Abstract
Background and Objective
Exhaustive pharmacokinetic (PK) studies in paediatric patients are unavailable for most antibiotics and feasibility of PK studies is limited by challenges, such as low blood volume and venipuncture-related pain. Microdialysis (MD) represents a promising method to overcome these obstacles. The aim of this proof-of-concept study was to develop and validate modified MD catheters that can be used to obtain concentration–time profiles of antibiotics in paediatric patients.
Methods
Following extensive in vitro MD experiments, a prospective open-labelled study in ten healthy adult volunteers (HVs) was conducted. Subjects received a single intravenous dose of 1000 mg vancomycin, then plasma and intravascular microdialysate were sampled over 24 h. In vivo MD probe calibration was conducted using the retrodialysis technique. Plasma protein binding was measured using ultrafiltration. Confirmation of the measurements was performed using a Bland-Altman plot, relevant PK parameters were calculated, and a pharmacometric model was established.
Results
No safety issues were encountered. The concentration–time curves of microdialysate and plasma measurements showed good alignment. The Bland-Altman plot yielded a mean bias of 0.19 mg/L and 95% limits of agreement of − 9.34 to 9.71 mg/L. A two-compartment model best described plasma PK, model-based estimates for recovery of the MD probes being in high agreement with the observed values. Quantified estimates of fraction unbound were comparable between plasma and microdialysate (p = 0.56).
Conclusions
An innovative MD catheter that can be inserted into small intravenous lines was successfully developed and applied in HV. This proof-of-concept study is encouraging and opens the way to further experiments leading towards future use of MD in paediatric patients.
Similar content being viewed by others
Performing pharmacokinetic studies in paediatric patients is difficult due to several challenges, such as low blood volume and venipuncture-related pain. We successfully developed an innovative intravascular microdialysis catheter that can be inserted into small intravenous lines for paediatric patients, which overcomes these challenges. |
This proof-of-concept study is encouraging and opens the way to further experiments leading towards future use of microdialysis in paediatric patients. |
1 Introduction
Compared to adults, the pharmacokinetics (PK) of drugs in paediatric patients, especially below the age of 2 years, differ considerably due to maturation-dependent differences in organ function, body weight, body water and protein composition [1]. Furthermore, these parameters continuously change with growth in paediatric patients. In the absence of PK data in the target population, in clinical practice pediatricians usually adapt the administered drug dose by down-scaling the adult dose according to total body weight. However, particularly for paediatric patients below the age of 2 years, this does not adequately account for the unique ontogenetic changes in drug disposition. Simply applying the PK characteristics of adults to paediatric patients involves the risk of potential overexposure and consequent toxicity or underexposure resulting in lack of efficacy [2].
To develop effective and safe treatment regimens for pediatrics, thorough pharmacokinetic/pharmacodynamic (PK/PD) models that integrate the dynamic changes during child development as well as confirmatory clinical data are necessary. However, PK sampling in the pediatric population is severely hampered by physiological, practical and ethical restrictions. On one hand the blood volume is limited in paediatric patients, in particular in preterm and full-term neonates and in infants, preventing the collection of sufficient sample numbers and volumes to estimate continuous blood concentration versus time profiles. On the other hand, repeated venipuncture is an unacceptable burden for paediatric patients, and measures to minimize pain are needed to reduce discomfort to the child and the concerns of parents. Conventional indwelling catheters are available but do not reduce the amount of removed blood, and repeated manipulation might cause contamination or malfunction of the device. Dried blood spot analysis only requires very small amounts of blood but can only be used to measure a few substances and is often more semi-quantitative than quantitative [3]. Quantitative analyses have, however, been performed using dried blood spots [4].
Moreover, all available techniques cannot distinguish between the bound (inactive) and unbound (active) fraction of a drug without relying on an additional method. However, differences in maturation of organs and inter-individual variability make PK/PD modelling without appropriate PK data impossible. Pharmacokinetic analysis of sparse sample data can be achieved using pharmacometric models. For vancomycin several such models have been developed using data from paediatric patients [5,5,7]. However, the development of pharmacometric models is time-consuming, computationally intensive, and usually relies to some degree on assumptions. Another approach involves scaling adult PK to get an estimate of paediatric PK and, thereby, determine the appropriate dose [8].
The difficulties in conducting exhaustive PK studies in paediatric patients have led to the current situation, where 30–50% of drugs that are used by pediatricians have insufficient dosing data and are unlicensed in paediatric patients or are used off-label [9, 10].
Microdialysis (MD) is a well-established method to measure unbound drug concentrations in adults and displays a unique opportunity to overcome the aforementioned obstacles of PK measurements in paediatric patients. It is a volume neutral method allowing continuous measurement of unbound (active) concentrations of pharmacological substances in blood. Furthermore, MD probes can be inserted in peripheral intravenous lines, which enables continuous dense PK sampling with no or minimal additional pain for the child [11,11,13]. However, MD is rarely used in paediatric patients, and the currently available MD probes do not fit the small intravenous lines used on pediatric wards. Therefore, the development and validation of MD probes that could be used in paediatric patients would enable the accumulation of invaluable information on the PK of drugs in paediatric patients.
The aim of our study was to develop and validate a practical method for PK measurements in paediatric patients. We investigated vancomycin as a representative drug because it is frequently used in the paediatric population.
2 Materials and Methods
2.1 Ethics
The clinical part of this study was conducted at the Department of Clinical Pharmacology at the Medical University of Vienna (Austria) in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. Approval of the Ethics Committee of the Medical University of Vienna and the Austrian Agency for Health and Food Safety was obtained, and the study was registered under the EudraCT number 2018-000010-38. Oral and written informed consent to participate was obtained from all study subjects before enrollment in the study.
2.2 Modified Microdialysis Catheters
The custom-made MD catheters were modified from the existing 67 Intravenous MD catheter (M Dialysis AB) to fit smaller intravenous lines (pink, 20 gauge) by shortening the length of the shaft to 10 mm and extending the length of the membrane to 60 mm (plus 4 mm glued tip). The part of the membrane within the blood stream is 19 mm long, with a glued tip of 4 mm and an active membrane of 15 mm. As a consequence of the increased membrane length, 45 mm of the membrane lies within the plastic tube of the intravenous line. The custom-made MD catheters fit intravenous lines with a gauge size of 20G, which is regularly used in children from 4 years of age and above.
The accessories used with the modified MD catheters were the same as recommended by the manufacturer for the unmodified 67 IV MD Catheter, and no modification of the accessories was performed.
A comparison between the modified MD catheter and the unmodified, commercially available catheter (67 Intravenous MD catheter by M Dialysis AB) is shown in Table 1.
2.3 In Vitro Experiments
In vitro MD experiments were performed prior to initiation of the clinical part of the study. The in vitro experiments served to assess if the recovery is similar in both directions (forward and retrodialysis) and constant over time. Furthermore, the in vitro MD experiments were conducted to choose an appropriate setting for the in vivo calibration procedure (retrodialysis technique). Two experiments (A and B) were performed in triplicate each (three MD probes). For experiment A physiological saline was used as the perfusion solution. Due to the dissatisfying results of experiment A (refer to the Sect. 3, Results), human albumin 1% was added to the perfusion solution and the procedures were repeated (experiment B).
The experiments were performed in a shaking water bath at approximately 37 °C. Three MD probes were placed separately in intravenous lines, which were placed in glass tubes containing Aqua a.i. The MD probes were inserted into the same intravenous lines that were used for the in vivo study. This was especially interesting since part of the MD membrane was lying within the plastic tube of the intravenous line. The probes were then perfused with either physiological saline or human albumin 1% solution at a flow rate of 1 µL/min for at least 12 h before the start of the forward experiment to coat the catheters and minimize adhesion of the drug to the catheter.
On day 1 of the experiments forward dialysis was performed. The coated MD probes were placed in separate glass vials containing a solution of 10 μg/mL vancomycin in human serum (pooled, sterile filtered) and then constantly perfused with either physiological saline or human albumin 1% solution at a flow rate of 1 µL/min using CMA 107 (M Dialysis AB) precision pumps.
After an equilibration period of at least 60 min, three consecutive microdialysate samples over 60-min intervals (0–60 min, 60–120 min, 120–180 min) were collected from each of the three probes. After completion of the last sampling period, all probes were placed in Aqua a.i. and perfused overnight with either physiological saline or human albumin 1% solution at a flow rate of 0.5 µL/min.
On the next day the retrodialysis experiments were performed. A solution of 10 μg/mL vancomycin in either physiological saline or human albumin 1% solution was used as perfusion solution. Pooled, sterile filtered serum was applied as immersion solution for all probes in separate glass vials. Probes were constantly perfused at a flow rate of 1 µL/min using CMA 107 precision pumps. After an equilibration period of at least 60 min, microdialysate sampling was performed identically to the sampling schedule described for forward dialysis.
Aliquots of immersion solutions in the forward dialysis experiments and aliquots of perfusion solutions in the retrodialysis experiments were collected before and at the end of each sampling period and stored together with the collected microdialysate samples at − 80 °C until analysis.
Vancomycin recovery during forward dialysis was calculated as the concentration in the MD vial of one time-interval divided by the concentration in the immersion solution:
Loss during retrodialysis was calculated as 1 minus the quotient of the concentration in the MD vial of one time-interval and the concentration in the perfusion solution:
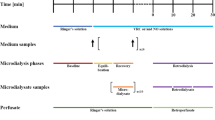
2.4 Trial Design and Study Population
The study was a prospective, open-label, single-centre PK study with ten healthy adult male volunteers.
Prior to inclusion, subjects were informed about the objectives, the procedures as well as the risks associated with the study. Inclusion and exclusion criteria and demographic data including underlying disease were assessed. Each enrolled subject underwent a general medical assessment including medical history, body mass index, blood sampling for laboratory screening tests and virology, vital signs and electrocardiogram.
Subjects were enrolled in the study provided the inclusion/exclusion criteria were fulfilled and upon receipt of signed informed consent.
On the morning of the study day, healthy volunteers (HVs) were admitted to the clinical research ward. Subjects received a total of four intravenous lines (Venflon®). One line was inserted into an antecubital vein of the arm for intravenous infusion of the study drug and three additional venous catheters were inserted on the contralateral arm. One of those was placed into the antecubital vein for repetitive blood sampling. Two 20-gauge venous catheters (BD Venflon, 20GA) were applied to forearm veins for the insertion of two modified MD probes, serving as duplicates of the same procedure. The MD systems were connected and perfused with human albumin 1% solution by means of microinfusion precision pumps (CMA 107, M Dialysis AB) at a flow rate of 2.0 μL/min. After MD probe insertion, an equilibration period of 30 min was allowed. Baseline samples of microdialysate and plasma were obtained prior to study drug administration.
A single dose of vancomycin 1000 mg (Vancomycin® ‘Hikma’ 1000 mg vials, Hikma Pharmaceutics, Portugal) was administered intravenously over 60 min. Plasma and microdialysate sampling time-points are given in the next section.
After the last microdialysate sample was obtained, in vivo recovery was estimated using the retrodialysis technique (30 min run-in, 2 × 30 min sampling). For retrodialysis a flow rate of 2.0 µL/min and a calibration solution with 10 μg/mL vancomycin in human albumin 1% solution were chosen based on the results of the in vitro experiments. Influence of the recovery/loss rate on final concentrations was limited by exclusion of all MD probes from the analysis that showed recoveries < 10%.
A final visit was performed in the study participants up to 7 days after the study day.
Since MD provides the concentration of the unbound drug fraction, the unbound fraction from the values obtained through blood sampling was calculated for comparison. Ultrafiltration was employed to determine the protein binding and calculate the unbound fraction of vancomycin for each HV separately.
2.5 In Vivo Sampling Time-Points and Handling of Samples
Blood was collected into lithium-heparin tubes (Vacuette® lithium-heparin) at baseline and the following time-points after drug administration: 30 min, 1, 2, 3, 4, 5, 6, 8, 12 and 24 h. These sampling time-points were chosen to cover the most important time-points of the concentration–time profile. One sample in the ascending part of the concentration–time curve, one point at Cmax, and then more dense sampling at the beginning of the descending part because more dynamic changes are expected here. The venous catheters were rinsed with physiological saline solution after each sampling. Blood samples were placed on ice immediately after collection and subsequently centrifuged (2000g, 10 min, 4 °C). Resulting plasma was divided into two aliquots and snap frozen at − 20 °C within 60 min from collection.
Sampling of dialysates was performed at baseline and at defined time-intervals to match the plasma sampling time-points: 15–45 min, 45–75 min, 1.5–2.5 h, 2.5–3.5 h, 3.5–4.5 h, 4.5–5.5 h, 5.5–6.5 h, 7.5–8.5 h, 11.5–12.5 h, 23.5–24.5 h. Dialysate samples were frozen at −20 °C.
At the end of the study day, plasma and microdialysate samples were transferred from − 20 to −80 °C and stored at − 80 °C until further analysis.
2.6 Sample Analysis
The concentration of vancomycin in plasma and MD was determined by high-performance liquid chromatography (HPLC) using a Dionex ‘UltiMate 3000’ system (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with ultraviolet (UV) detection at 220 nm. Frozen plasma samples were thawed at room temperature. After the addition of 200 µL of acetonitrile to 100 µL plasma, the samples were centrifuged (14000g for 5 min) and 100 µL of the clear supernatant was injected onto the HPLC column. Microdialysate samples (10 µL) were injected onto the column without any previous precipitation procedure. Chromatographic separation was carried out at 40 °C on a Hypersil BDS C18 column (5 µm, 250 × 4.6 mm internal diameter;
Thermo Fisher Scientific, Inc.) preceded by a Hypersil BDS-C18 precolumn (5 µm, 10 × 4.6 mm internal diameter) at a flow rate of 1 mL/min. The mobile phase consisted of a continuous gradient mixed from ion pair buffer, pH 3.0 (50 mM potassium phosphate with phosphoric acid and 5 mM heptane sulfonic acid) (mobile phase A) and acetonitrile (mobile phase B). The gradient started at 5% acetonitrile (0 min), was kept constant at 5% till 8 min, linearly increased to 25% at 17.5 min, and further increased within 2.5 min to 80%, at which point it was kept constant until 23 min. The percentage of acetonitrile was then decreased within 0.5 min to 5% to equilibrate the column for 6 min before injection of the next sample. Quantification of vancomycin was based on external calibration by spiking drug-free human plasma and microdialysate with standard solutions of vancomycin to give a concentration range of 0.01 mg to 100 mg/L (average correlation coefficients: > 0.999). The limit of quantification (LOQ) for vancomycin in plasma was 0.05 mg/L, and in microdialysate 0.02 mg/L. Coefficients of accuracy and precision for this compound were < 9.2%.
Plasma protein binding (PPB) of vancomycin was determined ex vivo by means of ultrafiltration. A baseline, drug-free blood sample from each HV was spiked with 2.5 mg/L and 50 mg/L of vancomycin. PPB was then determined for both concentrations using ultrafiltration. Briefly, a 200 µL plasma aliquot was transferred to a Centrisart filtration unit with a CTA membrane (MWCO: 5000 Da; Sartorius Stedim Biotech GmbH, Goettingen, Germany) and centrifuged at 1000g for 10 min at room temperature. Ultrafiltration efficiency of the filters used was strongly dependent on individual plasma samples (for some it was 220 µL, for some up to 380 µL). In order to use the exact protocol for all samples, 200 µL aliquots were used. The recovered ultrafiltrate was subsequently analysed by HPLC without extraction, to determine the concentration of free (unbound) drug in the plasma. Samples that did not undergo ultrafiltration were assayed to determine total (bound and unbound) drug concentration. Protein binding of vancomycin was then calculated according the following equation:
2.7 Statistics, Pharmacokinetic Analysis and Study Endpoints
Correlation of the observed concentrations measured with the two methods (MD and plasma sampling) was tested with Pearson correlation and agreement of the measurements was tested using a Bland-Altman plot [17].
Normal distribution of the differences in PK parameters between the two methods was assessed using the Kolmogorov–Smirnov test [18]. Statistical comparison of the PK parameters obtained with the two methods was performed using a paired, parametric t test (normally distributed parameters) or a Wilcoxon matched-pairs signed rank test (not normally distributed parameters) with a 0.05 two-sided significance level.
A pharmacometric data analysis was performed for plasma, ultrafiltration as well as MD measurements using non-linear mixed effects modelling in NONMEM® (ICON, Gaithersburg, MD, USA, version 7.4.3) together with PsN [14]. First, plasma data were analysed separately and one- and two-compartment models were evaluated. Second, ultrafiltration data were added to the model assuming a constant fraction unbound. Third, the raw MD measurements were added to the model. Here, the raw measurements were used and the recovery was estimated within the model using the integral sampling approach developed by Tunblad et al. [15].
Inter-individual variability of the PK parameters was assumed to be log-normally distributed. For the residual error, additive, proportional and combined additive/proportional error models were evaluated. Competing models were compared using the likelihood ratio test (dOFV: 3.84, apha = 0.05, df = 1) for nested models. In addition, goodness-of-fit plots as well as visual predictive checks were performed. Parameter uncertainty was assessed using the log-likelihood profiling-based sampling importance resampling (LLP-SIR) method, specifically suitable for evaluation of parameter uncertainty in small-n datasets [16].
The following PK parameters were calculated with noncompartmental analysis using a commercially available software program (Phoenix® WinNonlin® Build 8.0, Certara USA, Inc., Princeton, NJ, USA): Area under the concentration time curve (AUC) from 0 to 24 h (AUClast), area under the concentration time curve (AUC) from 0 to infinity (AUC∞), maximum drug concentration (Cmax), terminal half-life (t1/2), and clearance (Cl). Further outcome variables were safety and tolerability of MD measurement.
3 Results
3.1 In Vitro Experiments
Table 2 shows the mean recovery/loss rates of the three MD probes for each time-interval. Experiment A (physiological saline) yielded mean values (± standard deviation) of 28.6% (± 1.0%) and 13.4% (± 2.7%) for recovery (forward dialysis) and loss (retrodialysis), respectively. Because of the marked difference between forward and retrodialysis and the low rates of recovery/loss, the experiments were modified by adding human albumin 1% to the perfusion solution (experiment B). In these experiments the mean recovery (± standard deviation) during forward dialysis was 52.7% (± 3.8%) and the mean loss during retrodialysis was 45.8% (± 4.0%). In experiment B values for recovery/loss were similar between the sampling-intervals and in both directions (between retrodialysis and forward dialysis). Therefore, this experimental setting of experiment B was chosen for the calibration in the subsequent in vivo part of the study.
3.2 Demographics and Safety Analysis
Subjects’ demographics including estimated glomerular filtration rate (eGFR), calculated using the Chronic Kidney Disease Epidemiology Collaboration Equation, albumin and total protein are given in Table 3. Mean age was 27.3 ± 5.1 years and mean weight 75.5 ± 6.4 kg. All subjects had an eGFR and albumin within the normal range.
No safety issues were observed with the modified MD catheter. One subject had a mild allergic reaction to vancomycin (rash, chills). Apart from this, no adverse events occurred.
3.3 Pharmacokinetics Measured with Blood Sampling Versus Modified Microdialysis Catheters
3.3.1 Unbound Concentrations with Blood Sampling
For the plasma samples spiked with 2.5 and 50 mg/L vancomycin, mean PPB was 46.1 ± 0.02% and 52.0 ± 0.02%, respectively. Mean PPB overall was 49% with a standard deviation (SD) of 1.7% (Table 3). The mean PPB values for individual subjects were calculated based on the PPB values determined for the two concentrations and used to calculate the individual unbound fraction of the drug measured through blood sampling.
3.3.2 Unbound Concentrations with Microdialysis
Mean in vivo loss rates obtained during calibration with the retrodialysis technique for the two probes per HV are shown in Table 4. The mean loss rate over all subjects was 18.6% with a SD of 3.7%. In two subjects, one of the two MD probes showed loss rates < 10%. Therefore, we excluded the concentrations measured with these two probes from the analysis. Loss rates of each probe were used to back-calculate the unbound concentration present at the MD insertion site.
3.3.3 Comparison of Concentrations Measured with Blood Sampling Versus Microdialysis
Figure 1 shows the concentration time-profiles of the mean unbound (n = 10) plasma concentrations and the mean microdialysate concentrations (n = 18, two probes per HV) of vancomycin. The concentration–time curves show a good alignment, but concentrations measured with MD showed greater variability as demonstrated by the higher standard deviations compared to plasma sampling.
The correlation coefficient (Pearson) of the two methods was r = 0.783 with a p < 0.0001, indicating a significant positive correlation between the concentration measurements (Fig. 2).
The Bland-Altman plot in Fig. 3a compares the differences in concentrations (Cmicrodialysate – Cunbound plasma) with the average concentrations. The mean bias was 0.188 mg/L and the 95% limits of agreement were − 9.34 to 9.71 mg/L. In Fig. 3b the differences are plotted as percentage of the average concentration ((Cmicrodialysate – Cunbound plasma)/Caverage%). Mean bias was − 19.6 and 95% limits of agreement were − 102.8 to 63.61%. The 95% confidence interval (CI) of the mean bias is indicated in the graphs as a shaded area.
Bland-Altman plot for microdialysate and unbound plasma concentrations. In plot a the differences in concentrations (Cmicrodialysate − Cunbound plasma) vs. the average concentrations are plotted. In plot b the differences as percentage of the average concentration (Cmicrodialysate − Cunbound plasma)/Caverage%) vs. the average concentrations are plotted. Dotted lines represent 95% limits of agreement and lines of equality. Shaded areas represent 95% confidence intervals for mean bias
3.3.4 Comparison of Pharmacokinetic Parameters Calculated from Concentrations of the Two Methods
PK parameters calculated from the unbound vancomycin concentrations measured with plasma sampling and MD are given in Table 5. A statistically significant difference between the two methods was only observed for t1/2 (p value 0.022).
3.3.5 Pharmacometric Data Analysis
Plasma PK was best described by a two-compartment model and the unbound fraction by a constant fraction for both ultrafiltrate and MD. The model-based typical estimate for recovery of the MD probes (17.6%) were in high agreement with the observed values. The quantified estimates of fraction unbound were very similar: 0.48 (95% CI 0.40–0.59) for MD and 0.51 (95% CI 0.50–0.53) for ultrafiltration; the difference between the two was statistically insignificant in the model (p = 0.56). A visual predictive check of the pharmacometric model is presented in Fig. 4 indicating an overall adequate fit of the model with some slight underprediction at 8 and 12 h in the MD. Goodness-of-fit and residual plots are presented the Online Supplementary Material, Fig. 1, where the same pattern appears. The final parameter estimates of the model are given in Table 6.
Visual predictive check of the pharmacometric model for total plasma (left) and raw measurements of the microdialysis probes (unbound plasma concentrations); raw data are presented with median (solid line) and 10th to 90th percentiles (dashed lines). Shaded areas represent 95% confidence intervals of corresponding model predictions
4 Discussion
The present proof-of-concept study is unique in that it investigated a novel potential method for dense PK sampling in paediatric patients. In vitro experiments were conducted to evaluate the MD catheters that were modified for use in paediatric patients. The subsequent clinical study in HVs served to validate the measurements of the modified MD catheters and opened the way to further experiments leading towards a future use in paediatric patients. Thereby, we aimed at meeting the urgent need for a method that allows dense PK sampling in paediatric patients and ultimately improves their treatment.
A very promising MD setting could be established in the in vitro experiments. The addition of human albumin to the perfusion solution led to recovery/loss rates that were stable over time, similar in both directions (forward- and retrodialysis) and sufficiently high. The observed in vitro recovery/loss of about 50% leads to a correction factor of two, which in turn limits the influence of recovery/loss rate on the corrected concentration.
Because of these encouraging results, the clinical study was initiated and carried out successfully. No adverse events were reported that could be related to the modified MD catheters. The mean PPB of 49% measured in the present study lies well within the range of previously reported values for vancomycin PPB [19]. PK parameters calculated from plasma samples are similar to those previously reported [20]. Boeckh et al. performed a single dose study with administration of vancomycin 1 g to ten HVs. Only total concentrations were measured in this study, but a Cmax of 74.6 mg/L and t1/2 of 4.95 h were close to the values determined in the present study. Clearance showed greater discrepancies with 5.79 versus 9.7 L/h in the present study [21].
Concentrations measured with MD show higher inter-subject variability leading to higher standard deviations of PK parameters compared to blood sampling (Fig. 1). One possible factor contributing to the higher variability of the MD measurements is the high correction factor due to the low loss rates of vancomycin (mean 18.6 ± 3.7%) during retrodialysis. A high correction factor potentially amplifies analytical imprecision and corresponding variability. The low loss rate was an unexpected finding, since a mean loss rate of 45.8% was observed in the in vitro experiments. To increase the recovery (and loss rate) in future experiments a lower flow rate might prove valuable. This would potentially decrease variability. Schroepf et al. have recently published a MD study with vancomycin in which they included experiments with different flow rates in the in vitro setting. They demonstrated an increase in recovery from 27.1 to 51.1% by decreasing the flow rate from 2 to 0.5 µL/min [22]. However, the minimum volume required for sample analysis has to be taken into account when reducing the flow rate to such low values. Busse et al. have investigated MD-related variability with linear mixed-effects modelling of samples from 120 adult patients. The authors came to the conclusion that patient-associated variability accounted for 46.7%, catheter-associated variability for 16.8%, and residual unexplained variability for 36.4% of overall variability [23]. In our case the impact of patient associated variability is expected to be smaller, since the patient cohort was very homogenous, and such variability was not observed with conventional blood sampling.
A Bland–Altman plot was employed to directly assess agreement between the two methods. In relation to the observed concentrations (ranging from 0 to 47.3 mg/L), the mean bias of 0.19 mg/L seems small, but the 95% limits of agreement of − 9.34 to 9.71 mg/L are rather wide. This becomes even more evident when looking at the differences as percentage of the average concentration with a mean bias of − 19.6 and 95% limits of agreement of − 102.8 to 63.61% (Fig. 3b). The agreement between the two methods does not appear to be strong enough to replace individual concentrations measured by blood sampling with concentrations measured by the new MD catheters.
For the present study, two existing medical devices were combined (modified MD catheter and 20-gauge intravenous line). It is expected that specially designed MD probes could provide concentrations that could more reliably substitute for individual concentrations measured by blood sampling. Unfortunately, in the academic setting of this study, it was not feasible to develop MD probes specifically for use in smaller intravenous lines.
Apart from this, an inherent factor of MD that contributes to the difference between plasma and MD is that with MD each concentration represents a mean over a period of time (in the present study 30 or 60 min; see the methods, Sect. 2). To keep this factor as small as possible, shorter sampling intervals (30 min) were chosen for the beginning of the concentration–time profiles, where the greatest dynamic in the concentration–time profile is expected. In addition, blood sampling time-points were scheduled to lie in the middle of the MD sampling intervals.
Interestingly, with increasing concentrations, a trend reversal can be observed in the Bland-Altman plots. With low concentrations MD seems to underestimate the concentrations compared with plasma, whereas with higher concentrations, MD seems to slightly overestimate. Concentration-dependent protein binding could be an explanation for this finding. Wulkersdorfer et al. have demonstrated that the PPB of clindamycin decreases with increasing clindamycin concentrations [13]. In the present study ultrafiltration was performed with two different concentrations (2.5 and 50 mg/L) and the mean PPB was used to back calculate the unbound drug fraction for each HV (refer to the methods, Sect. 2). In contrast, MD directly samples the unbound drug concentration and therefore incorporates dynamic changes in PPB. Decreasing PPB with increasing vancomycin concentrations, as demonstrated for clindamycin, would explain the trend reversal observed in the concentration differences. However, when comparing PPB determined from the ultrafiltrate spiked with the two different concentrations, PPB of the low concentrations was smaller (46.1 vs. 52.0%). Furthermore, in the pharmacometric model, the unbound fraction in plasma and MD was best described by a constant fraction. This contradicts the aforementioned assumption that PPB might be concentration-dependent and a higher sample size might be needed to estimate a non-linear PPB of small magnitude. Ultimately, the influence of protein binding on concentration differences cannot be conclusively assessed by the present study. Regardless of whether PPB is concentration-dependent or not, the impact of PPB is most relevant in critically ill patients. In this patient population, on the one hand, it is crucial to achieve sufficiently high concentrations, and on the other hand, there are pronounced and dynamic changes in plasma protein concentration and thus in the free drug fraction. Therefore, especially for these patients, it is highly valuable to have a method with which the free drug fraction can be measured.
For the investigation of appropriate dosing schemes of drugs, PK parameters rather than individual concentrations are of primary importance. The differences in PK parameters between the two methods are much lower compared to the observed differences in concentrations (Table 5).
The antimicrobial effect of vancomycin is mainly driven by the area under the concentration–time curve divided by the minimal inhibitory concentration (AUC/MIC) [20]. In the present study, the mean AUC0–24 measured with MD and plasma was similar (87.8 ± 19.1 vs. 98.2 ± 9.1). Therefore, the assessment of the dosing scheme of vancomycin would yield similar if not identical conclusions regardless of the method used. For antimicrobial agents, the differences in PK parameters are further to be put into perspective in view of the inaccuracy of the MIC, which is the factor that is related to the PK parameters for PK/PD calculations (as one of the three PK/PD indices: AUC/MIC, Cmax/MIC or %T>MIC). The difference between two MICs of the same bacterial strain–antimicrobial drug combination determined at two different laboratories is commonly one twofold dilution, corresponding to a multiplication factor of 2 or 0.5 [24]. Compared to this, the observed differences in PK parameters are marginal.
Two MD probes yielded recoveries < 10% and the concentrations measured with these catheters were not included in the analysis. This is a limitation of our study, but we could not identify any specific reason for this and have to assume these two MD catheters were malfunctioning.
5 Conclusions
An innovative MD catheter that can be inserted into small intravenous lines was successfully developed and applied in initial experiments in healthy adult volunteers. This proof-of-concept study is encouraging and opens the way to further experiments leading towards future use of MD in paediatric patients.
References
Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395–404. https://doi.org/10.1111/bcp.12267.PubMedPMID:25855821;PubMedCentralPMCID:PMCPMC4345950.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. https://doi.org/10.1056/NEJMra035092.
Sharma A, Jaiswal S, Shukla M, Lal J. Dried blood spots: concepts, present status, and future perspectives in bioanalysis. Drug Test Anal. 2014;6(5):399–414. https://doi.org/10.1002/dta.1646 (Epub 20140401).
Linder C, Wide K, Walander M, Beck O, Gustafsson LL, Pohanka A. Comparison between dried blood spot and plasma sampling for therapeutic drug monitoring of antiepileptic drugs in children with epilepsy: a step towards home sampling. Clin Biochem. 2017;50(7–8):418–24. https://doi.org/10.1016/j.clinbiochem.2016.12.008 (PMID 28027888).
Kloprogge F, Hill LF, Booth J, Klein N, Irwin AD, Dixon G, et al. Revising pediatric vancomycin dosing accounting for nephrotoxicity in a pharmacokinetic-pharmacodynamic model. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/aac.00067-19 (Epub 20190425).
Germovsek E, Osborne L, Gunaratnam F, Lounis SA, Busquets FB, Standing JF, et al. Development and external evaluation of a population pharmacokinetic model for continuous and intermittent administration of vancomycin in neonates and infants using prospectively collected data. J Antimicrob Chemother. 2019;74(4):1003–11. https://doi.org/10.1093/jac/dky525.
Lu JJ, Chen M, Lv CL, Zhang R, Lu H, Cheng DH, et al. A population pharmacokinetics model for vancomycin dosage optimization based on serum cystatin C. Eur J Drug Metab Pharmacokinet. 2020;45(4):535–46. https://doi.org/10.1007/s13318-020-00621-9.
Germovsek E, Barker CIS, Sharland M, Standing JF. Pharmacokinetic-pharmacodynamic modeling in pediatric drug development, and the importance of standardized scaling of clearance. Clin Pharmacokinet. 2019;58(1):39–52. https://doi.org/10.1007/s40262-018-0659-0.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320(7227):79–82. https://doi.org/10.1136/bmj.320.7227.79.
Yackey K, Stukus K, Cohen D, Kline D, Zhao S, Stanley R. Off-label medication prescribing patterns in pediatrics: an update. Hosp Pediatr. 2019;9(3):186–93. https://doi.org/10.1542/hpeds.2018-0168 (Epub 20190211).
Muller M. Science, medicine, and the future: microdialysis. BMJ. 2002;324(7337):588–91. https://doi.org/10.1136/bmj.324.7337.588.
Al Jalali V, Wolfl-Duchek M, Taubert M, Matzneller P, Lackner E, Dorn C, et al. Plasma and soft tissue pharmacokinetics of ceftolozane/tazobactam in healthy volunteers after single and multiple intravenous infusion: a microdialysis study. J Antimicrob Chemother. 2021;76(9):2342–51. https://doi.org/10.1093/jac/dkab166.
Wulkersdorfer B, Wicha SG, Kurdina E, Carrion Carrera SF, Matzneller P, Al Jalali V, et al. Protein binding of clindamycin in vivo by means of intravascular microdialysis in healthy volunteers. J Antimicrob Chemother. 2021;76(8):2106–13. https://doi.org/10.1093/jac/dkab140.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57. https://doi.org/10.1016/j.cmpb.2005.04.005.
Tunblad K, Hammarlund-Udenaes M, Jonsson EN. An integrated model for the analysis of pharmacokinetic data from microdialysis experiments. Pharm Res. 2004;21(9):1698–707. https://doi.org/10.1023/b:pham.0000041468.00587.c6.
Broeker A, Wicha SG. Assessing parameter uncertainty in small-n pharmacometric analyses: value of the log-likelihood profiling-based sampling importance resampling (LLP-SIR) technique. J Pharmacokinet Pharmacodyn. 2020;47(3):219–28. https://doi.org/10.1007/s10928-020-09682-4 (Epub 20200404).
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82. https://doi.org/10.1080/10543400701329422.
Kolmogorov AN. Sulla Determinazione Empirica di Una Legge di Distribuzione. Giornale dell’Istituto Italiano degli Attuari. 1933;4:83–91.
Albrecht LM, Rybak MJ, Warbasse LH, Edwards DJ. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP. 1991;25(7–8):713–5. https://doi.org/10.1177/106002809102500701.
Al Jalali V, Zeitlinger M. Clinical pharmacokinetics and pharmacodynamics of telavancin compared with the other glycopeptides. Clin Pharmacokinet. 2018;57(7):797–816. https://doi.org/10.1007/s40262-017-0623-4.
Boeckh M, Lode H, Borner K, Hoffken G, Wagner J, Koeppe P. Pharmacokinetics and serum bactericidal activity of vancomycin alone and in combination with ceftazidime in healthy volunteers. Antimicrob Agents Chemother. 1988;32(1):92–5. https://doi.org/10.1128/AAC.32.1.92.
Schroepf S, Burau D, Muench HG, Derendorf H, Zeitlinger M, Genzel-Boroviczény O, et al. Microdialysis sampling to monitor target-site vancomycin concentrations in septic infants: a feasible way to close the knowledge gap. Int J Antimicrob Agents. 2021;58(4): 106405. https://doi.org/10.1016/j.ijantimicag.2021.106405 (Epub 20210718).
Busse D, Simon P, Michelet R, Ehmann L, Mehner F, Dorn C, et al. Quantification of microdialysis related variability in humans: clinical trial design recommendations. Eur J Pharm Sci. 2021;157: 105607. https://doi.org/10.1016/j.ejps.2020.105607 (Epub 20201022).
Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J. MIC-based dose adjustment: facts and fables. J Antimicrob Chemother. 2018;73(3):564–8. https://doi.org/10.1093/jac/dkx427.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Valentin al Jalali wrote the manuscript, designed the research, performed theresearch, and analysed data. Martin Bauer performed the research and critically revised the manuscript. Michael Wölfl-Duchek performed the research and critically revised the manuscript. Maysa Sarhan performed the research and critically revised the manuscript. Sebastian G. Wicha wrote manuscript and analysed data. Stefan Poschner analysed data and critically revised the manuscript. Walter Jäger analysed data and critically revised the manuscript. Franz König designed the research and critically revised the manuscript. Christoph Male designed the research and critically revised the manuscript. Markus Zeitlinger designed the research, analysed data and critically revised manuscript
Funding
Open access funding provided by Medical University of Vienna.
Conflict of interest
No conflicts of interest.
Ethics approval and informed consent
Ethics approval and informed consent of each study participant were obtained before study inclusion.
Consent to participate
Oral and written informed consent to participate was obtained from all study subjects before enrollment in the study.
Consent for publication
Not applicable.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The code was made available in the supplemental material.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
al Jalali, V., Bauer, M., Wölfl-Duchek, M. et al. Pilot Pharmacokinetic Study in Healthy Adults Using Intravascular Microdialysis Catheters Modified for Use in Paediatric Patients to Assess Vancomycin Blood Levels. Clin Pharmacokinet 62, 77–87 (2023). https://doi.org/10.1007/s40262-022-01190-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01190-7