim was to evaluate the current status of TDM guidelines and provide suggestions for their development and updates based on Appraisal of Guidelines for Research and Evaluation (AGREE) II.
Methods
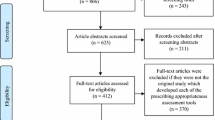
The TDM guidelines were systematically searched for among databases including PubMed, Embase, China National Knowledge Infrastructure, Wanfang Data, and the Chinese biomedical literature service system and the official websites of TDM-related associations. The search period was from inception to 6 April 2023. Four researchers independently screened the literature and extracted data. Any disagreement was discussed and reconciled by another researcher. The quality of guidelines was assessed using the AGREE II instrument.
Results
A total of 92 guidelines were included, including 57 technical guidelines, three management guidelines, and 32 comprehensive guidelines. The number of TDM guidelines has gradually increased since 1979. The United States published the most guidelines (20 guidelines), followed by China (15 guidelines) and the United Kingdom (ten guidelines), and 23 guidelines were developed by international organizations. Most guidelines are aimed at adult patients only, while 28 guidelines include special populations. With respect to formulation methods, there are 23 evidence-based guidelines. As for quality evaluation results based on AGREE II, comprehensive guidelines scored higher (58.16%) than technical guidelines (51.36%) and administrative guidelines (50.00%).
Conclusion
The number of TDM guidelines, especially technical and comprehensive ones, has significantly increased in recent years. Most guidelines are confronted with the problems of unclear methodology and low quality of evidence according to AGREE II. More evidence-based research on TDM and high-quality guideline development is recommended to promote individualized therapy.




Similar content being viewed by others
References
Zeng L, Yi Q, Huang L, et al. The guideline for therapeutic drug monitoring guidelines development. J Evid Based Med. 2022;15(3):272–83.
Wicha SG, Märtson AG, Nielsen EI, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021;109(4):928–41.
Tran D, Moore J. Analysis of Therapeutic Drug Monitoring in Drug Labels https://www.fda.gov/science-research/fda-stem-outreach-education-and-engagement/analysis-therapeutic-drug-monitoring-drug-labels. Accessed 29 Apr 2023.
Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. The Expert Consensus on the Standards of Therapeutic Drug Monitoring (2019 Edition). Evaluation and analysis of drug-use in hospitals of China. 2019;19(08):897-8+902.
Papamichael K, Afif W, Drobne D, et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol Hepatol. 2022;7(2):171–85.
Mueller-Schoell A, Groenland SL, Scherf-Clavel O, et al. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol. 2021;77(4):441–64.
Zeng M, Yi Q, Zeng L, et al. Quality of therapeutic drug monitoring guidelines is suboptimal: an evaluation using the Appraisal of Guidelines for Research and Evaluation II instrument. J Clin Epidemiol. 2020;120:47–58.
Huang L, Zhang L, Li Y, et al. Current status of guidelines for therapeutic drug monitoring: an evidence-based evaluation. Chin J Evid-based Med. 2016;16(04):451–9.
Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045–52.
Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472–8.
Burls A. AGREE II-improving the quality of clinical care. Lancet. 2010;376(9747):1128–9.
Lenzer J, Hoffman JR, Furberg CD, Ioannidis JP. Ensuring the integrity of clinical practice guidelines: a tool for protecting patients. BMJ. 2013;347: f5535.
Alonso-Coello P, Irfan A, Solà I, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies. Qual Saf Health Care. 2010;19(6): e58.
World Health Organization. WHO handbook for guideline development. 2nd ed. Geneva: World Health Organization; 2014. p. 2014.
Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–31.
Hammett-Stabler CA, Johns T. Laboratory guidelines for monitoring of antimicrobial drugs. National Academy of Clinical Biochemistry. Clin Chem. 1998;44(5):1129–40.
Acosta EP, Gerber JG. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res Hum Retroviruses. 2002;18(12):825–34.
Back D, Gatti G, Fletcher C, et al. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS. 2002;16(Suppl 1):S5-37.
Boffito M, Acosta E, Burger D, et al. Current status and future prospects of therapeutic drug monitoring and applied clinical pharmacology in antiretroviral therapy. Antivir Ther. 2005;10(3):375–92.
Boffito M, Acosta E, Burger D, et al. Therapeutic drug monitoring and drug-drug interactions involving antiretroviral drugs. Antivir Ther. 2005;10(4):469–77.
Higgins N, Tseng A, Sheehan NL, la Porte CJ. Antiretroviral therapeutic drug monitoring in Canada: current status and recommendations for clinical practice. Can J Hosp Pharm. 2009;62(6):500–9.
Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect. 2012;42(7):301–8.
Chau MM, Kong DC, van Hal SJ, et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy, 2014. Intern Med J. 2014;44(12b):1364–88.
He N, Su S, Ye Z, et al. Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese Pharmacological Society. Clin Infect Dis. 2020;71(Suppl 4):S363–71.
Lin B, Hu Y, Xu P, et al. Expert consensus statement on therapeutic drug monitoring and individualization of linezolid. Front Public Health. 2022;10: 967311.
Kim HY, Baldelli S, Märtson AG, et al. Therapeutic drug monitoring of the echinocandin antifungal agents: is there a role in clinical practice? A position statement of the anti-infective drugs committee of the international association of therapeutic drug monitoring and clinical toxicology. Ther Drug Monit. 2022;44(1):198–214.
Han J, Sauberan J, Tran MT, et al. Implementation of vancomycin therapeutic monitoring guidelines: focus on Bbayesian estimation tools in neonatal and pediatric patients. Ther Drug Monit. 2022;44(2):241–52.
Matsumoto K, Oda K, Shoji K, et al. Clinical practice guidelines for therapeutic drug monitoring of vancomycin in the framework of model-informed precision dosing: a consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics. 2022;14(3):489. https://doi.org/10.3390/pharmaceutics14030489.
Takesue Y, Hanai Y, Oda K, et al. Clinical practice guideline for the therapeutic drug monitoring of voriconazole in Non-Asian and Asian adult patients: consensus review by the japanese society of chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Clin Ther. 2022;44(12):1604–23.
Hanai Y, Takahashi Y, Niwa T, et al. Clinical practice guidelines for therapeutic drug monitoring of teicoplanin: a consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Antimicrob Chemother. 2022;77(4):869–79.
Liu X, Huang C, Bergen PJ, et al. Chinese consensus guidelines for therapeutic drug monitoring of polymyxin B, endorsed by the Infection and Chemotherapy Committee of the Shanghai Medical Association and the Therapeutic Drug Monitoring Committee of the Chinese Pharmacological Society. J Zhejiang Univ Sci B. 2023;24(2):130–42.
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98.
Hamada Y, Tokimatsu I, Mikamo H, et al. Practice guidelines for therapeutic drug monitoring of voriconazole: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19(3):381–92.
Matsumoto K, Takesue Y, Ohmagari N, et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19(3):365–80.
Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(5):1162–76.
Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective. Can J Infect Dis Med Microbiol. 2014;25(6):327–43.
Okada K, Kimura T, Mikamo H, et al. Clinical practice guidelines for therapeutic drug monitoring of arbekacin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2014;20(1):1–5.
Zhaochao H, Junyan W, Kaifeng Q. Guidance for Clinical pharmacists for Individualized administration of vancomycin. Pharm Today. 2015;25(02):78–82.
Ye ZK, Chen YL, Chen K, et al. Therapeutic drug monitoring of vancomycin: a guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. J Antimicrob Chemother. 2016;71(11):3020–5.
Chen K, Zhang X, Ke X, Du G, Yang K, Zhai S. Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese Pharmacological Society. Ther Drug Monit. 2018;40(6):663–74.
Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper(). Intensive Care Med. 2020;46(6):1127–53.
Reuter SE, Stocker SL, Alffenaar JC, et al. Optimal practice for vancomycin therapeutic drug monitoring: position statement from the anti-infectives committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit. 2022;44(1):121–32.
Kahan BD, Shaw LM, Holt D, Grevel J, Johnston A. Consensus document: Hawk’s Cay meeting on therapeutic drug monitoring of cyclosporine. Clin Chem. 1990;36(8 Pt 1):1510–6.
Keown P, Kahan BD, Johnston A, et al. Optimization of cyclosporine therapy with new therapeutic drug monitoring strategies: report from the International Neoral TDM Advisory Consensus Meeting (Vancouver, November 1997). Transplant Proc. 1998;30(5):1645–9.
Oellerich M, Armstrong VW, Schütz E, Shaw LM. Therapeutic drug monitoring of cyclosporine and tacrolimus. Update on Lake Louise Consensus Conference on cyclosporin and tacrolimus. Clin Biochem. 1998;31(5):309–16.
Shaw LM, Holt DW, Oellerich M, Meiser B, van Gelder T. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit. 2001;23(4):305–15.
Holt DW, Armstrong VW, Griesmacher A, Morris RG, Napoli KL, Shaw LM. International Federation of Clinical Chemistry/International Association of Therapeutic Drug Monitoring and Clinical Toxicology working group on immunosuppressive drug monitoring. Ther Drug Monit. 2002;24(1):59–67.
Morris RG, Ilett KF, Tett SE, et al. Cyclosporin monitoring in Australasia: 2002 update of consensus guidelines. Ther Drug Monit. 2002;24(6):677–88.
Morris RG, Holt DW, Armstrong VW, Griesmacher A, Napoli KL, Shaw LM. Analytic aspects of cyclosporine monitoring, on behalf of the IFCC/IATDMCT Joint Working Group. Ther Drug Monit. 2004;26(2):227–30.
Trevillian P. The CARI guidelines. Calcineurin inhibitors in renal transplantation: therapeutic drug monitoring. Nephrology (Carlton). 2007;12(Suppl 1):S57-65.
Kuypers DR, Le Meur Y, Cantarovich M, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010;5(2):341–58.
Cantarovich M, Brown NW, Ensom MH, et al. Mycophenolate monitoring in liver, thoracic, pancreas, and small bowel transplantation: a consensus report. Transplant Rev (Orlando). 2011;25(2):65–77.
Le Meur Y, Borrows R, Pescovitz MD, et al. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of The Transplantation Society consensus meeting. Transplant Rev (Orlando). 2011;25(2):58–64.
Seger C, Shipkova M, Christians U, et al. Assuring the proper analytical performance of measurement procedures for immunosuppressive drug concentrations in clinical practice: recommendations of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology Immunosuppressive Drug Scientific Committee. Ther Drug Monit. 2016;38(2):170–89.
NICE. Fluorouracil chemotherapy: The My5-FU assay for guiding dose adjustment https://www.nice.org.uk/guidance/dg36. Accessed 12 Jan 2023.
Wadhwa M, Bird C, Atkinson E, Cludts I, Rigsby P. The first WHO International Standard for adalimumab: dual role in bioactivity and therapeutic drug monitoring. Front Immunol. 2021;12: 636420.
Bergan S, Brunet M, Hesselink DA, et al. Personalized therapy for mycophenolate: consensus report by the international association of therapeutic drug monitoring and clinical toxicology. Ther Drug Monit. 2021;43(2):150–200.
Mockli G, Kabra PM, Kurtz TW. Laboratory monitoring of cyclosporine levels: guidelines for the dermatologist. J Am Acad Dermatol. 1990;23(6 Pt 2):1275–8 (discussion 8-9).
Jusko WJ, Thomson AW, Fung J, et al. Consensus document: therapeutic monitoring of tacrolimus (FK-506). Ther Drug Monit. 1995;17(6):606–14.
Yatscoff RW, Boeckx R, Holt DW, et al. Consensus guidelines for therapeutic drug monitoring of rapamycin: report of the consensus panel. Ther Drug Monit. 1995;17(6):676–80.
Shaw LM, Nicholls A, Hale M, et al. Therapeutic monitoring of mycophenolic acid. A consensus panel report. Clin Biochem. 1998;31(5):317–22.
Van Gelder T, Le Meur Y, Shaw LM, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006;28(2):145–54.
Shipkova M, Hesselink DA, Holt DW, et al. Therapeutic drug monitoring of everolimus: a consensus report. Ther Drug Monit. 2016;38(2):143–69.
Brunet M, van Gelder T, Åsberg A, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261–307.
Krieckaert CL, van Tubergen A, Gehin JE, et al. EULAR points to consider for therapeutic drug monitoring of biopharmaceuticals in inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2023;82(1):65–73.
Orsulak PJ, Schildkraut JJ. Guidelines for therapeutic monitoring of tricyclic antidepressant plasma levels. Ther Drug Monit. 1979;1(2):199–208.
Orsulak PJ. Therapeutic monitoring of antidepressant drugs: guidelines updated. Ther Drug Monit. 1989;11(5):497–507.
Guidelines for therapeutic monitoring on antiepileptic drugs. Commission on antiepileptic drugs, international league against epilepsy. Epilepsia. 1993;34(4):585–7.
Laux G, Baumann P, Hiemke C. Therapeutic drug monitoring of antidepressants–clinical aspects. J Neural Transm Suppl. 2007;72:261–7.
Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559–95.
Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19(1):97–117.
Shawahna R, Abdelfattah B, Shafei M, Ruzzeh S. Therapeutic monitoring of antiepileptic drugs: recommendations to improve care of patients with epilepsy in the Palestinian practice. Epilepsy Behav. 2020;111: 107215.
de Leon J, Schoretsanitis G, Smith RL, et al. An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry. 2022;55(2):73–86.
Riederer P, Laux G. Therapeutic drug monitoring of psychotropics: report of a consensus conference. Pharmacopsychiatry. 1992;25(6):271–2.
Baumann P, Hiemke C, Ulrich S, et al. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37(6):243–65.
Baumann P, Ulrich S, Eckermann G, et al. The AGNP-TDM Expert Group Consensus Guidelines: focus on therapeutic monitoring of antidepressants. Dialogues Clin Neurosci. 2005;7(3):231–47.
Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring. ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239–76.
Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195–235.
Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–02):9–62.
Reimers A, Berg JA, Burns ML, Brodtkorb E, Johannessen SI, Johannessen LC. Reference ranges for antiepileptic drugs revisited: a practical approach to establish national guidelines. Drug Des Devel Ther. 2018;12:271–80.
Schoretsanitis G, Kane JM, Correll CU, et al. Blood levels to optimize antipsychotic treatment in clinical practice: a Joint Consensus Statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;;81(3):19cs13169. https://doi.org/10.4088/JCP.19cs13169..
Guo W, Zhang L, Wang G. Expert consensus on clinical application of psychiatric therapeutic drug monitoring in China (2022 edition). Chin J Neurosci Mental Health. 2022;22(08):601–8.
Khanna R, Sattin BD, Afif W, et al. Review article: a clinician’s guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(5):447–59.
NICE. Therapeutic monitoring of TNF-alpha inhibitors in Crohn’s disease (LISA-TRACKER ELISA kits, IDKmonitor ELISA kits, and Promonitor ELISA kits) https://www.nice.org.uk/guidance/dg22. Accessed 12 Janu 2023.
Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827–34.
Expert consensus on therapeutic drug monitoring for inflammatory bowel disease in China. Chin J Dig. 2018;38(11):721-7.
Khan A, Berahmana AB, Day AS, Barclay ML, Schultz M. New Zealand Society of gastroenterology guidelines on therapeutic drug monitoring in inflammatory bowel disease. N Z Med J. 2019;132(1491):46–62.
Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655-68.e3.
Cheifetz AS, Abreu MT, Afif W, et al. A Comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am J Gastroenterol. 2021;116(10):2014–25.
Annese V, Nathwani R, Alkhatry M, et al. Optimizing biologic therapy in inflammatory bowel disease: a Delphi consensus in the United Arab Emirates. Therap Adv Gastroenterol. 2021;14:17562848211065328.
Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46(11–12):1037–53.
Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305–25.
NICE. Fluorouracil chemotherapy: The My5-FU assay for guiding dose adjustment. https://www.nice.org.uk/guidance/dg16. Accessed 12 Jan 2023.
Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102(5):765–76.
Beumer JH, Chu E, Allegra C, et al. Therapeutic drug monitoring in oncology: international association of therapeutic drug monitoring and clinical toxicology recommendations for 5-fluorouracil therapy. Clin Pharmacol Ther. 2019;105(3):598–613.
Chinese Pharmacological Society, China-Japan Friendship Hospital. Pharmacy Expert Consensus on the Therapeutic Drug Monitoring of Antitumor Biosimilars (2020 Edition). Evaluation and analysis of drug-use in hospitals of China. 2020;20(05):513-7+20.
Hamzic S, Aebi S, Joerger M, et al. Fluoropyrimidine chemotherapy: recommendations for DPYD genotyping and therapeutic drug monitoring of the Swiss Group of Pharmacogenomics and Personalised Therapy. Swiss Med Wkly. 2020;150: w20375.
Clarke WA, Chatelut E, Fotoohi AK, et al. Therapeutic drug monitoring in oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology consensus guidelines for imatinib therapy. Eur J Cancer. 2021;157:428–40.
Song Z, Hu Y, Liu S, et al. Medication therapy of high-dose methotrexate: an evidence-based practice guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Br J Clin Pharmacol. 2022;88(5):2456–72.
Aonuma K, Shiga T, Atarashi H, et al. Guidelines for therapeutic drug monitoring of cardiovascular drugs clinical use of blood drug concentration monitoring (JCS 2015)—digest version. Circ J. 2017;81(4):581–612.
Capiau S, Veenhof H, Koster RA, et al. Official International Association for therapeutic drug monitoring and clinical toxicology guideline: development and validation of dried blood spot-based methods for therapeutic drug monitoring. Ther Drug Monit. 2019;41(4):409–30.
The expert consensus on the interpretation of therapeutic drug monitoring. Chin J of Hosp Pharm. 2020;40(23):2389-95.
Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society, Evidence-Based Pharmacy Committee of Chinese Pharmaceutical Association. The guideline for therapeutic drug monitoring guideline development. Chin J Evid-based Med. 2021;21(02):125–32.
The Group of Clinical Pharmacology, Pediatrics Society of Chinese Medical Association. Expert Consensus on therapeutic drug monitoring in children. Chin J Pediatr. 2015;53(09):650–9.
The Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society, Pharmaceutical Bioanalysis Committee, Chinese Pharmaceutical Association, Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Expert consensus on chromatographic techniques for quality assurance in therapeutic drug monitoring (2021 editions). Chin Pharm J. 2021;56(17):1443–8.
Shekelle P, Woolf S, Grimshaw JM, et al. Developing clinical practice guidelines: reviewing, reporting, and publishing guidelines; updating guidelines; and the emerging issues of enhancing guideline implementability and accounting for comorbid conditions in guideline development. Implement Sci. 2012;7:62.
Fei W, Li T, Zeping Y, Lei Z, Juan H. Multiple antimicrobial therapeutic drug monitoring in adult critically infection patients: a clinical analysis. J Clin Emerg (China). 2022;23(11):781–6.
Matusik E, Boidin C, Friggeri A, et al. Therapeutic drug monitoring of antibiotic drugs in patients receiving continuous renal replacement therapy or intermittent hemodialysis: a critical review. Ther Drug Monit. 2022;44(1):86–102.
George R, Haywood A, Khan S, Radovanovic M, Simmonds J, Norris R. Enhancement and suppression of ionization in drug analysis using HPLC-MS/MS in support of therapeutic drug monitoring: a review of current knowledge of its minimization and assessment. Ther Drug Monit. 2018;40(1):1–8.
Tey HY, See HH. A review of recent advances in microsampling techniques of biological fluids for therapeutic drug monitoring. J Chromatogr A. 2021;1635: 461731.
Iacuzzi V, Posocco B, Zanchetta M, et al. Dried blood spot technique applied in therapeutic drug monitoring of anticancer drugs: a review on conversion methods to correlate plasma and dried blood spot concentrations. Pharm Res. 2021;38(5):759–78.
Peck Palmer OM, Dasgupta A. Review of the preanalytical errors that impact therapeutic drug monitoring. Ther Drug Monit. 2021;43(5):595–608.
Papamichael K, Vande Casteele N, Ferrante M, et al. Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. 2017;23(9):1510–5.
Menz BD, Stocker SL, Verougstraete N, et al. Barriers and opportunities for the clinical implementation of therapeutic drug monitoring in oncology. Br J Clin Pharmacol. 2021;87(2):227–36.
Widmer N, Bardin C, Chatelut E, et al. Review of therapeutic drug monitoring of anticancer drugs part two–targeted therapies. Eur J Cancer. 2014;50(12):2020–36.
Boulet LP, Reddel HK, Bateman E, et al. The Global INITIATIVE FOR ASTHMA (GINA): 25 years later. Eur Respir J. 2019;54(2):1900598.
Schouwenburg S, van der Klip RFJ, Smeets TJL, et al. Review of scavenged sampling for sustainable therapeutic drug monitoring: do more with less. Ther Drug Monit. 2022;44(1):215–23.
Acknowledgement
We extend special thanks to Ms. Yu-Meng Lv from Peking University Health Science Center for help with editing. This paper was invited to be presented as a poster at the 12th National Annual Conference on Therapeutic Drug Monitoring.
Author information
Authors and Affiliations
Contributions
RS Zhao, ZM Yi, and XY Li designed the study. ZM Yi and XY Li searched the literature. ZM Yi, XY Li, ZT Wang, JG Qin, D Jiang, and PH Tian screened the literature. XY Li, JG Qin, ZM Yi, and P Yang conducted data extraction and quality evaluation. ZM Yi and XY Li drafted the manuscript. RS Zhao revised for the finalization of the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Funding statement
This study was funded by the National Natural Science Foundation of China (72104003).
Conflict of Interest
Zhan-Miao Yi, Xinya Li, Zhitong Wang, Jiguang Qin, Dan Jiang, Panhui Tian, Ping Yang, and Rongsheng Zhao declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Patient Consent Statement
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Data Availability Statement
All data generated or analyzed during this study were included in this published article.
Ethics Approval
Not applicable.
Code Availability
Not applicable.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, ZM., Li, X., Wang, Z. et al. Status and Quality of Guidelines for Therapeutic Drug Monitoring Based on AGREE II Instrument. Clin Pharmacokinet 62, 1201–1217 (2023). https://doi.org/10.1007/s40262-023-01283-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01283-x