Abstract
Background and Objective
Multidrug-resistant tuberculosis has much poorer treatment outcomes compared with drug-susceptible tuberculosis because second-line drugs for treating multidrug resistant tuberculosis are less effective and are frequently associated with side effects. Optimization of drug treatment is urgently needed. Cycloserine is a second-line tuberculosis drug with variable pharmacokinetics and thus variable exposure when programmatic doses are used. The objective of this study was to develop a population pharmacokinetic model of cycloserine to assess drug exposure and to develop a limited sampling strategy for cycloserine exposure monitoring.
Material and Methods
Patients with multidrug-/extensively drug-resistant tuberculosis who were treated for > 7 days with cycloserine were eligible for inclusion. Patients received cycloserine 500 mg (body weight ≤ 50 kg) or 750 mg (body weight > 50 kg) once daily. MW/Pharm 3.83 (Mediware, Groningen, The Netherlands) was used to parameterize the population pharmacokinetic model. The model was compared with pharmacokinetic values from the literature and evaluated with a bootstrap analysis, Monte Carlo simulation, and an external dataset. Monte Carlo simulations were used to develop a limited sampling strategy.
Results
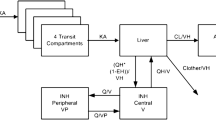
Cycloserine plasma concentration vs time curves were obtained from 15 hospitalized patients (nine male, six female, median age 35 years). Mean dose/kg body weight was 11.5 mg/kg (standard deviation 2.04 mg/kg). Median area under the concentration–time curve over 24 h (AUC0–24 h) of cycloserine was 888 h mg/L (interquartile range 728–1252 h mg/L) and median maximum concentration of cycloserine was 23.31 mg/L (interquartile range 20.14–33.30 mg/L). The final population pharmacokinetic model consisted of the following pharmacokinetic parameters [mean (standard deviation)]: absorption constant Ka_po of 0.39 (0.31) h−1, distribution over the central compartment (Vd) of 0.54 (0.26) L/kg LBM, renal clearance as fraction of the estimated glomerular filtration rate of 0.092 (0.038), and metabolic clearance of 1.05 (0.75) L/h. The population pharmacokinetic model was successfully evaluated with a bootstrap analysis, Monte Carlo simulation, and an external dataset of Chinese patients (difference of 14.6% and 19.5% in measured and calculated concentrations and AUC0–24 h, respectively). Root-mean-squared-errors found in predicting the AUC0–24 h using a one- (4 h) and a two- (2 h and 7 h) limited sampling strategy were 1.60% and 0.14%, respectively.
Conclusions
This developed population pharmacokinetic model can be used to calculate cycloserine concentrations and exposure in patients with multidrug-/extensively drug-resistant tuberculosis. This model was successfully validated by internal and external validation methods. This study showed that the AUC0–24 h of cycloserine can be estimated in patients with multidrug-/extensively drug-resistant tuberculosis using a 1- or 2-point limited sampling strategy in combination with the developed population pharmacokinetic model. This strategy can be used in studies to correlate drug exposure with clinical outcome. This study also showed that good target attainment rates, expressed by time above the minimal inhibitory concentration, were obtained for cycloserine with a minimal inhibitory concentration of 5 and 10 mg/L, but low rates with a minimal inhibitory concentration of 20 and 32.5 mg/L.




Similar content being viewed by others
References
World Health Organization. Fact sheet no 104, March 2017 . Available from: https://www.who.int/tb/challenges/mdr/MDR-RR_TB_factsheet_2017.pdf?ua=1. [https://www.who.int/mediacentre/factsheets/fs104/en/Accessed 18 Mar 2019].
Zumla A. Tuberculosis. N Engl J Med. 2013;368:745–55.
Dowdy DW. The persistence of tuberculosis in the age of DOTS: reassessing the effect of case detection. Bull World Health Organ. 2009;87(4):296–304.
Admad N. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392:821–34./
World Health Organization. Treatment guidelines for drug-resistant tuberculosis: 2011 update. Available from: https://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf?ua=1. [Accessed 26 Apr 2016]. [Accessed 12 Jan 2020].
World Health Organization. Management of MDR-TB: a field guide. Available from: https://whqlibdoc.who.int/publications/2009/9789241547765_eng.pdf?ua=1. [Accessed 26 Apr 2016].
Arbex MA. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 2: second line drugs. J Bras Pneumol. 2010;36:641–56.
Falzon D. World Health Organization (WHO) guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28.
Brunner M. Microdialysis versus other techniques for the clinical assessment of in vivo tissue drug distribution. AAPS J. 2006;8:E263–E271271.
Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb). 2010;90(5):279–92.
Nahid P. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195195.
Lange C. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44(1):23–63.
Park S-I. Pharmacokinetics of cycloserine of second-line antituberculosis drugs after multiple administrations in healthy volunteers. Antimicrobial Agents Chemother. 2015;59(8):4429–35.
Zhou H. Pharmacokinetic properties and tolerability of cycloserine following oral administration in healthy Chinese volunteers: a randomized, open-label, single- and multiple-dose 3-way crossover study. J Clin Ther. 2015;37(6):1292–300. https://doi.org/10.1016/j.clinthera.2015.03.015.
Court R. Steady state pharmacokinetics of cycloserine in patients on terizidone for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2018;22(1):30–3. https://doi.org/10.5588/ijtld.17.0475.
Alffenaar JWC. Making optimal use of available anti-tuberculosis drugs: first steps to investigate terizidone. Int J Tuberc Lung Dis. 2018;22(1):2. https://doi.org/10.5588/ijtld.17.0632.
Gumbo T. Pharmacokinetic/pharmacodynamic background and methods and scientific evidence base for dosing of second-line tuberculosis drugs. Clin Infect Dis. 2018;67(Suppl_3):S267–73. doi: 10.1093/cid/ciy608.
Deshpande D. d-Cycloserine pharmacokinetics/pharmacodynamics, susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin Infect Dis. 2018;67(Suppl_3):S308–16.
Mpagama SG. Plasma drug activity in patients on treatment for multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2014;58(2):782–8. https://doi.org/10.1128/AAC.01549-13.
Zhu H. Therapeutic drug monitoring of cycloserine and linezolid during anti-tuberulosis treatment in Beijing. China. Int J Tuberc Lung Dis. 2018;22(8):931–6. https://doi.org/10.5588/ijtld.17.0648.
van’t Boveneind-Vrubleuskaya N. Pharmacokinetics of levofloxacin in multidrug- and extensively drug-resistant tuberculosis patients. Antimicrob Agents Chemother. 2017;61(8):e00343–17. doi: 10.1128/AAC.00343–17.
Yan L. Pharmacokinetics of cycloserine in rats by HPLC-MS/MS. Med Chem. 2015;5(2):104–7.
Proost JH. Performance of an iterative two-stage Bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res. 2006;23(12):2748–59.
Milburn H. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;65:559–70. https://doi.org/10.1136/thx.2009.133173.
Delanaye P. Dosing adjustment and renal function: which equation(s)? Nephrol Ther. 2016;12(1):18–311. https://doi.org/10.1016/j.nephr.2015.07.472.
Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–23.
Dijkstra JA. Limited sampling strategies for therapeutic drug monitoring of amikacin and kanamycin in patients with multidrug-resistant tuberculosis. Int J Antimicrob Agents. 2015;46(3):332–7.
Dziak JJ. Sensitivity and specificity of information criteria. The Methodology Center and College of Health and Human Development, The Pennsylvania State University. June 27, 2012. Available from: https://www.methodology.psu.edu/files/2019/03/12-119-2e90hc6.pdf . [Accessed 27 May 2018].
Yew WW. Serum pharmacokinetics of antimycobacterial drugs in patients with multidrug-resistant tuberculosis during therapy. Int J Clin Pharmacol Res. 1999;19(3):65–71.
US FDA. Bioanalytical method validation, guidance for industry. Available from: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. [Assessed 30 Mar 2017].
Wang Y. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012;52:1601–6.
Hung W-Y. Serum concentrations of cycloserine and outcome of multidrug-resistant tuberculosis in Northern Taiwan. Int J Tuberc Lung Dis. 2014;18(5):601–6.
de Oliveira JL. Tuberculosis-associated chronic kidney disease. Am J Tropical Med Hygiene; 2011;84(6):843–4. doi: 10.4269/ajtmh.2011.11–0014.
Merchant S. Tuberculosis of the genitourinary system: urinary tract tuberculosis. Renal tuberculosis: part I. Indian J Radiol Imaging. 2013;23(1):46–63.
Mulubwa M. Amount of cycloserine emanating from terizidone metabolism and relationship with hepatic function in patients with drug-resistant tuberculosis. Drugs R D. 2019;19(3):289–96. https://doi.org/10.1007/s40268-019-00281-4.
Alghamdi WA. Cycloserine population pharmacokinetics and pharmacodynamics in patients with tuberculosis. Antimicrob Agents Chemother. 2019;63(5). pii: e00055–19. doi: 10.1128/AAC.00055–19.
Zhu M. Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy. 2001;21(8):891–7. https://doi.org/10.1592/phco.21.11.891.34524.
Kim K. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol. 2004;44(10):1083–105. https://doi.org/10.1177/0091270004268128.
Huang X. Random sparse sampling strategy using stochastic simulation and estimation for a population pharmacokinetic study. Saudi Pharm J. 2014;22(1):63–9. https://doi.org/10.1016/j.jsps.2013.01.010.
Barrasa GH. Pharmacokinetic/pharmacodynamic analysis of linezolid in critically ill patients with severe sepsis treated with renal replacement therapy. Intensive Care Med Exp. 2015;3(Suppl. 1):A880. https://doi.org/10.1186/2197-425X-3-S1-A880.
Owens RC Jr. Assessment of pharmacokinetic-pharmacodynamic target attainment of gemifloxacin against Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2005;51(1):45–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of Interest
Ruben van der Galiën, Natasha van’t Boveneind-Vrubleuskaya, Charles Peloquin, Alena Skrahina, Daan J. Touw, and Jan-Willem C. Alffenaar declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
van der Galiën, R., Boveneind-Vrubleuskaya, N.v., Peloquin, C. et al. Pharmacokinetic Modeling, Simulation, and Development of a Limited Sampling Strategy of Cycloserine in Patients with Multidrug-/Extensively Drug-Resistant Tuberculosis. Clin Pharmacokinet 59, 899–910 (2020). https://doi.org/10.1007/s40262-020-00860-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00860-8