Abstract
Background
The pharmacokinetic variability of ampicillin-sulbactam in adults has not been extensively described, particularly in patients with a reduced renal function (i.e., < 60 mL/min).
Objective
This study investigated the population pharmacokinetics of ampicillin and sulbactam in patients with a wide range of renal functions and sought to define dosing approaches that have a high likelihood for optimising drug exposure.
Methods
Serial blood samples were collected from 16 adult patients receiving intravenous ampicillin-sulbactam in general wards. Total ampicillin and sulbactam concentrations were measured by chromatographic assay and pharmacokinetic parameters were estimated using Pmetrics®. Monte Carlo simulations were used to evaluate the probability of target attainment (PTA) of free ampicillin and sulbactam concentrations exceeding the minimum inhibitory concentration (MIC) for 60% and 100% of the dosing interval. Fractional target attainment (FTA) was calculated against MIC distributions of common hospital pathogens. A threshold of ≥ 90% and ≥ 95% was used to define both optimal PTA and FTA, respectively.
Results
The median (range) age, weight, and serum creatinine of the study population was 68 (40–82) years, 62 (40–82) kg, and 1.4 (0.6–6.4) mg/dL, respectively. The pharmacokinetics of ampicillin and sulbactam were best described by a two-compartment model with serum creatinine most closely associated with clearance for both drugs. The estimated ampicillin and sulbactam clearances were 5.58 L/h and 4.79 L/h, respectively, while the volumes of distribution were 12.6 L and 15.36 L, respectively. Approved dosing regimens of ampicillin-sulbactam were sufficient against MICs ≤ 8 and ≤ 4 mg/L, respectively. A 4-h infusion enabled optimal PTA at higher MICs. For both dosing targets, optimal FTAs were obtained against Streptococcus pneumoniae.
Conclusion
Optimal FTAs were obtained against the susceptible MIC distributions of Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Applying a 4-h infusion will enhance PTA and FTA, particularly at higher MICs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this study, wide variations in ampicillin-sulbactam pharmacokinetics (~80 %) were observed among hospitalised patients and the final pharmacokinetics model established serum creatinine as the covariate to describe the clearance of ampicillin-sulbactam in our patient cohort. |
The highest dose in the approved product information may be required to achieve target exposure, particularly for hospitalised patients with preserved renal function infected with less susceptible causative pathogens. |
1 Introduction
Ampicillin is a penicillin antimicrobial with an extended spectrum of activity against Gram-negative bacteria. It remains an essential antimicrobial in the treatment of infections, including in low- and middle-income countries (LMICs) where there is limited access to newer broad-spectrum antimicrobials [1, 2]. However, there are concerns regarding its use due to the highly reported number of ampicillin-resistant pathogens [3, 4]. One mechanism of resistance to ampicillin is the production of bacterial enzymes that cleave the β-lactam ring, known as β-lactamases [5]. Use of ampicillin in combination with a β-lactamase inhibitor, such as sulbactam, can often overcome this resistance mechanism and maintain a broader spectrum of activity, including against Acinetobacter baumannii [5, 6]. The efficacy of ampicillin-sulbactam to treat various infections has been well documented, although registered indications and dosing regimens may vary across countries [6,7,8,9].
Optimising ampicillin and sulbactam exposures is essential to improve patient outcomes whilst potentially lowering the risk of developing resistant pathogens [10]. Ampicillin and sulbactam exhibit time-dependent killing, with pharmacokinetic/pharmacodynamic (PK/PD) targets best represented as the percentage of time in which the unbound fraction of ampicillin and sulbactam remain above the minimum inhibitory concentration (MIC) of the causative organism (% fT>MIC) [11,12,13]. The ability of a dosing regimen to attain desired PK/PD targets may be impacted by the PK variability in the population [10]. Although renal function has been identified as an important covariate, earlier PK studies have not included patients with a wide range of renal functions, particularly those with a reduced renal function (i.e., estimated glomerular filtration rate of < 60 mL/min), to adequately explain the PK variability of both ampicillin and sulbactam in relation to this covariate [14,15,16,17].
In addition to understanding PK, the MIC of the infecting pathogen should ideally be known to describe %fT>MIC target attainment [10]. Nevertheless, in resource-challenged hospitals in LMICs, laboratories to measure MICs are not widely available [18]. Therefore, it is important to evaluate whether commonly prescribed dosing regimens of ampicillin-sulbactam provide optimal %fT>MIC early in therapy (i.e., after the first dose) against a wide range of probable pathogens and MIC distributions. We are unaware of such data from previous studies [14,15,16,17].
Here, we studied the population PK of ampicillin and sulbactam in patients with a wide range of renal functions and sought to define dosing approaches that have a high likelihood for optimising drug exposure against the most frequently identified hospital-based pathogens [18,19,20].
2 Methods
2.1 Study Design and Setting
A prospective, observational PK study was conducted in a referral hospital in Surabaya, Indonesia. Patients aged ≥ 18 years receiving either first or multiple doses of intravenous ampicillin-sulbactam in general wards (excluding the intensive care unit [ICU]) were enrolled in this study. Patients on, or planned for, renal replacement therapy (RRT) at the time of sampling were excluded. Pregnant women were also excluded from the study.
Ethical approvals for the study were granted by the Ethics Committee of Dr Ramelan Navy Hospital (approval number 76/EC/KERS/2019) and The University of Queensland Human Research Ethics Committee (approval number 2018001592). Before collecting blood samples, written informed consent was obtained from either the patients themselves or their legally authorised representatives.
2.2 Drug Administration, Sampling Procedure and Data Collection
Ampicillin-sulbactam dosing regimens were prescribed at the discretion of the treating team. At the time of this study, there was only one product of ampicillin-sulbactam at the research site which consisted of 1000 mg of ampicillin and 500 mg of sulbactam. Doses given, administration times, and number of doses before sampling were documented at the time of sampling.
Doses of ampicillin-sulbactam were diluted with 0.9% sodium chloride just before administration as a bolus injection (over approximately 3 min). Multiple blood samples per patient were collected during one dosing interval (5 min, 20 min, 120 min, 240 min after injection and just before the next dose) with 3 mL of venous blood collected per blood sample using lithium heparin tubes. Each blood sample was placed on ice and immediately transferred to in-house laboratory for centrifugation (3000 rpm for 15 min). After centrifugation, the aliquot was stored in a – 80 °C freezer until analysis.
Relevant patient characteristics (age, gender, body weight, and body mass index) and laboratory data (serum albumin and serum creatinine, SeCr) were collected from the medical records at the time of recruitment. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to determine the estimated glomerular filtration rate (eGFRCKD-EPI) for each patient [21, 22]. All medications administered concomitantly with ampicillin-sulbactam were recorded and checked for potential clinically significant interactions with ampicillin-sulbactam [26]. Interactions classified as “avoid combination” and “usually avoid combination” in the reference used in our study were considered as clinically significant interactions.
2.3 Ampicillin-sulbactam assay
Total concentrations of ampicillin and sulbactam were measured simultaneously in plasma using a validated ultra-high-performance chromatography-tandem mass spectrometry (UHPLC-MS/MS) method on a Nexera UHPLC system coupled to a 8030+ triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). Test samples were assayed in batches alongside plasma calibrators and quality controls, and results were subjected to batch acceptance criteria [27]. The assay methods were linear from 1 to 200 mg/L and 0.5 to 100 mg/L for ampicillin and sulbactam, respectively. The precision at lower limit of quantification (LLOQ) of 1 mg/L (ampicillin) and 0.5 mg/L (sulbactam) was reported as 2.4% and 0.3%, respectively, while the accuracy at the same LLOQ was reported as − 1.1% and 5.8%, respectively. For ampicillin, precision of 2.5%, 1.4%, and 1.9%, and accuracy of 1.5%, − 3.0%, and − 6.4% were reported at concentrations of 3, 30 and 150 mg/L, respectively. For sulbactam, precision of 5.1%, 0.7%, and 8.2%, and accuracy of − 6.7%, 6.3%, and − 1.2% was reported at concentrations of 1.5, 15 and 75 mg/L, respectively.
2.4 Population PK Modelling
2.4.1 Structural Model
Ampicillin and sulbactam concentration data were fitted to generate a population PK model using Non-Parametric Adaptive Grid (NPAG) programme in Pmetrics version 1.9.7. (Laboratory of Applied Pharmacokinetics and Bioinformatics, Los Angeles, CA, USA) for R (version 4.0.1) [23]. A structural model of ampicillin and sulbactam was first developed by comparing the one- and two-compartment model without introducing covariates. Both lambda and gamma error models were tested for ampicillin and sulbactam PK models.
The best structural model was chosen according to the goodness-of-fit of both the population- and individual-observed versus predicted concentration plots, the value of the − 2*Log-likelihood (− 2LL), and the Akaike Information Criterion (AIC). The goodness-of-fit of the models was assessed by visual inspection of the observed versus predicted concentration plots both in population- or individual-scatter plots, the coefficient of determination (R2), slopes, intercept, and bias of the linear regression [28, 29]. For AIC and − 2LL, models with lower values were considered to be better than the comparator with a decrease of 3.84 unit of − 2LL considered statistically significant.
2.4.2 Covariate Analysis
The effect of several biologically plausible demographic and clinical characteristics on the PK of ampicillin and sulbactam was assessed. The variables tested for inclusion were age, gender, weight, body mass index, serum albumin, SeCR, and eGFRCKD-EPI. The final estimated PK parameters from the final model with covariates were presented as mean, standard deviation (SD), percentage coefficient of variation (%CV), median value, and the percentage of shrinkage. The %CV represented the inter-individual variability.
2.4.3 PK Model Diagnostics
The best structural model was chosen according to the goodness-of-fit of both the population- and individual-observed versus predicted concentration plots, the value of the − 2*Log-likelihood (− 2LL), and the AIC. The goodness-of-fit of the models was assessed by visual inspection of the observed versus predicted concentration plots both in population- or individual-scatter plots, the coefficient of determination (R2), slopes, intercept, and bias of the linear regression [23]. For AIC and − 2LL, models with lower values were considered to be better than the comparator with a decrease of 3.84 unit of − 2LL considered statistically significant. Once the structural model was chosen, each covariate was separately added to that particular model and only covariates resulting in a statistical decrease of − 2LL (a decrease of 3.84 units) and AIC, whilst improving the goodness-of-fit of the scatter plots, were retained in the final model. Internal validation was conducted via a visual predictive check (VPC) to evaluate the predictive performance of the final model with covariates using 1000 simulations. The distribution of the observed concentration in this simulation was plotted and visually inspected.
2.4.4 Monte Carlo Dosing Simulation
The final model with covariates was used in the Monte Carlo dosing simulations to identify the dosing strategy of ampicillin and sulbactam with the highest likelihood of achieving target drug exposures. Dosing regimens simulated were selected according to the approved product information that was based on creatinine clearance (CLCr) ranges [11, 12]:
-
1.
Ampicillin: 1000 mg (corresponds to 1500 mg ampicillin-sulbactam) and 2000 mg (corresponds to 3000 mg ampicillin-sulbactam) every 24 h (for CLCr 10 mL/min/1.73 m2), every 12 h (for CLCr 20 mL/min/1.73 m2), every 6 and 8 h for (for CLCr ≥ 30 mL/min/1.73 m2).
-
2.
Sulbactam: 500 mg (corresponds to 1500 mg ampicillin-sulbactam) and 1000 mg (corresponds to 3000 mg ampicillin-sulbactam) every 24 h (for CLCr 10 mL/min/1.73 m2), every 12 h (for CLCr 20 mL/min/1.73 m2), every 6 and 8 h for (for CLCr ≥ 30 mL/min/1.73 m2).
In order to better identify the influence of renal function on the achievement of PK/PD targets, the typical simulated patients with CLCr ≥ 30 mL/min/1.73 m2 were further divided into three (3) groups including: 30, 70, 100 mL/min/1.73 m2. Each dosing regimen was simulated in Pmetrics® (version 1.9.7), with 1000 subjects as either receiving a bolus injection or prolonged infusion over 4 h.
Fixed protein binding values of 28% and 38% for ampicillin and sulbactam, respectively, were used in the dosing simulations [24, 25]. In general, %fT>MIC value of ≥ 50% provided maximal bactericidal effect for ampicillin based on preclinical studies [13]. While for sulbactam, it was found in an in vivo murine thigh infection model that ≥ 60% of fT>MIC would result in maximal bactericidal effect (3 log10 kill against A. baumannii) [11]. However, a recently published consensus paper on antimicrobial therapeutic drug monitoring in critically ill patients recommended a PK/PD target of 100% fT>MIC [26]. It is worth mentioning that even though our study was conducted predominantly in a non-ICU setting, due to limited ICU capacity, critically ill patients were often also treated in general wards. Therefore, the achievement of 60% fT>MIC and 100 % fT>MIC were both used as a priori PK/PD targets for ampicillin-sulbactam in our study.
The percentage of simulated patients that could attain the PK/PD targets was used to calculate the probability of target attainment (PTA). Since adequate PK/PD exposures attained from the beginning of treatment would be important for therapeutic success, the PTAs in our study were calculated both after the first administration and at steady state (i.e., after the fifth administration) [27]. The optimal PTA for a specific MIC was defined as ≥ 90% [34]. To calculate the fractional target attainment (FTA), the PTA of each dosing regimen was compared against the MIC distribution of relevant pathogens. The FTAs of ampicillin were calculated against Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumoniae, while for sulbactam, the FTA was calculated against A. baumannii. Two FTA assessments were performed. First, FTAs of ampicillin and sulbactam were calculated against the whole MIC distribution of bacteria (ranging from 0.002 to 512 mg/L) derived from European Committee on Antimicrobial Susceptibility Testing (EUCAST) database [35]. The intention of evaluating PK/PD exposures against entire MIC distributions was to select optimal dosing regimens in such situations where the MIC of the pathogen was unknown (i.e., when used as empiric therapy). Second, FTAs of ampicillin were calculated against a fixed MIC range for susceptible strains of selected pathogens (i.e., directed therapy), including: ≤ 8 mg/L, ≤ 8 mg/L, and ≤ 0.5 mg/L for E. coli, K. pneumoniae, and S. pneumoniae, respectively [35]. The directed FTAs for sulbactam against A. baumannii were calculated against MIC ≤ 4 mg/L [28]. Findings from the second assessment are relevant where susceptibility reports are made without including the actual MIC value, as often seen in LMIC countries [18, 20]. Any dosing regimen that achieved optimal FTA (i.e., defined as ≥ 95%) was considered a successful dosing regimen either for directed or empirical based therapy.
2.4.5 Statistical Analysis
Descriptive analysis using frequencies (%) for categorical data and mean (± standard deviation; SD) for continuous data in the demographic of patients were conducted using Microsoft Excel v2016.
3 Results
3.1 Patient and Sampling Characteristics
A total of 59 and 60 samples from 16 patients were included in the population PK analyses of ampicillin and sulbactam, respectively. The characteristics of patients included in the study are presented in Table 1.
3.2 Population PK Model
The plasma concentration-time profiles for both ampicillin and sulbactam were best described by a two-compartment model. The improvement of both population- or individual-scatter plots (graphs are not shown) and the value of − 2LL and AIC (Table 2) after the addition of eGFRCKD-EPI on CL of ampicillin and sulbactam were not as good as the addition of SeCr on CL of both antimicrobials. The final model for both ampicillin and sulbactam consisted of SeCr normalised to the median value of patients (1.4 mg/dL) on CL:
where CLAMP and CLSUL stands for clearance of ampicillin and sulbactam, respectively.
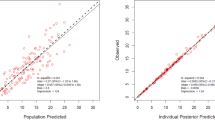
The value of − 2LL and AIC of all compartmental models and estimated population PK parameters obtained from the final model with covariates for ampicillin and sulbactam are presented in Table 2. The goodness-of-fit of both the population- and individual-observed versus predicted concentration plots of final models are shown in Fig. 1A, B, while the VPC plots are presented in Fig. 2A, B.
Visual predictive check plot of the final covariate two-compartment model; y axis indicated concentrations of ampicillin and sulbactam (mg/L). Percentiles (with shaded 95% confidence interval) are the lines shown as 0.95, 0.75, 0.5, 0.25, and 0.05 values. Individual circles represent the observed concentration; A ampicillin; B sulbactam
3.3 Probability of Target Attainment
Dosing simulations were conducted in five groups of simulated patients with different SeCr values. Efforts to correlate SeCr to CLCr were made when SeCr values were found as a significant covariate in the final model, including: SeCr 6, 2, 1.5, 1, 0.7 mg/dL to represent CLCr 10, 20, 30, 70, 100 mL/min/1.73 m2, respectively [29].
The probability of simulated dosing regimens achieving a 60% fT>MIC for ampicillin and sulbactam is presented in Figs. 3 and 4, respectively. To maintain clarity, only the most commonly prescribed dosing regimens at the research site (ampicillin-sulbactam 1500 mg every 8 h) as a bolus injection as well as 4-h prolonged infusion dosing regimens are presented for SeCr 1.5, 1, and 0.7 mg/L, respectively. For the target of 60% fT>MIC, there was at least one dosing regimen of ampicillin-sulbactam in each group of SeCr that attained optimal PTA for ampicillin at MIC of 8 mg/L (MIC value to define susceptible E. coli and K. pneumoniae) [35] and for sulbactam at MIC of 4 mg/L (MIC value to define susceptible A. baumannii) [28], respectively, from the first administration (Table 3).
Probability of target attainment of several dosing regimens of ampicillin-sulbactam (in mg) to attain fT>MIC ≥60% for the ampicillin component at (i) the first 24 hours and (ii) fifth dose for patients with SeCr a 6 mg/dL, b 2 mg/dL, c 1.5 mg/dL, d 1 mg/dL, and e 0.7 mg/dL; q24 h, 12 h, 8 h, 6 h indicate the dose is given q24 h, 12 h, 8 h, 6 h, respectively; B bolus injection. 4hI 4-h infusion, MIC minimum inhibitory concentration
Probability of target attainment of several dosing regimens of ampicillin-sulbactam (in mg) to attain fT>MIC ≥60% for the sulbactam component at (i) the first 24 hours and (ii) fifth dose for patients with SeCr a 6 mg/dL, b 2 mg/dL, c 1.5 mg/dL, d 1 mg/dL, and e 0.7 mg/dL; q24 h, 12 h, 8 h, 6 h indicate the dose is given q24 h, 12 h, 8 h, 6 h, respectively; B bolus injection. 4hI 4-h infusion, MIC minimum inhibitory concentration
For a target of 100% fT>MIC, the PTAs of ampicillin and sulbactam are shown in the Supplementary Figs. 1 and 2, respectively; while the highest MIC at which optimal PTA was attained for all simulated dosing regimens of ampicillin and sulbactam are presented in the Supplementary Table 1.
3.4 Fractional Target Attainment
For 60% fT>MIC, the empiric and directed FTAs of all simulated ampicillin dosing regimens against E. coli, K. pneumoniae, S. pneumoniae are presented in Table 4; while those for sulbactam against A. baumannii are presented in Table 5. Based on our dosing simulations, almost all dosing regimens of ampicillin-sulbactam achieved optimal FTA for both empirical and directed therapy against S. pneumoniae (Table 4). However, no ampicillin-sulbactam dose attained optimal FTA for empirical therapy against E. coli and K. pneumoniae (Table 4). Similar to this, optimal FTA for empirical therapy could not be obtained by any ampicillin-sulbactam dosing regimen against A. baumannii (Table 5). While for directed therapy, optimal FTA was difficult to attain among patients with SeCr ≤ 1 mg/dL against K. pneumoniae (Table 4).
For 100% fT>MIC, the empiric and directed FTAs of ampicillin and sulbactam are presented in Supplementary Tables 2 and 3, respectively.
4 Discussion
4.1 Key Findings
This study included patients with a relatively reduced renal function (11 of 16 patients had eGFRCKD-EPI of < 60 mL/min/1.73 m2), a sub-population that has not been commonly investigated previously. The PK data for both ampicillin and sulbactam were best described using a two-compartment model with SeCr as the covariate for CL. For 60% fT>MIC, dosing regimens recommended in the approved product information for ampicillin-sulbactam attained optimal PTAs against MIC values used to define susceptible strains of the studied bacteria. However, when a target of 100% fT>MIC was used, optimal PTAs were relatively harder to attain. Findings of our dosing simulations emphasised that standard dosing of ampicillin-sulbactam can potentially be used in empirical therapy against S. pneumoniae but not necessarily against E. coli, K. pneumoniae, and A. baumannii even for the lower target of 60% fT>MIC. While for directed therapy, limited options of dosing regimens could attain an acceptable FTA if ampicillin-sulbactam is used to treat infections caused by K. pneumoniae among hospitalised patients with good renal function (SeCr ≤ 1 mg/dL). The highest dose in the approved product information may be required to achieve target exposure, particularly for hospitalised patients with preserved renal function infected with less susceptible causative pathogens.
4.2 Relationship with Previous Studies
The two-compartment PK model for both ampicillin and sulbactam described in the present study was similar when compared with other published ampicillin [18, 30] and sulbactam studies [14, 15]. The covariates affecting the final model, however, are different. Previous studies on ampicillin [16] and sulbactam [14,15,16] found renal function measures as the preferred covariate for CL calculated by using the Cockcroft-Gault equation. We also tested renal function calculated using eGFRCKD-EPI as the covariate for CL for both ampicillin and sulbactam, but the −2LL, AIC, and the goodness-of-fit of models for both ampicillin and sulbactam with the inverse of SeCr on CL was seen to statistically outperform the models with eGFRCKD-EPI on CL.
Although SeCr is generally not considered to be the most accurate measurement of renal function [40], SeCr concentrations may still be valuable to dose optimise ampicillin-sulbactam in LMIC countries for several reasons. First, measured urinary CLCr may not be readily available in some LMIC clinical settings [31]. Second, the association between SeCr and CL, as well as PK/PD exposure, has been observed for other antimicrobials with similar physiochemical properties to both ampicillin and sulbactam [32,33,34]. Finally, the findings of our PK/PD simulation emphasised that PK/PD exposures of a specific dosing regimen were influenced by changes in SeCr values.
The estimated PK parameters for ampicillin and sulbactam described in our study were relatively different compared with other studies. The CLAMP (5.58 L/h) and CLSUL (4.79 L/h) in our study are relatively lower compared to the reported CLAMP (10.7 L/h) and CLSUL (10.4 L/h) among patients in a study by Soto et al. [16]. These differences could be related to observed differences in renal function between the two populations. The study by Soto et al recruited patients with relatively higher renal function (median SeCr = 0.73 mg/dL) compared to our patients (1.4 mg/dL) [16]. Regardless of the differences in reported estimates, the CLAMP among patients in our study and that of Soto et al were relatively lower compared to healthy subjects (CL = 13.28 L/h) [16, 30]. In addition, the CLSUL in our study is also lower compared with those described by Yokoyama et al (7.23 L/h) and Jaruratanasirikul et al (7.75 L/h) [14, 15]. The differences between each of the two studies is attributable to differences in reported median renal function descriptor [14, 15]. Furthermore, the value of CLSUL in our study is also in line with the reported CLSUL in another study by Jaruratanasirikul et al. [35]. In this study, the age of patients included in the study ranged from 21 to 81 years and the CLSUL was 2.26 L/h and 7.64 L/h for patients aged > 65 and ≤ 65 years, respectively. We included patients aged 40–82 years and it could be suggested that the CLSUL in our study, i.e., 4.79 L/h, is in line with those reported by Jaruratanasirikul et al. [35].
In our PTA simulations, we observed superiority in achieving the PK/PD targets if ampicillin-sulbactam was administered as a 4-h prolonged infusion. Even though this superiority was observed in all SeCr groups, it was more prominent in patients with SeCr ≤ 1.5 mg/dL. For both 60% fT>MIC and 100% fT>MIC, any dosing regimen of ampicillin-sulbactam in these patients had a PTA exceeding 90% at a higher MIC value if simulated as a 4-h infusion compared to bolus injection. Consistent with our findings, Jaruratanasirikul et al. also found the superiority of a 4-h infusion of sulbactam to provide adequate PK/PD exposures [14]. However, whether 4-h infusions result in better clinical outcomes in non-ICU patients requires further investigation [36, 37].
Our first FTA assessment demonstrated that all ampicillin-sulbactam dosing regimens (i.e., the ampicillin component) in all SeCr groups were optimal (> 95 %) after both the first and fifth administration against the whole MIC distribution of S. pneumoniae. Furthermore, the optimal FTA was maintained for both PK/PD targets (60% and 100% fT>MIC). However, this was not the case against E. coli and K. pneumoniae. One reason is that 28.1% of E. coli and 87.2% of K. pneumoniae have reported MICs of > 8 mg/L, in which case almost all of our simulated dosing regimens did not attain the optimal PTA [35]. Meanwhile, only one strain of S. pneumoniae was reported with an MIC of 8 mg/L [35]. In contrast, our second assessment of directed therapy found that among all SeCr groups, an ampicillin-sulbactam dosing regimen of 3000 mg as a 4-h infusion provided adequate exposure against S. pneumoniae and E. coli from the first dose even for the higher PK/PD target. While against K. pneumoniae, this dosing regimen would generally be sufficient for the lower target of 60% fT>MIC for the ampicillin component.
All simulated ampicillin-sulbactam dosing regimens were found to be inadequate in providing optimal FTA for either 60% fT>MIC and 100% fT>MIC for the sulbactam component against the whole MIC distribution of A. baumannii. This could be related to the high percentage (41.5%) of A. baumannii with an MIC > 4 mg/L. We did find that most 1500 mg dosing regimens were sufficient to achieve optimal FTA at the lower target of 60% fT>MIC for the sulbactam component against susceptible strains of A. baumannii. A dose of 1500 mg given every 6 h as a 4-h infusion would be appropriate for patients with a relatively preserved renal function (SeCr ranging from 0.7 to 1.5 mg/dL). Our findings, however, were not supported by others [15]. Yokoyama et al. reported that although 90% of A. baumannii had an MIC ≤ 4 mg/L (MIC90 4 mg/L), sulbactam dosing regimens below 2000 mg every 6 h were not enough to attain optimal FTA for a target of 60% fT>MIC in patients with preserved renal function (CLCr 90 mL/min). There could be two reasons for the need for higher doses. First, the doses in Yokoyama et al were simulated as 1-h infusions while ours were as 4-h prolonged infusions. According to Jaruratanasirikul et al., 4-h infusion of sulbactam achieves better PK/PD exposures than 1-h infusion [14]. Second, the population CLSUL of 7.23 L/h reported in Yokoyama et al. was higher than what was found in our study [15].
4.3 Limitations
There are a few limitations that require attention when interpreting our findings. First, our PK/PD simulations were based on applied fixed protein binding percentages (i.e., 28% and 38% for ampicillin and sulbactam, respectively). Simulations based on actual measured free fractions of ampicillin and sulbactam are indeed important because only the free fraction of the drug is responsible for antibacterial activity [38]. Second, as the 60% fT>MIC target for sulbactam against A. baumannii was chosen based on only one study [11], further pre-clinical PK-PD studies are needed to better define the exposure required for maximal sulbactam activity and these investigations need to include a wider range of A. baumannii strains. Third, our dosing simulation trials have not been validated in real clinical settings. Whether SeCr-based dosing adjustment of ampicillin and sulbactam could result in better clinical and microbiological outcomes is yet to be determined. While awaiting such validation, dosing regimens suggested in our simulations could serve to guide clinicians on which dosing regimens are likely to provide a high probability in attaining optimal PK/PD exposures.
5 Conclusion
Both ampicillin and sulbactam PK were well described using a two-compartment model with SeCr being the only covariate on CL. Currently approved dosing regimens may provide adequate exposure against S. pneumoniae, E. coli, K. pneumoniae, and A. baumannii provided that these pathogens are within the MIC distributions of susceptible strains and a target of 60% fT>MIC is deemed acceptable. Administering both ampicillin and sulbactam as 4-h infusions will enhance attainment of PK/PD exposures, even for the higher target of 100% fT>MIC.
References
Lobanovska M, Pilla G. Penicillin’s discovery and antibiotic resistance: lessons for the future? Yale J Biol Med. 2017;90(1):135–45.
Rolinson GN, Stevens S. Microbiological studies on a new broad-spectrum penicilin, “Penbritin.” Br Med J. 1961;2(5246):191–6.
Reed TAN, Krang S, Miliya T, Townell N, Letchford J, Bun S, et al. Antimicrobial resistance in Cambodia: a review. Int J Infect Dis. 2019;85:98–107.
Mortazavi-Tabatabaei SAR, Ghaderkhani J, Nazari A, Sayehmiri K, Sayehmiri F, Pakzad I. Pattern of antibacterial resistance in urinary tract infections: a systematic review and meta-analysis. Int J Prev Med. 2019;10:169.
Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39(6):1211–33.
Williams D, Perri M, Zervos MJ. Randomized comparative trial with ampicillin/sulbactam versus cefamandole in the therapy of community acquired pneumonia. Eur J Clin Microbiol Infect Dis. 1994;13(4):293–8.
Kohno S, Tateda K, Mikamo H, Kadota J, Niki Y, Itamura R. Efficacy and safety of intravenous sulbactam/ampicillin 3 g 4 times daily in Japanese adults with moderate to severe community-acquired pneumonia: a multicenter, open-label, uncontrolled study. J Infect Chemother. 2015;21(3):182–8.
Talan DA, Summanen PH, Finegold SM. Ampicillin/sulbactam and cefoxitin in the treatment of cutaneous and other soft-tissue abscesses in patients with or without histories of injection drug abuse. Clin Infect Dis. 2000;31(2):464–71.
Chan JC. Ampicillin/sulbactam versus cefazolin or cefoxitin in the treatment of skin and skin-structure infections of bacterial etiology. Adv Ther. 1995;12(2):139–46.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509.
Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Shigemi A, Umezaki Y, et al. Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int J Antimicrob Agents. 2014;43(6):547–52.
Rodríguez-Hernández MJ, Cuberos L, Pichardo C, Caballero FJ, Moreno I, Jiménez-Mejías ME, et al. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J Antimicrob Chemother. 2001;47(4):479–82.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10 (quiz 1–2).
Jaruratanasirikul S, Nitchot W, Wongpoowarak W, Samaeng M, Nawakitrangsan M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur J Pharm Sci. 2019;1(136): 104940.
Yokoyama Y, Matsumoto K, Ikawa K, Watanabe E, Morikawa N, Takeda Y. Population pharmacokinetic-pharmacodynamic target attainment analysis of sulbactam in patients with impaired renal function: dosing considerations for Acinetobacter baumannii infections. J Infect Chemother. 2015;21(4):284–9.
Soto E, Shoji S, Muto C, Tomono Y, Marshall S. Population pharmacokinetics of ampicillin and sulbactam in patients with community-acquired pneumonia: evaluation of the impact of renal impairment. Br J Clin Pharmacol. 2014;77(3):509–21.
Blum RA, Kohli RK, Harrison NJ, Schentag JJ. Pharmacokinetics of ampicillin (2.0 grams) and sulbactam (1.0 gram) coadministered to subjects with normal and abnormal renal function and with end-stage renal disease on hemodialysis. Antimicrob Agents Chemother. 1989;33(9):1470–6.
Sugianli AK, Ginting F, Kusumawati RL, Pranggono EH, Pasaribu AP, Gronthoud F, et al. Antimicrobial resistance in uropathogens and appropriateness of empirical treatment: a population-based surveillance study in Indonesia. J Antimicrob Chemother. 2017;72(5):1469–77.
Zilberberg MD, Nathanson BH, Sulham K, Shorr AF. Antimicrobial susceptibility and cross-resistance patterns among common complicated urinary tract infections in U.S. hospitals, 2013 to 2018. Antimicrob Agents Chemother. 2020;64(8).
Budayanti NS, Aisyah DN, Fatmawati NND, Tarini NMA, Kozlakidis Z, Adisasmito W. Identification and distribution of pathogens in a major tertiary hospital of Indonesia. Front Public Health. 2019;7:395.
Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011;58(1):56–63.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–76.
Foulds G. Pharmacokinetics of sulbactam/ampicillin in humans: a review. Rev Infect Dis. 1986;8(Suppl 5):S503–11.
Rolinson GN, Sutherland R. The binding of antibiotics to serum proteins. Br J Pharmacol Chemother. 1965;25(3):638–50.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46(6):1127–53.
Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56(6):2795–805.
Yang Y, Fu Y, Lan P, Xu Q, Jiang Y, Chen Y, et al. Molecular epidemiology and mechanism of sulbactam resistance in Acinetobacter baumannii isolates with diverse genetic backgrounds in China. Antimicrob. Agents Chemother. 2018;62(3).
Park EY, Kim TY. Where are cut-off values of serum creatinine in the setting of chronic kidney disease? Kidney Int. 2010;77(7):645–6.
Rho JP, Jones A, Woo M, Castle S, Smith K, Bawdon RE, et al. Single-dose pharmacokinetics of intravenous ampicillin plus sulbactam in healthy elderly and young adult subjects. J Antimicrob Chemother. 1989;24(4):573–80.
Dowling TC, Matzke GR, Murphy JE, Burckart GJ. Evaluation of renal drug dosing: prescribing information and clinical pharmacist approaches. Pharmacotherapy. 2010;30(8):776–86.
Öbrink-Hansen K, Juul RV, Storgaard M, Thomsen MK, Hardlei TF, Brock B, et al. Population pharmacokinetics of piperacillin in the early phase of septic shock: does standard dosing result in therapeutic plasma concentrations? Antimicrob Agents Chemother. 2015;59(11):7018–26.
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50(2):99–110.
Cutler RE, Gyselynck AM, Fleet WP, Forrey AW. Correlation of serum creatinine concentration and gentamicin half-life. JAMA. 1972;219(8):1037–41.
Jaruratanasirikul S, Wongpoowarak W, Wattanavijitkul T, Sukarnjanaset W, Samaeng M, Nawakitrangsan M, et al. Population pharmacokinetics and pharmacodynamics modeling to optimize dosage regimens of sulbactam in critically ill patients with severe sepsis caused by Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60(12):7236–44.
Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal beta-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108–20.
Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, et al. Continuous versus intermittent beta-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194(6):681–91.
Kunin CM. Clinical pharmacology of the new penicillins. 1. The importance of serum protein binding in determining antimicrobial activity and concentration in serum. Clin Pharmacol Ther. 1966;7(2):166–79.
Acknowledgements
J.A. Roberts would like to acknowledge funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065) as well as an Advancing Queensland Clinical Fellowship. All other authors: none to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflict of interest
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Setiawan, E., Cotta, M.O., Abdul-Aziz, MH. et al. Population Pharmacokinetics and Dosing Simulations of Ampicillin and Sulbactam in Hospitalised Adult Patients. Clin Pharmacokinet 62, 573–586 (2023). https://doi.org/10.1007/s40262-023-01219-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01219-5