Abstract
Background and Objective
Ceftriaxone is a cornerstone antibiotic for critically ill children with severe infections. Despite its widespread use, information on the pharmacokinetics of ceftriaxone is lacking in this population. We aimed to determine ceftriaxone pharmacokinetics in critically ill children and to propose ceftriaxone dosing guidelines resulting in adequate target attainment using population pharmacokinetic modeling and simulation.
Methods
Critically ill children (aged 0–18 years) treated with intravenous ceftriaxone (100 mg/kg once daily, infused in 30 minutes) and a central or arterial line in place were eligible. Opportunistic blood sampling for total and unbound ceftriaxone concentrations was used. Population pharmacokinetic analysis was performed using non-linear mixed-effects modeling on NONMEM™ Version 7.4.3. Simulations were performed to select optimal doses using probability of target attainment for two pharmacokinetic targets of the minimum inhibitory concentration (MIC) reflecting the susceptibility of pathogens (f T > MIC 100% and fT > 4 × MIC 100%).
Results
Two hundred and five samples for total and 43 time-matched samples for unbound plasma ceftriaxone concentrations were collected from 45 patients, median age 2.5 (range 0.1–16.7) years. A two-compartment model with bodyweight as the co-variate for volume of distribution and clearance, and creatinine-based estimated glomerular filtration rate as an additional covariate for clearance, best described ceftriaxone pharmacokinetics. For a typical patient (2.5 years, 14 kg) with an estimated glomerular filtration rate of 80 mL/min/1.73 m2, the current 100-mg/kg once-daily dose results in a probability of target attainment of 96.8% and 60.8% for a MIC of 0.5 mg/L and 4 × MIC (2 mg/L), respectively, when using fT > MIC 100% as a target. For a 50-mg/kg twice-daily regimen, the probability of target attainment was 99.9% and 93.4%, respectively.
Conclusions
The current dosing regimen of ceftriaxone provides adequate exposure for susceptible pathogens in most critically ill children. In patients with an estimated glomerular filtration rate of > 80 mL/min/1.73 m2 or in areas with a high prevalence of less-susceptible pathogens (MIC ≥ 0.5 mg/L), a twice-daily dosing regimen of 50 mg/kg can be considered to improve target attainment.
Clinical Trial Registration
POPSICLE study (ClinicalTrials.gov, NCT03248349, registered 14 August, 2017), PERFORM study (ClinicalTrials.gov, NCT03502993, registered 19 April, 2018).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In a relevant cohort of critically ill children, with an age range of 1–17 years and highly varying kidney function, we quantified the impact of weight and estimated glomerular filtration rate on ceftriaxone pharmacokinetics. This has led to the development of a rational dosing regimen of ceftriaxone for critically ill children |
Target attainment with the currently used dose of 100 mg/kg once daily is sufficient for the majority of pathogens (minimum inhibitory concentration ≤ 0.5 mg/L) in critically ill children with a normal estimated glomerular filtration rate |
An alternative dosing regimen of 50 mg/kg twice daily could be considered in patients with a higher estimated glomerular filtration rate and/or regions with a high prevalence of less-susceptible pathogens (minimum inhibitory concentration ≥ 0.5 mg/L) |
1 Introduction
Ceftriaxone is a third-generation, broad-spectrum, cephalosporin antibiotic agent frequently used in critically ill children owing to its wide antibiotic spectrum, high penetration in tissues [1], and convenient once-daily dosing regimen [2]. However, despite its widespread use in critically ill children, ceftriaxone pharmacokinetics has not been studied in this vulnerable population [3].
Critically ill patients show several pathophysiological changes that can affect the volume of distribution (Vd) or clearance (Cl) of drugs, such as extensive fluid retention and liver and kidney failure or the opposite, augmented kidney clearance (AKC) [4]. These changes in pharmacokinetic (PK) parameters can result in increased or decreased drug exposure, potentially causing drug toxicity or therapy failure [5].
Ceftriaxone is highly protein bound in human plasma (85% in healthy volunteers) and primarily excreted by glomerular filtration, with a relatively long half-life (6 h) compared with other β-lactam antibiotics (1 h) [1, 6]. In critically ill adults, ceftriaxone pharmacokinetics shows large interindividual variability (IIV), mainly caused by variability in kidney function, such as acute kidney injury and AKC affecting drug disposition [7,8,9]. In addition, in critically ill children, the interplay with age-related variation in most processes involved in drug disposition, including kidney function, further impacts the pharmacokinetics, adding to the complexity of adequate dosing [10]. This is also indicated by the high rates of non-target attainment up to 95% in this population for several other antibiotic agents, potentially resulting in undertreatment [3, 11].
We therefore hypothesized large IIV in ceftriaxone pharmacokinetics in critically ill children, resulting in a significant proportion of children with inadequate drug concentrations using standard doses. With this study, we aimed to elucidate the PK properties of ceftriaxone in critically ill children, and to identify and quantify relevant covariates in ceftriaxone pharmacokinetics. Moreover, we aimed to study target attainment of current dosing regimens and, if needed, provide optimized alternative dosing strategies.
2 Materials and Methods
2.1 Study Design
This was a two-center prospective population PK (Pop-PK) study.
2.2 Setting and Patients
Children aged 0–18 years were eligible if they were admitted to a level 3, pediatric intensive care unit (Radboudumc, Nijmegen, The Netherlands, POPSICLE study, NCT03248349 or Erasmus MC, PERFORM study, NCT03502993, www.perform2020.org), received intravenous antibiotics, had a central venous or arterial line in place for clinical reasons, and informed or deferred consent was provided by all parents/caregivers with the consent/assent of the child depending on the child’s age and/or capabilities. Exclusion criteria consisted of inability to understand the informed consent form, or concomitant treatment with extra-corporeal membrane oxygenation or kidney replacement therapy.
The PERFORM study protocol, and local amendment for the PK sub-study at Erasmus MC, were approved by the local medical ethics review board (CMO Arnhem-Nijmegen, NL58103.091.16, 2016-3085). The medical ethics review board waived the need for formal ethics approval for the POPSICLE study according to the Dutch Law on Human Research (REB 2016-2689).
2.3 Patient Data Collection
The following patient characteristics were collected from electronic health records: postnatal age, current weight, height, sex, and main reason for intensive care unit admission. Clinical data included co-medication, mechanical ventilation (yes/no), validated pediatric disease severity scores after 24 h of admission (Pediatric Index of Mortality 2 [PIM-II, range 0–100%], Pediatric RISK for Mortality-3 scores [PRISM-3, range 0–74]) or scored daily (Pediatric Logistic Organ Dysfunction-2 score [PELOD-2, range 0–31]) [12,13,14]. Data from laboratory work taken during the study period included serum creatinine (SCr), cystatin C (CysC), urea (BUN), aspartate aminotransferase, alanine aminotransferase, albumin, and C-reactive protein. Glomerular filtration rate (eGFR) was estimated using two equations by Schwartz, which include height (meters), SCr (mg/dL), CysC (mg/L), BUN (mg/dL), and/or sex (male = 1) as determinants of eGFR [15]:
2.4 Ceftriaxone Dosing
Ceftriaxone was prescribed as a once-daily intravenous dose of 100 mg/kg (maximum 2000 mg for prophylaxis, and 4000 mg for therapeutic indications), administered over 30 min [16]. Exact date, time, and duration of ceftriaxone infusion were collected prospectively on a dedicated case report form for the POPSICLE study. For patients in the PERFORM study, timing of ceftriaxone doses was obtained from electronic health records.
2.5 Blood Sampling and Handling
Patients included in the POPSICLE study underwent an opportunistic blood sampling scheme for the PK analysis. This included 4 times/day 0.5-mL anticoagulated ethylenediaminetetraacetic acid samples in the first 3 study days, and once-daily 0.5-mL ethylenediaminetetraacetic acid samples from day 4 until the end of the study. The end of the study period was marked by cessation of antibiotic therapy, removal of arterial or central venous line, discharge from a pediatric intensive care unit, or after a maximum number of 14 sampling days.
Patients included in the PERFORM study underwent sparse opportunistic sampling with a single 0.5-mL ethylenediaminetetraacetic acid sample on days 1, 2, and/or 3 of the PERFORM study period. All samples were centrifuged at 1900g for 5 min at 20 °C [17]. Plasma was stored at – 8 °C until drug analysis in November 2019.
Total ceftriaxone concentrations were measured using a validated high-performance liquid chromatography mass spectrometry. Additional details on drug analysis are described in the Electronic Supplementary Material (ESM). The lower limit of quantification and upper limit of quantification were 1.0 mg/L and 250 mg/L, respectively. Samples with concentrations greater than the upper limit of quantification were diluted and reanalyzed.
Unbound ceftriaxone concentrations were measured in one selected sample per patient closest to the end of the dosing interval. These samples were deliberately chosen to optimize accurate model predictions of unbound trough concentrations, as these correspond best with the pharmacologic effect [18]. Samples were centrifuged at 37 °C using tubes containing an ultrafiltration filter, followed by the previously mentioned, high-performance liquid chromatography mass spectrometry method.
2.6 PK Analysis
Ceftriaxone concentration–time data were analyzed using non-linear mixed-effect modeling software (NONMEM). Model building included two main steps: (1) identifying the structural and statistical model and (2) covariate analysis on PK parameters. Models were compared using the objective function value (OFV) as a metric for a general model fit. Additionally, general goodness-of-fit plots and other model diagnostics (distribution of IIV and weighted residuals for potential covariates, goodness of fit in weight or kidney function quartiles, precision and shrinkage of parameter estimates were considered in model development) were used to evaluate model performance and potential bias. Tested covariates on PK parameters included metrics of age and body size, disease severity indications, clinical data, kidney function parameters, and other laboratory data. Protein binding of ceftriaxone was tested both as a fixed value of 85% [1], and using a function describing saturable protein binding, which estimates the maximum binding capacity and dissociation constant of ceftriaxone [19]. The final model was internally validated using normalized prediction distribution errors and parameter precision was analyzed by a bootstrap analysis. Additional details of the PK model development and covariate analysis are presented in the ESM.
2.7 Dosing Simulations
Dosing simulations were performed to explore the impact of selected covariates on ceftriaxone exposure and to evaluate the target attainment of existing ceftriaxone dosing regimens. Individual ceftriaxone concentrations were simulated for a resample of the original dataset, imputed with fixed values eGFRcreat (30, 80, and 120 mL/min/1.73 m2, n = 1000 each). To identify the impact of weight, the output of simulations was stratified to weight tertiles observed in our study population (< 10 kg, 10–25 kg, and > 25 kg). Simulations were performed for a selection of national and international dosing regimens (50, 80, and 100 mg/kg once daily and 50 mg/kg twice daily [20]. Extended or continuous infusion of ceftriaxone was not tested given the relatively small benefit for long half-life drugs [21].
2.8 PTA
The desired PK target for ceftriaxone is the time unbound ceftriaxone concentrations are above the minimal inhibitory concentration (MIC) of the pathogen expressed as a percentage (%fT > MIC). For critically ill patients, a common PK target for β-lactam antibiotics is 100% of %fT > MIC [22], while 100% fT > 4 × MIC has been proposed for immunocompromised patients [23]. Simulations were performed using MIC values from 0.002 to 128 mg/L to cover a wide range of pathogens. For our final estimation of the probability of target attainment (PTA), we identified a primary target MIC (0.5 mg/L) corresponding with the highest EUCAST epidemiological cut-off value, except for Staphylococcus aureus (EUCAST epidemiological cut-off 8 mg/L), thereby covering the majority of Gram-positive (like Streptococcus pneumoniae) and Gram-negative (like Escherichia coli or Haemophilus influenzae) pathogens [24]. In addition to 100% fT > 0.5 mg/L, we tested 100% fT > 2.0 mg/L, corresponding with four times the primary target and representing a ‘worst-case scenario’ for the majority of pathogens [24]. Individual-predicted steady-state trough concentrations after 72 h were used to assess the PTA, where a PTA of > 90% was desired to select the optimal dosing regimen.
3 Results
Forty-five patients were included between 2017 and 2019, 26 from the POPSICLE study and 19 from the PERFORM study, respectively (Table 1). Ceftriaxone plasma concentrations were determined in 213 plasma samples. Eight samples were excluded from the analysis; four were taken during the infusion of ceftriaxone, and four samples after a missed dose of ceftriaxone, leaving 205 samples in the final dataset. Concentrations ranged from 1.89 to 500 mg/L with seven samples above the upper limit of quantification and none below the lower limit of quantification.
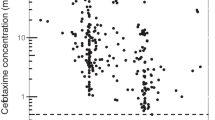
Unbound ceftriaxone concentrations were measured in 45 samples, but two samples had insufficient plasma available, leaving 43 samples for analysis. Median unbound fraction (FU) was 13.6%, with a range of 7.6–70.3% with only two samples showing a FU > 30% (Fig. 1 of the ESM). Both outliers were measured in patients with severe hypoalbuminemia and severe kidney dysfunction, with one patient also showing severe hyperbilirubinemia of 343 µmol/L [25].
Goodness of fit of predicted total (gray) and unbound ceftriaxone (white) concentrations. Diagnostic plots for ceftriaxone: observed ceftriaxone concentrations vs population predictions and individual predictions and conditional weighted residuals vs time after the last dose and population predictions. Dots represent individual total (gray) and unbound (white) ceftriaxone concentrations. The red line represents the regression line, the blue line represents the correlation of our observations and predictions. CWRES conditional weighted residuals
3.1 PopPK Analysis
Ceftriaxone concentrations were best described with a two-compartment model with IIV on ceftriaxone Cl and central Vd, and using a proportional error model for residual variability (Fig. 1). Unbound concentrations were best described using saturable protein binding (p < 0.01 compared to the model with fixed protein binding).
In the covariate analysis, ceftriaxone Cl was best described using both weight and eGFRcreat as power functions with estimated exponents of 0.67 and 0.575, respectively, which resulted in a OFV decrease of 11.2 and 26 points, respectively (Table 2). The model showed no inherent bias in different weight or eGFR quartiles (Fig.2 of the ESM). The impact of weight and eGFRcreat on ceftriaxone Cl is presented in Fig. 2, showing a large variation in estimated Cl with a range of 0.15–2.72 L/h as a result of these two covariates. Additionally, we observed that data points of individual Cl values of the group with eGFRcreat > 120 mL/min/1.73 m2 largely overlapped with the 80–120 mL/min/1.73 m2 group, and that the data of the high eGFRcreat group were limited to patients <23 kg. To test the impact of high eGFR on Cl values, we ran models with capped values of eGFRcreat at 120 and 100 mL/min/1.73 m2, which only showed marginal increases in OFV of 1.7 and 4.0 points, respectively (data not shown). This underlines that the extrapolation of the model to individuals with an eGFR > 120 mL/min/1.73 m2 and > 25 kg should be interpreted with caution, owing to the small range of our observed data. Therefore, we have refrained from drawing firm conclusions or dose advice on these extrapolations.
Impact of the final covariates weight and estimated glomerular filtration (eGFR) on ceftriaxone clearance. Dots represent the individual estimated clearance values, with colors and shapes representing different categories of median eGFRcreat during the study period (red squares <30 mL/min/1.73 m2, yellow dots 30–80 mL/min/1.73 m2, blue triangles 80–120 mL/min/1.73 m2, and green diamonds > 120 mL/min/1.73 m2. Solid lines represent the range of weight in each eGFR category observed in our dataset. Dotted lines represent extrapolated clearance for each category for a weight of 0–80 kg
Additionally, weight was also the most significant covariate on central Vd using a power function with an estimated exponent of 1.29 (OFV − 31 points). Last, albumin level was a significant covariate on maximum binding capacity using a power function (OFV − 20 points), for which an estimated exponent performed not significantly different from a fixed exponent of 1. After the addition of these covariates, no remaining correlation between covariates and IIV remained (Figs. 3 and 4 of the ESM).
Parameter estimates of the final model and bootstrap evaluation are presented in Table 2. Final parameter estimates were within the 95% confidence interval of the bootstrap, indicating a low model bias. Additional detailed results regarding the PK analysis and the final model are described in the ESM.
3.2 Dosing Simulations
For patients with eGFR values and albumin levels at the median level (85.22 mL/min/1.73 m2 and 27 g/L, respectively), the PTA at an MIC of 0.5 and 2.0 mg/L was 96.8% and 60.8% for the current dosing regimen (100 mg/kg once daily), respectively (Fig. 3). The PTA for once-daily dosing regimens of 50 and 80 mg/kg for the 0.5–2.0 mg/L targets was 86.5–29.9% and 94.6–51.3%, respectively. A twice-daily dosing regimen of 50 mg/kg resulted in higher ceftriaxone trough concentrations and reached a PTA of 99.9 and 93.4% for MICs of 0.5 and 2.0 mg/L (Fig. 3), respectively, both at 24 and 72 h after start of treatment.
Probability of target attainment (PTA) for different ceftriaxone dosing regimens for patients with a median estimated glomerular filtration rate for different MICs. The PTA for patients with p50 estimated glomerular filtration rate (85.22 mL/min/1.73 m2) and albumin levels (27 g/L) for the four tested dosing regimens (red circles 100 mg/kg once daily; yellow squares 50 mg/kg once daily; blue diamonds 80 mg/kg once daily, and green triangles 50 mg/kg twice daily). The dashed horizontal line represents target PTA of 90%. Shaded areas represent MICs selected for the primary (0.5 mg/L, gray) and high (2.0 mg/L, blue) MIC target
When stratifying PTA for different categories of eGFR and weight, the current 100-mg/kg once-daily and alternative 50-mg/kg twice-daily regimen show a PTA > 90% for the 0.5-mg/L target in all weight groups and an eGFR up to 80 mL/min/1.73 m2 (Fig. 7 of the ESM). Overall, patients <10 kg and with an eGFR > 80 mL/min/1.73 m2 showed the lowest PTA, making these patients more at risk of subtherapeutic trough concentrations (Fig. 7 of the ESM). For patients with an eGFR of > 80 mL/min/1.73 m2 and/or a less susceptible MIC (≥0.5 mg/L), the 50-mg/kg twice-daily regimen can be considered to improve target attainment.
4 Discussion
To the best of our knowledge, we present the first pop-PK parameters, PTA, and resulting dose advice for ceftriaxone in a pediatric intensive care unit setting. Ceftriaxone pharmacokinetics shows large IIV in this patient population, indicated by a ten-fold range in estimated Cl, which is largely explained by bodyweight and eGFR variability. Despite this large IIV, target attainment with 100 mg/kg once daily seems adequate for the majority of patients and pathogens. For patients with an eGFR of > 80 mL/min/1.73 m2 and/or less susceptible pathogens (MIC ≥0.5 mg/L), a 50-mg/kg twice-daily dosing regimen could improve ceftriaxone target attainment because of higher trough concentrations.
Much of the IIV in ceftriaxone pharmacokinetics in our cohort was explained by weight and kidney function, which is in line with adult data [25, 26]. Garot et al. studied 54 critically ill adults, showing large IIV and a significant correlation between SCr and ceftriaxone Cl [26]. Another two-compartment pop-PK model by Ollivier et al. shows similar findings in 21 critically ill adults, of which the majority (18/21) showed AKC (eGFR > 150 mL/min/1.73 m2) [7]. In this population, target attainment was similar to our results (45–76% compared with 60.8% for an MIC of 2 mg/L), but using a lower maximum daily dose of 2 g. Schleibinger et al. also identified eGFR as a significant covariate for ceftriaxone Cl among 17 critically ill adults [25]. Additionally, they identified ceftriaxone FU was higher in patients with acute kidney injury and severe hyperbilirubinemia. We observed a similar trend in children with acute kidney injury (Fig. 1 of the ESM), but not with severe hyperbilirubinemia.
Compared with other studies in non-critically ill pediatric patients, PK parameters in our critically ill pediatric cohort show some differences. Hayton and Stoeckel describe age-related changes in the pharmacokinetics of ceftriaxone from neonates to adulthood, with a median Vd and Cl of 0.399 L/kg and 0.045 L/kg/h in children of 1–6 years of age [27]. Additionally, a recent Pop-PK model among non-critically ill children with cystic fibrosis showed even lower population values for both Vd and Cl (0.164 L/kg and 0.029 L/kg/h, respectively), even though their population was slightly older [20]. Compared to the population values in our study, both median Vd and Cl in our model are higher when normalized for bodyweight (total Vd 0.407 L/kg and Cl 0.051 L/kg/h). This can be explained by pathophysiological alterations during a critical illness, such as edema or fluid overload, resulting in a higher Vd and increased kidney perfusion and AKC, which may result in higher Cl rates [4].
Although SCr and/or eGFRcreat are included in several adult Pop-PK models, it is known that SCr is a suboptimal marker of GFR, especially in critically ill patients and children [28, 29]. Other biomarkers, such as CysC, seem to correlate better with actual GFR than SCr-based estimations [15]. As ceftriaxone is predominantly cleared by glomerular filtration [1], we hypothesized CysC to be a superior covariate of drug Cl. However, in our final model, eGFRcreat was superior to eGFRcysc. We only collected eGFRcysc in the richly sampled patients of the POPSICLE study (57.8% of our total cohort), which might have limited the power of eGFRcysc as a covariate. In a recent Pop-PK model of ceftriaxone in non-critically ill children, adding SCr as a covariate did not improve the model fit [20]. However, this might be because of the lack of patients with severe kidney dysfunction in their cohort [20].
The PTA of ceftriaxone in critically ill children with standard doses is markedly higher than what we hypothesized based on the literature [3]. Studies on different β-lactam antibiotics in critically ill children universally show low target attainment with standard doses, and all advise extended or continuous infusion to ensure adequate trough concentrations [3, 11, 30,31,32,33]. This discrepancy between ceftriaxone and other β-lactam antibiotics can be explained by differences in both pharmacokinetics and pharmacodynamics. First, ceftriaxone is highly protein bound and, as only the Fu is available for Cl, it has a particularly long half-life of 6 h in children [1]. This is in contrast with other β-lactam antibiotics such as cefotaxime, piperacillin, or amoxicillin, which show half-lives of roughly 1 h in critically ill children and who are generally on a multiple-dose per day regimen by default [33,34,35]. β-Lactam antibiotics are classified as time-dependent antibiotics, meaning the time drug concentrations are above the MIC determines the effect, thus drugs with shorter half-lives will inherently be more prone to underdosing. Second, the pharmacodynamics of ceftriaxone plays a role, as the majority of pathogens ceftriaxone aims to cover are in a low MIC range (0.016–0.5 mg/L). This favorable susceptibility profile in turn reduces the chance of ceftriaxone trough concentrations dropping below the MIC threshold.
There are some limitations to address. First, we validated our model internally, using a resampling of the patients in our study. Using an external dataset to test model performance could have increased the external validity of our model even further. We chose to enhance the power and heterogeneity of our study by using samples from two separate studies, on different locations, and using different sampling strategies, therefore increasing the external validity of our model. Second, because of a difference in research protocol, location, and electronic health records between the POPSICLE study and the PERFORM study, we encountered some missing data, such as eGFRcysc, in a significant proportion of our cohort. Ideally, similar data and samples would be collected in all patients, but this was not possible within the context of the PERFORM study. Third, the number of patients in our cohort with AKC or an eGFR > 120 mL/min/1.73 m2 was relatively small and limited to a relatively small weight range. Therefore, the exact impact of high eGFR on ceftriaxone Cl might be overestimated in our model. Capping eGFR at 120 mL/min/1.73 m2 did not show a significant increase in OFV (+1.7 points, p > 0.05) while a cap at 100 mL/min/1.73 m2 did increase OFV significantly (+4.0 points, p < 0.05). Future research with additional patients with AKC should reveal whether a further increase in ceftriaxone Cl can be expected in patients with an eGFR of > 120 mL/min/1.73 m2. Last, we made a selection of one sample closest to 24 h after dosing for each patient to determine unbound ceftriaxone concentrations, to accurately predict unbound trough concentrations and PTA. However, not all unbound concentrations were taken at 24 h after a dose. Having a larger number of concentrations at 24 h could have made our PTA findings more robust. However, this would also have caused an even higher uncertainty of our maximum binding capacity estimation, which is already relatively uncertain, which can be estimated more accurately using higher concentrations.
5 Conclusions
To the best of our knowledge, this study represents the first ceftriaxone PK model in a dedicated cohort of critically ill children, in which we identified that standard doses of 100 mg/kg once daily result in adequate exposure for the majority of patients and pathogens. For children with an eGFR > 80 mL/min/1.73 m2 or in settings with increased prevalence of ceftriaxone resistance with less susceptible pathogens (MIC ≥ 0.5 mg/L), a 50-mg/kg twice-daily regimen could be advised to improve target attainment.
References
Steele RW, Eyre LB, Bradsher RW, Weinfeld RE, Patel IH, Spicehandler J. Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob Agents Chemother. 1983;23(2):191–4.
Fusco NM, Parbuoni KA, Morgan JA. Time to first antimicrobial administration after onset of sepsis in critically ill children. J Pediatr Pharmacol Ther. 2015;20(1):37–44. https://doi.org/10.5863/1551-6776-20.1.37.
Hartman SJF, Brüggemann RJ, Orriëns L, Dia N, Schreuder MF, de Wildt SN. Pharmacokinetics and target attainment of antibiotics in critically ill children: a systematic review of current literature. Clin Pharmacokinet. 2020;59(2):173–205. https://doi.org/10.1007/s40262-019-00813-w.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509. https://doi.org/10.1016/S1473-3099(14)70036-2.
De Waele JJ, Lipman J, Akova M, Bassetti M, Dimopoulos G, Kaukonen M, et al. Risk factors for target non-attainment during empirical treatment with beta-lactam antibiotics in critically ill patients. Intensive Care Med. 2014;40(9):1340–51. https://doi.org/10.1007/s00134-014-3403-8.
Schaad UB, Stoeckel K. Single-dose pharmacokinetics of ceftriaxone in infants and young children. Antimicrob Agents Chemother. 1982;21(2):248–53. https://doi.org/10.1128/aac.21.2.248.
Ollivier J, Carrie C, d’Houdain N, Djabarouti S, Petit L, Xuereb F, et al. Are standard dosing regimens of ceftriaxone adapted for critically ill patients with augmented creatinine clearance? Antimicrob Agents Chemother. 2019;63(3):e02134-e2218. https://doi.org/10.1128/AAC.02134-18.
Udy AA, De Waele JJ, Lipman J. Augmented renal clearance and therapeutic monitoring of beta-lactams. Int J Antimicrob Agents. 2015;45(4):331–3. https://doi.org/10.1016/j.ijantimicag.2014.12.020.
Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol. 2020;35(1):25–39. https://doi.org/10.1007/s00467-018-4120-2.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. https://doi.org/10.1056/NEJMra035092.
Cies JJ, Moore WS 2nd, Enache A, Chopra A. Beta-lactam therapeutic drug management in the PICU. Crit Care Med. 2018;46(2):272–9. https://doi.org/10.1097/CCM.0000000000002817.
Slater A, Shann F, Group APS. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5(5):447–54. https://doi.org/10.1097/01.PCC.0000138557.31831.65.
Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–52. https://doi.org/10.1097/00003246-199605000-00004.
Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761–73. https://doi.org/10.1097/CCM.0b013e31828a2bbd.
Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–53. https://doi.org/10.1038/ki.2012.169.
Kinderformularium. Dutch Pediatric Formulary: ceftriaxone. 2020. https://www.kinderformularium.nl/geneesmiddel/129/ceftriaxon. Accessed 6 May 2021.
Nickolai DJ, Lammel CJ, Byford BA, Morris JH, Kaplan EB, Hadley WK, et al. Effects of storage temperature and pH on the stability of eleven beta-lactam antibiotics in MIC trays. J Clin Microbiol. 1985;21(3):366–70.
Tsai D, Lipman J, Roberts JA. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr Opin Crit Care. 2015;21(5):412–20. https://doi.org/10.1097/MCC.0000000000000229.
De Cock RF, Smits A, Allegaert K, de Hoon J, Saegeman V, Danhof M, et al. Population pharmacokinetic modelling of total and unbound cefazolin plasma concentrations as a guide for dosing in preterm and term neonates. J Antimicrob Chemother. 2014;69(5):1330–8. https://doi.org/10.1093/jac/dkt527.
Khan MW, Wang YK, Wu YE, Tang BH, Kan M, Shi HY, et al. Population pharmacokinetics and dose optimization of ceftriaxone for children with community-acquired pneumonia. Eur J Clin Pharmacol. 2020;76(11):1547–56. https://doi.org/10.1007/s00228-020-02939-4.
Grupper M, Kuti JL, Nicolau DP. Continuous and prolonged intravenous beta-lactam dosing: implications for the clinical laboratory. Clin Microbiol Rev. 2016;29(4):759–72. https://doi.org/10.1128/CMR.00022-16.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46(6):1127–53. https://doi.org/10.1007/s00134-020-06050-1.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–51. https://doi.org/10.1097/CCM.0b013e3181961bff (quiz 59).
European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. http://www.eucast.org. Accessed 6 May 2021.
Schleibinger M, Steinbach CL, Topper C, Kratzer A, Liebchen U, Kees F, et al. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol. 2015;80(3):525–33. https://doi.org/10.1111/bcp.12636.
Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, et al. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol. 2011;72(5):758–67. https://doi.org/10.1111/j.1365-2125.2011.04005.x.
Hayton WL, Stoeckel K. Age-associated changes in ceftriaxone pharmacokinetics. Clin Pharmacokinet. 1986;11(1):76–86. https://doi.org/10.2165/00003088-198611010-00005.
Soveri I, Berg UB, Bjork J, Elinder CG, Grubb A, Mejare I, et al. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64(3):411–24. https://doi.org/10.1053/j.ajkd.2014.04.010.
Guignard JP, Drukker A. Why do newborn infants have a high plasma creatinine? Pediatrics. 1999;103(4):e49.
De Cock PA, Standing JF, Barker CI, de Jaeger A, Dhont E, Carlier M, et al. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob Agents Chemother. 2015;59(11):7027–35. https://doi.org/10.1128/AAC.01368-15.
De Cock PA, van Dijkman SC, de Jaeger A, Willems J, Carlier M, Verstraete AG, et al. Dose optimization of piperacillin/tazobactam in critically ill children. J Antimicrob Chemother. 2017;72(7):2002–11. https://doi.org/10.1093/jac/dkx093.
Béranger A, Benaboud S, Urien S, Moulin F, Bille E, Lesage F, et al. Piperacillin population pharmacokinetics and dosing regimen optimization in critically ill children with normal and augmented renal clearance. Clin Pharmacokinet. 2019;58(2):223–33. https://doi.org/10.1007/s40262-018-0682-1.
Béranger A, Oualha M, Urien S, Genuini M, Renolleau S, Aboura R, et al. Population pharmacokinetic model to optimize cefotaxime dosing regimen in critically ill children. Clin Pharmacokinet. 2018;57(7):867–75. https://doi.org/10.1007/s40262-017-0602-9.
Cies JJ, Shankar V, Schlichting C, Kuti JL. Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J. 2014;33(2):168–73. https://doi.org/10.1097/INF.0b013e3182a743c7.
Jones AE, Barnes ND, Tasker TC, Horton R. Pharmacokinetics of intravenous amoxycillin and potassium clavulanate in seriously ill children. J Antimicrob Chemother. 1990;25(2):269–74. https://doi.org/10.1093/jac/25.2.269.
Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F. Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta. 2008;396(1–2):49–55.
Acknowledgments
We acknowledge Elke H.J. Krekels from the Leiden Academic Center for Drug Research for her support during model evaluation and finalization of the model, Koen Ten Hove from the Radboudumc for assistance with the preparation of the final modeling dataset, and Dennis T.D. van der Laan from the Amsterdam UMC for his work during the sample analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The PERFORM study has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 668303.
Conflicts of interest/Competing interests
Roger J. Brüggemann has no conflicts of interest that are directly relevant to the content of this article. Outside of this work, he has served as consultant to and has received unrestricted research grants from Astellas Pharma Inc., F2G, Amplyx, Gilead Sciences, Merck Sharpe and Dohme Corp., and Pfizer Inc. All payments were invoiced by the Radboud University Medical Centre. Saskia N. de Wildt has no conflicts of interest that are directly relevant to the content of this article. Outside of this work she has served as consultant to and had received unrestricted research grant or in kind support from UCB Pharma, Spingotec, and Pfizer Inc. Saskia N. de Wildt is the director of the Dutch Pediatric Formulary and its internationally licensed versions (Stichting Nederlands Kenniscentrum Pharmacotherapy voor Kinderen & Kinderformularium B.V.) All payments were invoiced by the Radboud University Medical Centre. Stan J.F. Hartman, Parth J. Upadhyay, Nienke N. Hagedoorn, Ron A.A. Mathôt, Henriëtte A. Moll, Michiel van der Flier, Michiel F. Schreuder, and Catherijne A. Knibbe have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The PERFORM study protocol, and local amendment for the PK sub-study at Erasmus MC, were approved by the local medical ethics review board (CMO Arnhem-Nijmegen, NL58103.091.16, 2016-3085). The medical ethics review board waived the need for formal ethics approval for the POPSICLE study according to the Dutch Law on Human Research (REB 2016-2689).
Consent to participate
Informed or deferred consent was provided by all parents/caregivers with the consent/assent of the child depending on the child’s age and/or capabilities.
Consent for publication
Not applicable.
Availability of data and material
Data and material are available upon request with the corresponding author.
Code availability
The model code is provided in the ESM.
Authors’ contributions
Development of the POPSICLE research protocol (SH, RB, MvdF, MS, SdW), development of the PERFORM sub-study protocol amendment (NH, MvdF, HM, SdW, SH), including patients and collection of samples from the POPSICLE study (SH), including patients and collecting samples from the PERFORM study (NH), bioanalysis of samples (RM), data analysis and pop-PK modeling (SH, PU, CK, SdW), writing first version of manuscript (SH, PU, CK, SdW), and completing final version of manuscript (SH, PU, NH, RM, HM, MvdF, MS, RB, CK, SdW).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hartman, S.J.F., Upadhyay, P.J., Hagedoorn, N.N. et al. Current Ceftriaxone Dose Recommendations are Adequate for Most Critically Ill Children: Results of a Population Pharmacokinetic Modeling and Simulation Study. Clin Pharmacokinet 60, 1361–1372 (2021). https://doi.org/10.1007/s40262-021-01035-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01035-9