Abstract
Background and Objective
Abiraterone is a first-in-class inhibitor of cytochrome P450 17A1 (CYP17A1), and its pharmacokinetic (PK) profile is susceptible to intrinsic and extrinsic variabilities. Potential associations between abiraterone concentrations and pharmacodynamic consequences in prostate cancer may demand further dosage optimization to balance therapeutic outcomes. Consequently, we aim to develop a physiologically based pharmacokinetic (PBPK) model for abiraterone via a middle-out approach to prospectively interrogate the untested, albeit clinically relevant, scenarios.
Methods
To characterize in vivo hydrolysis of prodrug abiraterone acetate (AA) and supersaturation of abiraterone, in vitro aqueous solubility data, biorelevant measurements, and supersaturation and precipitation parameters were utilized for mechanistic absorption simulation. CYP3A4-mediated N-oxidation and sulfotransferase 2A1-catalyzed sulfation of abiraterone were subsequently quantified in human liver subcellular systems. Iterative PBPK model refinement involved evaluation of potential organic anion transporting polypeptide (OATP)-mediated abiraterone uptake in transfected cells in the absence and presence of albumin.
Results
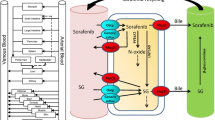
The developed PBPK model recapitulated the duodenal concentration–time profile of both AA and abiraterone after simulated AA administration. Our findings established abiraterone as a substrate of hepatic OATP1B3 to recapitulate its unbound metabolic intrinsic clearance. Further consideration of a transporter-induced protein-binding shift established accurate translational scaling factors and extrapolated the sinusoidal uptake process. Subsequent simulations effectively predicted the PK of abiraterone upon single and multiple dosing.
Conclusion
Our systematic development of the abiraterone PBPK model has demonstrated its application for the prospective interrogation of the individual or combined influences of potential interindividual variabilities influencing the systemic exposure of abiraterone.






Similar content being viewed by others
References
Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85.
Huggins C, Hodges CV. Studies on Prostatic Cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7.
US FDA. Clinical pharmacology and biopharmaceutics review: Zytiga (abiraterone acetate); 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202379orig1s000clinpharmr.pdf.
Mueller-Schoell A, Groenland SL, Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R, et al. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol. 2021;77:441–64.
Carton E, Noe G, Huillard O, Golmard L, Giroux J, Cessot A, et al. Relation between plasma trough concentration of abiraterone and prostate-specific antigen response in metastatic castration-resistant prostate cancer patients. Eur J Cancer. 2017;72:54–61.
van Nuland M, Groenland SL, Bergman AM, Steeghs N, Rosing H, Venekamp N, et al. Exposure–response analyses of abiraterone and its metabolites in real-world patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:244–51.
Szmulewitz RZ, Peer CJ, Ibraheem A, Martinez E, Kozloff MF, Carthon B, et al. Prospective international randomized phase ii study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer. J Clin Oncol. 2018;36:1389–95.
Marbury T, Lawitz E, Stonerock R, Gonzalez M, Jiao J, Breeding J, et al. Single-dose pharmacokinetic studies of abiraterone acetate in men with hepatic or renal impairment. J Clin Pharmacol. 2014;54:732–41.
Janssen. ZYTIGA® (abiraterone acetate) highlights of prescribing information; 2019.
Tolcher AW, Chi KN, Shore ND, Pili R, Molina A, Acharya M, et al. Effect of abiraterone acetate plus prednisone on the QT interval in patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2012;70:305–13.
Bernard A, Vaccaro N, Acharya M, Jiao J, Monbaliu J, De Vries R, et al. Impact on abiraterone pharmacokinetics and safety: open-label drug-drug interaction studies with ketoconazole and rifampicin. Clin Pharmacol Drug Dev. 2015;4:63–73.
Stappaerts J, Geboers S, Snoeys J, Brouwers J, Tack J, Annaert P, et al. Rapid conversion of the ester prodrug abiraterone acetate results in intestinal supersaturation and enhanced absorption of abiraterone: in vitro, rat in situ and human in vivo studies. Eur J Pharm Biopharm. 2015;90:1–7.
Geboers S, Stappaerts J, Mols R, Snoeys J, Tack J, Annaert P, et al. The effect of food on the intraluminal behavior of abiraterone acetate in man. J Pharm Sci. 2016;105:2974–81.
Solymosi T, Ötvös Z, Angi R, Ordasi B, Jordán T, Semsey S, et al. Development of an abiraterone acetate formulation with improved oral bioavailability guided by absorption modeling based on in vitro dissolution and permeability measurements. Int J Pharm. 2017;532:427–34.
Hens B, Pathak SM, Mitra A, Patel N, Liu B, Patel S, et al. In silico modeling approach for the evaluation of gastrointestinal dissolution, supersaturation, and precipitation of posaconazole. Mol Pharm. 2017;14:4321–33.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57.
European Medicines Agency. Assessment Report For Zytiga (abiraterone); 2017.
Acharya M, Gonzalez M, Mannens G, De Vries R, Lopez C, Griffin T, et al. A phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjects. Xenobiotica. 2013;43:379–89.
Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–9.
Rostami-Hodjegan A. Physiologically based pharmacokinetics joined with in vitro-in vivo extrapolation of ADME: a marriage under the arch of systems pharmacology. Clin Pharmacol Ther. 2012;92:50–61.
Abduljalil K, Cain T, Humphries H, Rostami-Hodjegan A. Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: an analysis based on in vivo observations. Drug Metab Dispos. 2014;42:1478–84.
Mostaghel EA, Cho E, Zhang A, Alyamani M, Kaipainen A, Green S, et al. Association of tissue abiraterone levels and SLCO genotype with intraprostatic steroids and pathologic response in men with high-risk localized prostate cancer. Clin Cancer Res. 2017;23:4592–601.
Zamek-Gliszczynski MJ, Taub ME, Chothe PP, Chu X, Giacomini KM, Kim RB, et al. Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin Pharmacol Ther. 2018;104:890–9.
US FDA. Drug development and drug interactions: table of substrates, inhibitors and inducers. center for drug evaluation and research; 2017.
Bowman CM, Okochi H, Benet LZ. The presence of a transporter-induced protein binding shift: a new explanation for protein-facilitated uptake and improvement for in vitro-in vivo extrapolation. Drug Metab Dispos. 2019;47:358–63.
Sissung TM, Ley AM, Strope JD, McCrea EM, Beedie S, Peer CJ, et al. Differential expression of OATP1B3 mediates unconjugated testosterone influx. Mol Cancer Res. 2017;15:1096–105.
Müller P, Pomorski T, Porwoli S, Tauber R, Herrmann A. Transverse movement of spin-labeled phospholipids in the plasma membrane of a hepatocytic cell line (HepG2): implications for biliary lipid secretion. Hepatology. 1996;24:1497–503.
Badée J, Achour B, Rostami-Hodjegan A, Galetin A. Meta-analysis of expression of hepatic organic anion-transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab Dispos. 2015;43:424–32.
Acharya M, Bernard A, Gonzalez M, Jiao J, De Vries R, Tran N. Open-label, phase I, pharmacokinetic studies of abiraterone acetate in healthy men. Cancer Chemother Pharmacol. 2012;69:1583–90.
Pathak SM, Schaefer KJ, Jamei M, Turner DB. Biopharmaceutic IVIVE—mechanistic modeling of single- and two-phase in vitro experiments to obtain drug-specific parameters for incorporation into PBPK models. J Pharm Sci. 2019;108:1604–18.
Kourentas A, Vertzoni M, Stavrinoudakis N, Symillidis A, Brouwers J, Augustijns P, et al. An in vitro biorelevant gastrointestinal transfer (BioGIT) system for forecasting concentrations in the fasted upper small intestine: design, implementation, and evaluation. Eur J Pharm Sci. 2016;82:106–14.
Stover JT, Moore RA, Davis K, Harrison MR, Armstrong AJ. Reversal of PSA progression on abiraterone acetate through the administration with food in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:161–6.
Chien C, Smith M, De PP. Effect of food on abiraterone pharmacokinetics: a review. Int J Pharmacokinet. 2017;2:183–93.
Minekus M. The TNO gastro-intestinal model (TIM). Impact food bioact heal vitr ex vivo model. Berlin: Springer International Publishing; 2015. p. 37–46.
Cho E, Montgomery RB, Mostaghel EA. Minireview: SLCO and ABC transporters: a role for steroid transport in prostate cancer progression. Endocrinology. 2014;155:4124–32.
Solymosi T, Tóth F, Orosz J, Basa-Dénes O, Angi R, Jordán T, et al. Solubility measurements at 296 and 310 K and physicochemical characterization of abiraterone and abiraterone acetate. J Chem Eng Data. 2018;63:4453–8.
Bowman CM, Benet LZ. An examination of protein binding and protein-facilitated uptake relating to in vitro-in vivo extrapolation. Eur J Pharm Sci. 2018;123:502–14.
Miyauchi S, Masuda MM, Kim SJ, Tanaka Y, Lee KR, Iwakado S, et al. The phenomenon of albumin-mediated hepatic uptake of organic anion transport polypeptide substrates: prediction of the in vivo uptake clearance from the in vitro uptake by isolated hepatocytes using a facilitated-dissociation model. Drug Metab Dispos. 2018;46:259–67.
Kim SJ, Lee KR, Miyauchi S, Sugiyama Y. Extrapolation of in vivo hepatic clearance from in vitro uptake clearance by suspended human hepatocytes for anionic drugs with high binding to human albumin: improvement of in vitro-to-in vivo extrapolation by considering the “albumin-mediated” hepatic u. Drug Metab Dispos. 2019;47:94–103.
El-Khateeb E, Achour B, Al-Majdoub ZM, Barber J, Rostami-Hodjegan A. Non-uniformity of changes in drug-metabolizing enzymes and transporters in liver cirrhosis: implications for drug dosage adjustment. Mol Pharm. 2021;18:3563–77.
Gufford BT, Robarge JD, Eadon MT, Gao H, Lin H, Liu Y, et al. Rifampin modulation of xeno- and endobiotic conjugating enzyme mRNA expression and associated microRNAs in human hepatocytes. Pharmacol Res Perspect. 2018;6:386.
Acknowledgements
The authors thank Dr Bruno Stieger (Division of Clinical Pharmacology and Toxicology, University Hospital, Zurich, Switzerland) for the kind donation of both wild type and human OATP1B1/1B3/2B1-transfected Chinese hamster ovary cell lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by the Singapore Ministry of Education Tier 1 Academic Research Funding (grant R-148-000-249-114) and the National University of Singapore (NUS) President’s Graduate Fellowship (PGF) to E.J.Y.C and the National University of Singapore, Department of Pharmacy, final year project funding provided to Z.W.N, T.J.Y and E.Z.B.C.
Author contributions
All authors participated in research design, performed data analysis, and wrote or contributed to the writing of the manuscript. Cheong, Ng, Chin, and Wang conducted the experiments. All authors read and approved the final manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
40262_2023_1266_MOESM1_ESM.tif
Figure S1. Schematic presentation of the interplay between supersaturation and precipitation of abiraterone in intestinal fluids. Region A: The dissolved concentration of abiraterone is permitted to rise above equilibrium solubility until the critical supersaturation concentration (CSC) is attained. Region B: Above the CSC, there exists a metastable time window of supersaturated concentrations. Region C: Supersaturated abiraterone will precipitate as a function of time following a first-order kinetic process governed by the precipitation rate constant (PRC). Precipitation continues until dissolved concentration is equal to equilibrium solubility (TIF 101 kb)
40262_2023_1266_MOESM2_ESM.tif
Figure S2. Determination of the intrinsic solubility of abiraterone via a saturation shake flask experiment. Measurements after 14 and 24 h of incubation indicated that the difference in mean intrinsic solubility of abiraterone was not statistically significant, p>0.05. Hence, taking the average of 12 measurements, the intrinsic solubility of abiraterone was calculated to be 0.0058 mg/mL (TIF 592 kb)
40262_2023_1266_MOESM3_ESM.tif
Figure S3. Examining how the magnitude of the secondary precipitation rate constant (sPRC) will affect (a) the simulated duodenal concentration-time profile of abiraterone and (b) the simulated plasma Cmax (TIF 562 kb)
40262_2023_1266_MOESM4_ESM.tif
Figure S4. Comparing the impact of utilizing a perfusion-limited liver model versus a permeability-limited liver model on the simulated plasma (solid lines) and liver (dashed lines) concentration-time profiles of abiraterone. Open symbols represent the observed clinical plasma concentration-time profile following a single 250 mg dose of abiraterone acetate administered under fasted conditions (TIF 982 kb)
40262_2023_1266_MOESM5_ESM.tif
Figure S5. Time-dependent uptake of (a) testosterone 5 μM and (b) abiraterone 2.5 μM in OATP1B3-expressing CHO cells. Linearity of OATP1B3-mediated testosterone and abiraterone uptake was demonstrated between 1 to 2 min and 0.5 to 1 min respectively. Each point represents the mean ± SD of triplicate determinations. (TIF 553 kb)
40262_2023_1266_MOESM6_ESM.tif
Figure S6. Evaluating the source and quality of drug-dependent parameters utilized to parameterize the absorption models of abiraterone acetate and abiraterone. (a) Depletion profile of abiraterone acetate in fasted state human intestinal fluid, fasted state simulated intestinal fluid supplemented with pancreatin (10 mg/mL) and fed state human intestinal fluid after the addition of 1 µM of abiraterone acetate to the media. Simulated (solid line) plasma drug concentration-time profiles of abiraterone in the fed state following (b) a single 250 mg dose of AA as reported by Geboers et al (TIF 642 kb)
40262_2023_1266_MOESM7_ESM.tif
Figure S7. Comparison between the simulated geometric mean (solid and dashed lines) and observed PK profiles (open symbols) of abiraterone in healthy controls versus moderately hepatic impaired subjects following single dose administration of 1000 mg AA (TIF 629 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheong, E.J.Y., Chin, S.Y., Ng, Z.W. et al. Unraveling Complexities in the Absorption and Disposition Kinetics of Abiraterone via Iterative PBPK Model Development and Refinement. Clin Pharmacokinet 62, 1243–1261 (2023). https://doi.org/10.1007/s40262-023-01266-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01266-y