Abstract
Background and Aims
The pharmacokinetics (PK) and single-dose tolerability of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist being developed for once-weekly treatment of type 2 diabetes (T2D), weight management, and nonalcoholic steatohepatitis, was evaluated in subjects with renal impairment versus healthy subjects with normal renal function.
Methods
Forty-five subjects, categorized by baseline renal status, i.e. mild (n = 8, estimated glomerular filtration rate [eGFR] 60–89 mL/min/1.73m2), moderate (n = 8, eGFR 30–59 mL/min/1.73m2), severe renal impairment (n = 7, eGFR < 30 mL/min/1.73m2), end-stage renal disease requiring dialysis (n = 8), and normal renal function (n = 14, eGFR ≥ 90 mL/min/1.73m2), received a single subcutaneous dose of tirzepatide 5 mg. Tirzepatide plasma concentrations up to 648 h postdose were measured to compute PK parameters. The primary analysis evaluated the ratios of area under the plasma concentration–time curves (AUCs) and maximum plasma drug concentration (Cmax) of renal impairment versus the normal renal function group (90% confidence interval [CI]). In addition, the relationship between PK parameters and continuous variables of renal function was assessed by linear regression.
Results
Tirzepatide exposure was similar across renal impairment groups and healthy subjects. The 90% CI of ratios of AUCs and Cmax comparing each renal impairment group versus normal renal function spanned unity, except for a 25–29% increase in AUCs in the moderate renal impairment group. There was no significant relationship between tirzepatide exposure and eGFR. Few adverse events were reported across the renal impairment and normal renal function groups. The majority were mild in severity and of a gastrointestinal nature in the renal impairment groups.
Conclusion
There were no clinically relevant effects of renal impairment on tirzepatide PK. Dose adjustment may not be required for patients with renal impairment.
Clinical Trial Registration
ClinicalTrials.gov NCT03482024.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Participant renal impairment status did not appear to result in clinically relevant effects on tirzepatide pharmacokinetics following a single subcutaneous dose of 5 mg. |
A single dose of tirzepatide 5 mg was well-tolerated regardless of the degree of renal impairment. |
Treatment of type 2 diabetes, weight management, and nonalcoholic steatohepatitis with tirzepatide in patients with renal impairment, including patients undergoing dialysis, may not require dose adjustment. |
1 Introduction
Approximately one-third of patients with diabetes mellitus (type 1 or type 2) report diabetic nephropathy [1, 2]. Diabetic nephropathy is characterized by either persistent albuminuria and/or progressive renal impairment. Renal impairment is associated with arterial hypertension and increased cardiovascular morbidity and mortality [3, 4] and can eventually lead to end‐stage renal disease (ESRD), in which patients require dialysis or renal transplantation [5, 6].
The pharmacokinetics (PK) of standard antidiabetic drugs, such as metformin, sulfonylureas, dipeptidyl peptidase-IV inhibitors, sodium-glucose cotransporter-2 inhibitors, and insulin, may be affected in subjects with impaired renal function [7]. Reduced drug clearance due to renal impairment may lead to increased risk of adverse events (AEs), such as hypoglycemia, in patients with type 2 diabetes (T2D) [8]. Some antidiabetic drugs are, therefore, unsuitable or are used with caution in this particular patient group, thereby limiting their treatment options.
Newer therapies such as glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are increasingly being used in the treatment of T2D. Previous studies have evaluated the effect of renal impairment on the PK of the GLP-1 RAs exenatide [9], lixisenatide [10], liraglutide [11], albiglutide [12], dulaglutide [13], and semaglutide [14, 15]. In patients with mild-to-moderate renal impairment versus subjects with normal renal function, observed PK changes of subcutaneous exenatide were reported as clinically acceptable, while these PK changes were significant in patients with severe renal impairment or ESRD [9]. There were no significant differences reported in the PK of lixisenatide in patients with mild-to-moderate renal impairment versus subjects with normal renal function, but drug exposure may be increased in patients with severe renal impairment [10]. The PK of liraglutide and albiglutide were not significantly altered in patients with mild-to-moderate renal impairment versus subjects with normal renal function, but there is limited reported evidence of experience in patients with more severe renal dysfunction [11, 12]. The PK of dulaglutide was not influenced by the extent of renal impairment [13]. The PK profile of subcutaneous semaglutide has also been reported as similar between patients with mild-to-severe renal impairment or ESRD and subjects with normal renal function, when adjusted for differences in sex, age, and body weight [14]. Similar results were also noted for oral semaglutide, with lack of impact of renal impairment on plasma PK [15].
Improving glycemic control has beneficial effects on the development and progression of renal impairment [16,17,18]. In short-term studies, once-daily liraglutide reduced urinary albumin excretion and increased urinary sodium excretion [19, 20]. In the LEADER trial investigating the influence of liraglutide on renal outcomes in patients with T2D, fewer cases of new-onset persistent macroalbuminuria and fewer cases of death due to renal disease occurred and the risk of increased serum creatinine levels and the risk of ESRD did not differ compared with placebo [21]. In addition, liraglutide reduced the risk of new or worsening nephropathy by 21% and once-weekly semaglutide reduced the risk by 38% [22, 23]. In the AWARD-7 study investigating the efficacy and safety of once-weekly dulaglutide in patients with T2D and moderate-to-severe chronic kidney disease, results showed that glycemic control in patients treated with dulaglutide was similar to that in patients treated with insulin glargine. Furthermore, treatment with dulaglutide was associated with a reduced decline in estimated glomerular filtration rate (eGFR) [24, 25]. Long-term use of dulaglutide in patients with T2D was associated with reduced composite renal outcomes (new macroalbuminuria, sustained decline in eGFR of 30% or more from baseline, and chronic renal replacement therapy), suggesting a protective effect of dulaglutide on renal outcomes [26]. In summary, there appears to be a benefit associated with the use of GLP-1 RA agents in patients with T2D and renal disease. Therefore, it is imperative to understand the influence of renal disease on drug PK before conducting these investigations.
Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide and GLP-1 RA that consists of a 39-amino acid synthetic peptide. Tirzepatide is in development for the treatment of T2D, chronic weight management issues, and nonalcoholic steatohepatitis [27,28,29]. In a phase Ib study, once-weekly administration of tirzepatide demonstrated significant, dose-dependent reductions in body weight in both healthy subjects and subjects with T2D (up to 4.52 kg reduction) and reductions of glycated hemoglobin A1c (HbA1c) up to 0.84% (subjects with T2D only) following 4 weeks of treatment [27]. In a phase IIb study, tirzepatide 5, 10, and 15 mg showed superior efficacy in HbA1c reduction compared with once-weekly dulaglutide 1.5 mg in patients with T2D (up to 2.4% vs. 1.1% reduction), while tirzepatide 10 and 15 mg demonstrated superior efficacy in body weight reduction (up to 11.3 kg vs. 2.7 kg reduction) [28].
The molecular weight of tirzepatide is 4.8 kDa, which, while greater than that of nonbiologic drugs, is still notably lower than the glomerular filtration cut-off of 30–50 kDa. Therefore, it is important to understand the impact of renal impairment on tirzepatide PK.
The aim of this study was to examine the PK and tolerability of tirzepatide in subjects with or without T2D and varying degrees of renal impairment compared with healthy subjects. A 5-mg dose of tirzepatide was chosen for investigation in this organ-disease study since this dose was previously identified as a maximum tolerated initial dose [27] and can be administered as a single dose without stepwise dose escalation.
2 Methods
2.1 Study Design and Participants
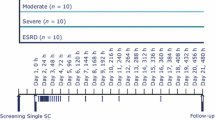
This phase I, parallel-design, open-label, multicenter, single-dose study assessed the PK and tolerability of tirzepatide in 45 subjects with mild (n = 8), moderate (n = 8), or severe renal impairment (n = 7), or ESRD undergoing dialysis (n = 8), and control subjects with normal renal function (n = 14). Study eligibility included adults aged 18–85 years inclusive, with a body mass index (BMI) ≥ 19.0 and ≤ 40.0 kg/m2 at screening. Subjects were classified into groups of varying degree of renal function based on eGFR values determined by the Modification of Diet in Renal Disease (MDRD) abbreviated equation [30] using serum creatinine levels obtained at screening and on day − 1 (Fig. 1). The renal function groups are normal renal function (eGFR ≥ 90 mL/min/1.73m2), mild renal impairment (eGFR 60–89 mL/min/1.73m2), moderate renal impairment (eGFR 30–59 mL/min/1.73m2), severe renal impairment (eGFR < 30 mL/min/1.73m2, and not requiring dialysis), and ESRD (requiring dialysis) [electronic supplementary Table 1]. Only high-flux polysulfone membranes were used for dialysis to eliminate potential variation between different types of dialysis membrane. In addition, subjects were also re-categorized into renal function groups using eGFR calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [31] and also using creatinine clearance (CLCR) determined by the Cockcroft–Gault equation [32] for exploratory analyses (electronic supplementary Table 1).
The control subjects were selected in order that the mean and distribution were comparable with each group with renal impairment, as far as was practically possible, for age (± 10 years), sex, race, weight (± 10 kg), and BMI (± 20%). Subjects with T2D were not included in the control group but were permitted to enroll in the renal impairment groups. Subjects with renal impairment, including ESRD, and T2D were allowed to be treated with stable doses of metformin and/or insulins for at least 8 weeks. In addition, the use of concomitant medications such as lipid-lowering, antihypertensive agents, and aspirin was permitted for subjects with renal impairment if treatment was stable for at least 4 weeks.
Key exclusion criteria for all subjects included organ transplantation; personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2; significant history or presence of cardiovascular, respiratory, hepatic, or gastrointestinal disorders; and pancreatitis or elevation in serum amylase or lipase.
All subjects provided written informed consent prior to the start of any study-related activities. The study was conducted in accordance with the ethical standards of the appropriate research committee, the Declaration of Helsinki, and current guidelines for studies in patients with renal impairment [33, 34].
2.2 Tirzepatide Administration, Sample Collection, and Other Assessments
A single subcutaneous injection of tirzepatide 5 mg (lot number: C860410) was administered on day 1 (Fig. 1). Plasma samples for tirzepatide PK analysis were collected at predose (0 h) and at 8, 12, 24, 48, 72, 96, 168, 336, and 648 h postdose (Fig. 1) and stored at approximately −70 °C. Plasma samples were analyzed for tirzepatide using a validated liquid chromatography/mass spectrometry method at Q2 Solutions (Ithaca, NY, USA). Tirzepatide was extracted from human plasma by immunoprecipitation and measured by high resolution mass spectrometry with a Thermo Q-Exactive Orbitrap utilizing Heated Electrospray IonizationTM (HESI) operated in the positive ion mode; LCquan version 2.9 was used for all data integrations. The range of quantification was from 2.00 to 500.00 ng/mL. The interassay accuracy (% relative error) during validation ranged from − 0.5 to 10.9%, and the interassay precision (% relative standard deviation) during validation was ≤12.2%.
Tirzepatide plasma concentrations were used to determine PK parameters using standard noncompartmental methods in a validated software program (Phoenix WinNonlin version 8.1; Pharsight, a Certara Company, Princeton, NJ, USA). The primary PK parameters for analysis include area under the plasma concentration–time curve (AUC) from time zero to infinity (AUC∞), AUC from time zero to the time of the last measurable concentration (AUClast), and the maximum observed plasma drug concentration (Cmax). Additional PK parameters, namely time to reach Cmax following drug administration (tmax), elimination half-life (t½), and apparent total body clearance of drug from plasma after extravascular administration (CL/F) were also computed. AUClast was calculated using the trapezoidal method, and AUC∞ was computed based on AUClast and additional area extrapolated from the time of the last measurable concentration to infinity using AUClast–∞ = C(t)/λz. The terminal rate constant (λz) was approximated by log-linear regression on the terminal part of the plasma drug concentration versus time profile. Cmax and tmax were derived from the observed plasma drug concentration versus time profiles. The t½ was calculated as ln2/λz, and CL/F was estimated as dose/AUC∞. Actual sampling times were used in the calculation of all PK parameters and plasma drug concentration versus time profiles.
Serum samples obtained during this study for immunogenicity (Fig. 1) were analyzed for the presence of antidrug antibodies (ADAs) at BioAgilytix (Durham, NC, USA) [35,36,37,38,39].
2.3 Safety Parameters
Safety parameters were assessed throughout the study. AEs were evaluated at screening and on days 1, 2, 3, 4, 5, 8, 15, and ≥ 28. Hypoglycemic events were verified by plasma glucose concentration and/or required third-party assistance according to the American Diabetes Association guidance [40]. Plasma glucose was monitored at predose, at 12, 24, 48, 72, 96, 168, 336 h postdose, and on day ≥ 28. Clinical laboratory parameters were evaluated at screening and on days 3, 5, 8, 15, and ≥ 28, including serum amylase and lipase measurements. Vital signs were monitored at screening, predose, at 12, 24, 48, 72, 96, 168, 336 h postdose, and on day ≥ 28. Injection-site reactions (ISRs) were assessed at predose and at 0, 6, 12, 24, and 48 h postdose. Electrocardiograms (ECGs) were collected at screening, predose, and on days 3, 5, and ≥ 28. Physical examination findings were also monitored and reviewed at screening and on days 8, 15, and ≥ 28.
2.4 Statistical Analyses
The planned sample size (16 subjects for the control group and 8 subjects in each of the renal impairment groups) provided a 90% coverage probability of the half-width of the 90% confidence interval (CI) within 0.29 on a log scale for comparison of each of the renal impairment groups with the control group for AUCs or Cmax, assuming the variability (coefficient of variation) is 32%. CIs and other descriptive statistics were the main form of interpretation of results.
The primary analysis evaluated log-transformed Cmax and AUC parameters using an analysis of variance model to estimate ratios between each impaired renal function group versus the control group and the corresponding 90% CIs. This analysis was conducted three times for the different renal function group assignments classified using eGFR (MDRD abbreviated equation) for the primary analysis and CLCR (Cockcroft–Gault equation) and eGFR (CKD-EPI equation) for the exploratory analysis. Additional supporting analyses were conducted to evaluate the relationship between the PK of tirzepatide and the MDRD eGFR. The PK parameters versus eGFR were presented graphically and a linear regression line fitted, with eGFR as a continuous variable [33]. Similar graphical analysis of PK parameters versus CLCR and CKD-EPI eGFR was also conducted. Summaries of tmax were computed using Hodges–Lehmann methodology.
3 Results
3.1 Baseline Characteristics and Demographics and Subject Disposition
A total of 45 subjects (30 males and 15 females) aged between 40 and 84 years, participated in this study (Table 1). Overall, baseline characteristics, namely means of age, weight, and BMI, were similar across the control and renally impaired groups. The control and renally impaired groups included subjects of different races, with the exception of the ESRD group, in which all subjects were Black or African American. Each of the renally impaired groups included at least one subject with T2D, while the mild and severe renal impairment groups had two subjects each with T2D. Five of the six subjects with T2D received stable concomitant treatment for diabetes during the course of the study. One subject with T2D, in the ESRD group, received no treatment for diabetes throughout the course of the study.
All but one subject with normal renal function completed the study. This subject was discontinued at the discretion of the physician due to positive ‘drugs of abuse’ tests. Data from this subject have been included in the safety analysis set but have been excluded from the PK analysis sets. Out of the remaining study cohort, one was classified as having mild renal impairment based on eGFR (MDRD and CKD-EPI) but normal renal function based on CLCR on day − 1. Two unscheduled serum creatinine measurements were collected the following day. The first unscheduled measurement gave a normal renal function classification based on CLCR and the CKD-EPI eGFR, but mild impairment based on MDRD eGFR. The second unscheduled measurement showed normal renal function for all classification methods: CLCR, MDRD eGFR, and CKD-EPI eGFR. Thus, this subject was assigned to the control group and was administered tirzepatide the next day.
3.2 Pharmacokinetics of Tirzepatide
The overall exposure to tirzepatide, based on AUClast, AUC∞, and Cmax, was comparable across the control subjects (with normal renal function) and subjects with mild, moderate, and severe renal impairment or ESRD (Table 2).
Statistical analysis showed no difference in the geometric mean Cmax between subjects in the control group and the renal impairment groups, with the 90% CIs for the ratios of geometric least-squares means (LSM) spanning unity (Table 2). Comparison of the AUClast and AUC∞ showed no difference between the mild renal impairment, severe renal impairment, and ESRD groups compared with the control group, with the 90% CIs spanning unity (Table 2). The geometric means for AUClast and AUC∞ were higher by 25% (90% CI 1.04–1.52) and 29% (90% CI 1.07–1.56), respectively, for subjects in the moderate renal impairment group when compared with the control group, where 90% CIs did not span unity. There was no notable difference in the median tmax of tirzepatide between the renal impairment groups (Table 2); however, the median tmax did appear to occur earlier for the severe renal impairment group (18 h) compared with the control, mild renal impairment, moderate renal impairment, and ESRD groups (median range 48–60 h). The geometric mean t½ values were similar across the control and renal impairment groups. This is supported by the overlapping terminal part of the plasma drug concentration versus time profile (Fig. 2) for all categories.
Overall, the mean plasma concentration versus time profile of tirzepatide appeared comparable between the renally impaired and normal renal function groups (Fig. 2).
3.3 Relationship between Pharmacokinetic Parameters of Tirzepatide and Renal Function
Linear regression analysis did not show a relationship between the exposure of tirzepatide, based on Cmax, AUClast, and AUC∞, and MDRD eGFR (Fig. 3a–c). Similar results were observed for the exploratory analysis based on CKD-EPI eGFR (electronic supplementary Fig. 1a–c). AUC∞ appeared to increase marginally with decreasing values of CLCR. While the p values of the slope for this assessment was small (p = 0.0873), the fit of the regression line was poor (r2 = 0.0696) [electronic supplementary Fig. 2b]. There was no significant relationship (p > 0.1) between CLCR and AUClast or Cmax (electronic supplementary Fig. 2a, c).
Relationship between pharmacokinetic parameters of tirzepatide 5 mg and renal function calculated by MDRD eGFR. AUC∞ area under the plasma concentration–time curve from time zero to infinity, AUClast area under the plasma concentration–time curve from time zero to the time of the last measurable concentration, CI confidence interval, Cmax maximum plasma drug concentration, eGFR estimated glomerular filtration rate, MDRD Modification of Diet in Renal Disease, R2 regression coefficient
3.4 Safety Parameters
Safety assessment following a single subcutaneous dose of tirzepatide 5 mg is presented in Table 3. The most commonly reported AEs were diarrhea, nausea, vomiting, and back pain. The number of subjects with AEs related to tirzepatide was 3 (37.5%) in each of the mild and moderate renal impairment groups and the ESRD group, whereas only 1 subject (7.1%) reported a treatment-related AE in the control group (Table 3). Most treatment-related AEs were mild in severity, with the exception of one event of nausea in the ESRD group that was considered to be related to treatment and was moderate in severity. No treatment-related AEs were reported in the severe renal impairment group. No discontinuations due to AEs, serious AEs, or deaths occurred during this study. Thus, overall, few AEs were reported and the majority were mild in severity.
One hypoglycemic event was recorded during the course of the study. A blood glucose level of 53 mg/dL was recorded approximately 29 h following administration of tirzepatide to a subject in the severe renal impairment group. This subject was being treated for T2D with insulin glargine and insulin lispro. The subject self-treated with food and drink and recovered promptly. There were no severe hypoglycemic events.
No ISRs were reported, and there were no clinically significant alterations in laboratory, vital signs values, ECGs, or blood glucose monitoring. No treatment-emergent ADAs were detected following the administration of a single dose of tirzepatide 5 mg to a maximum of 30 days of ADA monitoring postdose.
4 Discussion
This parallel-design, open-label study aimed to evaluate the PK parameters of tirzepatide in subjects with mild, moderate, or severe renal impairment, or ESRD (requiring dialysis) compared with control subjects with normal renal function, after the administration of a single subcutaneous dose of tirzepatide 5 mg. In addition, the safety and tolerability of tirzepatide in subjects with varying degrees of renal impairment or ESRD was also assessed.
This study was conducted under the auspices of regulatory guidance for renal impairment studies [33, 34] with a full study design. The study included an adequate number of subjects in the mild, moderate, severe impairment, or ESRD groups, while comparing with an appropriately baseline-matched and sized control reference group.
Generally, a regression analysis approach, where estimated renal function and PK parameters are treated as continuous variables, is preferred to an analysis where eGFR or CLCR are treated as categorical variables [33]. Regression analyses of PK parameters AUClast, AUC∞, and Cmax against the continuous variable of eGFR (MDRD and CKD-EPI) indicated that tirzepatide exposure was independent of the renal function. When this analysis was conducted by plotting PK parameters against a continuous variable of CLCR, there was evidence of a significant relationship between AUC∞ values and CLCR. The AUC∞ values appeared to increase with decreasing rates of CLCR. However, a poor fit of the regression line, indicated by a low R2 value, suggested a lack of meaningful association.
A higher tirzepatide exposure was observed in the moderate renal impairment group irrespective of the renal impairment classification equation used, in which the geometric LSM increased between 23% and 28% for AUClast and 26% and 32% for AUC∞ compared with control subjects. Numerically higher exposure was also noted for the mild renal impairment group compared with control when classified by CLCR, i.e. 23% and 24% for AUClast and AUC∞, respectively. While these values appeared notable, they fell within the observed range of intersubject variability, which was determined as approximately 30%. Overall, given the thorough characterization of tirzepatide PK over a wide dose range from 0.5 to 15 mg at single-dose levels and under steady-state conditions, in which PK were observed to be linear and dose-proportional [27, 28], these increases of approximately 25% observed in this study are not considered clinically relevant. No notable difference in AUC parameters was detected between the control group and the severe renal impairment or ESRD groups regardless of the renal impairment classification method. In addition, the t½ of tirzepatide was comparable across the renal impairment and control groups. The observed differences in the median tmax between the severe renal impairment group and the other renal categories could be due to the smaller sample size in this particular group (Table 2). Therefore, no changes in the dose or frequency of tirzepatide dosing would be required for patients with varying degrees of renal impairment.
A single subcutaneous dose of tirzepatide 5 mg was well-tolerated by all treatment groups. The higher proportion of patients reporting treatment-emergent AEs in the mild and moderate renal impairment groups, compared with the control group, may be due to the overall disease state or use of other concomitant antidiabetic medication. However, no AEs were observed in the severe renal impairment group, in which the two patients with T2D were also taking stable concomitant antidiabetic medication. Thus, the small sample size and the fact that this is a single-dose study may limit further conclusions. In the SURPASS clinical program (phase III development programs for T2D), doses of 5, 10, and 15 mg, which are attained following a stepwise dose escalation, are being studied. The stepwise dose escalation starts at a 2.5-mg dose every week for 4 weeks, with 2.5-mg dose escalations every 4 weeks to attain the target dose of 5, 10, or 15 mg. These stepwise dose escalations are expected to minimize tolerability concerns and AEs. Ongoing phase III studies include patients with T2D with an eGFR of at least 30–45 mL/min/1.73m2, while also permitting severe renally impaired and ESRD patients in one of the studies. Thus, SURPASS phase III studies will provide further insights on the tirzepatide tolerability over a wide range of clinical doses and exposure in patients with renal impairment.
Further limitations of this study include the open-label design and the inclusion criterion of T2D in the renal impairment groups, while this was an exclusion criterion in the control group. In addition, while all efforts were made to match the different races included in the control and renally impaired groups, only Black or African American were enrolled in the ESRD group. The impact of race on PK will be evaluated based on data collected in phase III SURPASS studies.
Irrespective of the classification system used for renal impairment categorization or categorical versus continuous analysis, the results consistently indicated that tirzepatide PK were not significantly influenced by renal impairment; hence, dose adjustment is not warranted. These outcomes are similar to the lack of relationship between the PK of long-acting GLP-1 RAs, namely dulaglutide and semaglutide, and renal function [13,14,15].
5 Conclusion
Since there were no clinically relevant effects of renal impairment on the PK of tirzepatide, adjustment to the dose of tirzepatide, based on PK parameters, may not be required in patients with renal impairment or patients undergoing dialysis.
References
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group US. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–9.
Costacou T, Orchard TJ. Cumulative kidney complication risk by 50 years of type 1 diabetes: the effects of sex, age, and calendar year at onset. Diabetes Care. 2018;41(3):426–33.
Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn study. Kidney Int. 2002;62(4):1402–7.
Colyer WRJ, Cooper CJ. Cardiovascular morbidity and mortality and renal artery stenosis. Prog Cardiovasc Dis. 2009;52(3):238–42.
Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4):510–33.
Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl. 1):S79-83.
Davies M, Chatterjee S, Khunti K. The treatment of type 2 diabetes in the presence of renal impairment: what we should know about newer therapies. Clin Pharmacol. 2016;8:61–81.
Arnouts P, Bolignano D, Nistor I, Bilo H, Gnudi L, Heaf J, et al. Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29(7):1284–300.
Linnebjerg H, Kothare PA, Park S, Mace K, Reddy S, Mitchell M, et al. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64(3):317–27.
Liu YH, Ruus P. Pharmacokinetics and safety of the GLP-1 agonist AVE0010 in patients with renal impairment. Diabetes. 2009;58(Suppl 1):abstract 557.
Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68(6):898–905.
Young MA, Wald JA, Matthews JE, Yang F, Reinhardt RR. Effect of renal impairment on the pharmacokinetics, efficacy, and safety of albiglutide. Postgrad Med. 2014;126(3):35–46.
Loghin C, De la Pena A, Cui X, Zhang X, Geiser JS, Chien JY. Pharmacokinetics of once weekly dulaglutide in special populations. Diabetologia. 2014;57(1):S358.
Marbury TC, Flint A, Jacobsen JB, Derving Karsbol J, Lasseter K. Pharmacokinetics and tolerability of a single dose of semaglutide, a human glucagon-like peptide-1 analog, in subjects with and without renal impairment. Clin Pharmacokinet. 2017;56(11):1381–90.
Granhall C, Sondergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57(12):1571–80.
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290(16):2159–67.
DCCT/EDIC Research Group, de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–76.
von Scholten BJ, Lajer M, Goetze JP, Persson F, Rossing P. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabet Med. 2015;32(3):343–52.
Skov J, Pedersen M, Holst JJ, Madsen B, Goetze JP, Rittig S, et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab. 2016;18(6):581–9.
Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32(8):776–90.
Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–17.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–8.
Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14.
Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–93.
Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352–5.
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–72.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
US FDA. Draft guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. 2010. https://www.fda.gov/media/78573/download. Accessed 20 Jan 2020.
European Medicines Agency. Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function. 17 Dec 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-decreased-renal-function_en.pdf. Accessed 1 Sep 2020.
Bourdage JS, Cook CA, Farrington DL, Chain JS, Konrad RJ. An Affinity Capture Elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. J Immunol Methods. 2007;327(1–2):10–7.
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81.
US FDA. Guidance for industry: immunogenicity testing of therapeutic protein products—developing and validating assays for anti-drug antibody detection. 2019. https://www.fda.gov/media/119788/download. Accessed 11 Nov 2019.
European Medicines Agency. Guideline on immunogenicity assessment of therapeutic proteins. 18 May 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf. Accessed 11 Nov 2019.
Devanarayan V, Smith WC, Brunelle RL, Seger ME, Krug K, Bowsher RR. Recommendations for systematic statistical computation of immunogenicity cut points. AAPS J. 2017;19(5):1487–98.
Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
Acknowledgements
The authors thank Chrisanthi A. Karanikas, MS (Eli Lilly and Company) and Amelia Torcello Gomez, PhD (Eli Lilly and Company) for writing and editorial contributions. The authors also express their gratitude to Charles Benson, MD, Parag Garhyan, PhD, Paul Owens, PhD, Laura Fernandez, MD, and Jing Li, PhD, from Eli Lilly and Company, for providing scientific review. The US centers where the study was performed are gratefully acknowledged: Orlando Clinical Research Center, Clinical Pharmacology of Miami, High Point Clinical Trials Center, and Orange County Research Center. Partial data were presented at the 80th Scientific Sessions of the American Diabetes Association, held 12–16 June 2020, as a virtual poster presentation and encored at the 56th Annual Meeting of the European Association Study of Diabetes, held 21–25 September 2020 in Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was received from Eli Lilly and Company.
Conflict of interest
Shweta Urva, Tonya Quinlan, John Landry, Jennifer Martin, and Corina Loghin are employees and shareholders of Eli Lilly and Company.
Ethics approval
The study was approved by the appropriate institutional and/or national research ethics committee, and was conducted in accordance with the ethical standards of the institutional and/or national research committee, the Declaration of Helsinki, and current guidelines for studies in patients with renal impairment.
Consent to participate
All participants gave their written informed consent prior to the start of any study-related activities.
Availability of data and material
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of PK or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author contributions
SU, JL, JM, and CL contributed to the study design; CL provided medical oversight during the trial; JL was responsible for the statistical analyses; and TQ was responsible for oversight of the PK analyses. SU and CL are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in interpretation of the data and critical review of the manuscript, had full access to all the data in the study, and approved this manuscript to be submitted for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Urva, S., Quinlan, T., Landry, J. et al. Effects of Renal Impairment on the Pharmacokinetics of the Dual GIP and GLP-1 Receptor Agonist Tirzepatide. Clin Pharmacokinet 60, 1049–1059 (2021). https://doi.org/10.1007/s40262-021-01012-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01012-2