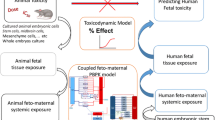
Abstract
Background
Fetal blood and plasma volume and binding components are critical parameters in a fetal physiologically based pharmacokinetic model. To date, a comprehensive review of their changes during fetal development has not been reported.
Objective
The objective of this work was to collate and analyze physiological information on fetal blood and plasma volume and binding component data during development and to provide a mathematical description of these parameters that can be integrated within a fetal physiologically based pharmacokinetic model.
Methods
A comprehensive literature search was conducted on fetal blood and plasma volume and binding component parameters and their changes during growth from apparently healthy fetuses from uncomplicated pregnancies. Collated data were assessed, integrated, and analyzed to establish continuous mathematical functions describing their growth trends with fetal age and weight.
Results
Data were available from 14 studies for blood, ten studies for hematocrit, 12 studies for albumin, and four studies for alpha-1-acid glycoprotein, while plasma and red blood cell volumes were described based on blood and hematocrit data. Fetal physiologically based pharmacokinetic parameters, including blood, plasma and red blood cell volumes, hematocrit, serum albumin, and acid glycoprotein were quantified as a function of fetal age and weight. Variability around the mean parameters at different fetal ages was also investigated. The growth of each of these parameters was different (with respect to direction and monotonicity).
Conclusions
Despite the limitations identified in the availability of some values, the collected data presented in this article provide a useful resource for fetal physiologically based pharmacokinetic modeling. Potential applications include predicting xenobiotic exposure and risk assessment in the fetus following maternally administered drugs or unintended exposure to environmental toxicants.






Similar content being viewed by others
References
Schalkwijk S, Buaben AO, Freriksen JJM, Colbers AP, Burger DM, Greupink R, et al. Prediction of fetal darunavir exposure by integrating human ex-vivo placental transfer and physiologically based pharmacokinetic modeling. Clin Pharmacokinet. 2018;57:705–16.
Koren G, Ornoy A. The role of the placenta in drug transport and fetal drug exposure. Expert Rev Clin Pharmacol. 2018;11:373–85.
De Sousa Mendes M, Lui G, Zheng Y, Pressiat C, Hirt D, Valade E, et al. A physiologically-based pharmacokinetic model to predict human fetal exposure for a drug metabolized by several CYP450 pathways. Clin Pharmacokinet. 2017;56:537–50.
Zhang Z, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model II: verification of the model for passive placental permeability drugs. Drug Metab Dispos. 2017;45:939–46.
Yoon M, Schroeter JD, Nong A, Taylor MD, Dorman DC, Andersen ME, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicol Sci. 2011;122:297–316.
Papantoniou N, Ismailos G, Daskalakis G, Karabinas C, Mesogitis S, Papapanagiotou A, et al. Pharmacokinetics of oral cefatrizine in pregnant and non-pregnant women with reference to fetal distribution. Fetal Diagn Ther. 2007;22:100–6.
Luecke RH, Wosilait WD, Pearce BA, Young JF. A physiologically based pharmacokinetic computer model for human pregnancy. Teratology. 1994;49:90–103.
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51:365–96.
Young JF, Branham WS, Sheehan DM, Baker ME, Wosilait WD, Luecke RH. Physiological “constants” for PBPK models for pregnancy. J Toxicol Environ Health. 1997;52:385–401.
Lu G, Abduljalil K, Jamei M, Johnson TN, Soltani H, Rostami-Hodjegan A. Physiologically-based pharmacokinetic (PBPK) models for assessing the kinetics of xenobiotics during pregnancy: achievements and shortcomings. Curr Drug Metab. 2012;13:695–720.
Gentry PR, Covington TR, Clewell HJ 3rd. Evaluation of the potential impact of pharmacokinetic differences on tissue dosimetry in offspring during pregnancy and lactation. Regul Toxicol Pharmacol. 2003;38:1–16.
Gaohua L, Abduljalil K, Jamei M, Johnson TN, Rostami-Hodjegan A. A pregnancy physiologically based pharmacokinetic (p-PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br J Clin Pharmacol. 2012;74:873–85.
Corley RA, Mast TJ, Carney EW, Rogers JM, Daston GP. Evaluation of physiologically based models of pregnancy and lactation for their application in children’s health risk assessments. Crit Rev Toxicol. 2003;33:137–211.
Andrew MA, Hebert MF, Vicini P. Physiologically based pharmacokinetic model of midazolam disposition during pregnancy. In: Conf Proc IEEE Eng Med Biol Soc. 2008. p. 5454–7.
Ke AB, Rostami-Hodjegan A, Zhao P, Unadkat JD. Pharmacometrics in pregnancy: an unmet need. Annu Rev Pharmacol Toxicol. 2014;54:53–69.
Johnson RF, Herman NL, Johnson HV, Arney TL, Paschall RL, Downing JW. Effects of fetal pH on local anesthetic transfer across the human placenta. Anesthesiology. 1996;85:608–15.
Savona-Ventura C, Sammut M, Sammut C. Pethidine blood concentrations at time of birth. Int J Gynaecol Obstet. 1991;36:103–7.
Rothberg RM, Rieger CH, Hill JH, Danielson J, Matadial L. Cord and maternal serum meperidine concentrations and clinical status of the infant. Biol Neonate. 1978;33:80–9.
Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy; a point to ponder! Indian J Pharm Sci. 2009;71:1–7.
Colbers A, Greupink R, Burger D. Pharmacological considerations on the use of antiretrovirals in pregnancy. Curr Opin Infect Dis. 2013;26:575–88.
Cox PB, Marcus MA, Bos H. Pharmacological considerations during pregnancy. Curr Opin Anaesthesiol. 2001;14:311–6.
Abduljalil K, Johnson TN, Rostami-Hodjegan A. Fetal physiologically-based pharmacokinetic models: systems information on fetal biometry and gross composition. Clin Pharmacokinet. 2018;57:1149–71.
Abduljalil K, Jamei M, Johnson TN. Fetal Physiologically based pharmacokinetic models: systems information on the growth and composition of fetal organs. Clin Pharmacokinet. 2019;58:235–62.
Silverwood RJ, Cole TJ. Statistical methods for constructing gestational age-related reference intervals and centile charts for fetal size. Ultrasound Obstet Gynecol. 2007;29:6–13.
Blackburn ST. Maternal, fetal and neonatal physiology: a clinical perspective. 3rd ed. Philadelphia (PA): Saunders Elsevier; 2007.
de Alarcon P, Werner E. Neonatal hematology. Cambridge: Cambridge University Press; 2005.
Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–14.
Moore KL, Persaud TVN, Torchia MG. The developing human: clinically oriented embryology. 9th ed. Philadelphia: Saunders, Elsevier; 2013.
Ohls RK. Erythropoietin treatment in extremely low birth weight infants: blood in versus blood out. J Pediatr. 2002;141:3–6.
McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;7:CD004074.
Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2:871–3.
Yagel S, Silverman NH, Ulrich Gembruch U. Fetal cardiography: embryology, genetics, physiology, echocardiographic evaluation, diagnosis and perinatal management of cardiac diseases. London: Martin Dunitz, Taylor & Francis Group; 2003.
Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117:93–8.
Linderkamp O. Placental transfusion: determinants and effects. Clin Perinatol. 1982;9:559–92.
Sisson TR, Whalen LE. The blood volume of infants. J Pediatr. 1960;56:43–7.
Pasman SA, van den Brink CP, Kamping MA, Adama van Scheltema PN, Oepkes D, Vandenbussche FP. Total blood volume is maintained in nonhydropic fetuses with severe hemolytic anemia. Fetal Diagn Ther. 2009;26:10–5.
Mollison PL, Veall N, Cutbush M. Red cell and plasma volume in newborn infants. Arch Dis Child. 1950;25:242–53.
Nicolaides KH, Economides DL, Soothill PW. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;161:996–1001.
Berrebi A, Benichou AC, Sarramon MF, Bessieres MH, Rolland M, Kobuch WE, et al. Biological reference values in the human fetus. 106 cord blood sampling in utero. J Gynecol Obstet Biol Reprod (Paris). 1992;21:355–9.
Nava S, Bocconi L, Zuliani G, Kustermann A, Nicolini U. Aspects of fetal physiology from 18 to 37 weeks’ gestation as assessed by blood sampling. Obstet Gynecol. 1996;87:975–80.
International Commission on Radiological Protection. Report of the Task Group on Reference Man. ICRP Publication 23. Oxford: Pergamon Press; 1975.
White DR, Widdowson EM, Woodard HQ, Dickerson JW. The composition of body tissues (II): fetus to young adult. Br J Radiol. 1991;64:149–59.
Hoffman R, Benz EJ, Silberstein LE, Heslop H, Weitz J, Anastasi J. Hematology: basic principles and practice. 6th ed. New York: Churchill Livingstone Inc., Elsevier; 2013.
Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123:e333–7.
Saigal S, O’Neill A, Surainder Y, Chua LB, Usher R. Placental transfusion and hyperbilirubinemia in the premature. Pediatrics. 1972;49:406–19.
Linderkamp O, Versmold HT, Riegel KP, Betke K. Estimation and prediction of blood volume in infants and children. Eur J Pediatr. 1977;125:227–34.
Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. Ann ICRP. 2002;32(3–4):5–265.
Bardy AH, Hiilesmaa VK, Teramo K, Neuvonen PJ. Protein binding of antiepileptic drugs during pregnancy, labor, and puerperium. Ther Drug Monit. 1990;12:40–6.
Belpaire FM, Wynant P, Van Trappen P, Dhont M, Verstraete A, Bogaert MG. Protein binding of propranolol and verapamil enantiomers in maternal and foetal serum. Br J Clin Pharmacol. 1995;39:190–3.
Krauer B, Dayer P, Anner R. Changes in serum albumin and alpha 1-acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. Br J Obstet Gynaecol. 1984;91:875–81.
Andreoli M, Robbins J. Serum proteins and thyroxineprotein interaction in early human fetuses. J Clin Invest. 1962;41:1070–7.
Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown-rump length: reevaluation of relation to menstrual age (5-18 weeks) with high-resolution real-time US. Radiology. 1992;182:501–5.
Moniz CF, Nicolaides KH, Bamforth FJ, Rodeck CH. Normal reference ranges for biochemical substances relating to renal, hepatic, and bone function in fetal and maternal plasma throughout pregnancy. J Clin Pathol. 1985;38:468–72.
Gitlin D, Kumate J, Urrusti J, Morales C. The selectivity of the human placenta in the transfer of plasma proteins from mother to fetus. J Clin Invest. 1964;43:1938–51.
Dancis J, Braverman N, Lind J. Plasma protein synthesis in the human fetus and placenta. J Clin Invest. 1957;36:398–404.
van den Akker CH, Schierbeek H, Rietveld T, Vermes A, Duvekot JJ, Steegers EA, et al. Human fetal albumin synthesis rates during different periods of gestation. Am J Clin Nutr. 2008;88:997–1003.
Perkins SL, Livesey JF, Belcher J. Reference intervals for 21 clinical chemistry analytes in arterial and venous umbilical cord blood. Clin Chem. 1993;39:1041–4.
Seta N, Tissot B, Forestier F, Feger J, Daffos F, Durand G. Changes in alpha 1-acid glycoprotein serum concentrations and glycoforms in the developing human fetus. Clin Chim Acta. 1991;203:167–75.
Mandelbrot L, Daffos F, Forestier F, MacAleese J, Descombey D. Assessment of fetal blood volume for computer-assisted management of in utero transfusion. Fetal Ther. 1988;3:60–6.
Leduc L, Moise KJ Jr, Carpenter RJ Jr, Cano LE. Fetoplacental blood volume estimation in pregnancies with Rh alloimmunization. Fetal Diagn Ther. 1990;5:138–46.
MacGregor SN, Socol ML, Pielet BW, Sholl JT, Minogue JP. Prediction of fetoplacental blood volume in isoimmunized pregnancy. Am J Obstet Gynecol. 1988;159:1493–7.
Smith GC, Cameron AD. Estimating human fetal blood volume on the basis of gestational age and fetal abdominal circumference. BJOG. 2002;109:721–2.
Abbasi N, Johnson J-A, Ryan G. Fetal anemia. Ultrasound Obstet Gynecol. 2017;50:145–53.
Nicolini U, Nicolaidis P, Tannirandorn Y, Fisk NM, Nasrat H, Rodeck CH. Fetal liver dysfunction in Rh alloimmunization. Br J Obstet Gynaecol. 1991;98:287–93.
Gojnic M, Petkovic S, Papic M, Mostic T, Jeremic K, Vilendecic Z, et al. Plasma albumin level as an indicator of severity of preeclampsia. Clin Exp Obstet Gynecol. 2004;31:209–10.
Morris JA, Hustead RF, Robinson RG, Haswell GL. Measurement of fetoplacental blood volume in the human previable fetus. Am J Obstet Gynecol. 1974;118:927–34.
Linderkamp O, Nelle M, Kraus M, Zilow EP. The effect of early and late cord-clamping on blood viscosity and other hemorheological parameters in full-term neonates. Acta Paediatr. 1992;81:745–50.
Salem F, Johnson TN, Abduljalil K, Tucker GT, Rostami-Hodjegan A. A re-evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin Pharmacokinet. 2014;53:625–36.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Valentin J, editor. Basic anatomical and physiological data for use in radiological protection: reference values. ICRP Publication 89. Oxford: Pergamon; 2002.
Usher R, Shephard M, Lind J. The blood volume of the newborn infant and placental transfusion. Acta Paediatr. 1963;52:497–512.
Hoogeveen M, Meerman RH, Pasman S, Egberts J. A new method to determine the feto-placental volume based on dilution of fetal haemoglobin and an estimation of plasma fluid loss after intrauterine intravascular transfusion. BJOG. 2002;109:1132–6.
Marx JA, Hockberger RS, Walls RM, Adams JG, Barsan WG. Rosen’s emergency medicine: concepts and clinical practice. 7th ed. Philadelphia: Elsevier; 2009.
Marx JA, Hockberger RS, Walls RM. Rosen’s emergency medicine: concepts and clinical practice. 8th ed. Philadelphia: Saunders, Elsevier; 2013.
Nicolaides KH, Clewell WH, Rodeck CH. Measurement of human fetoplacental blood volume in erythroblastosis fetalis. Am J Obstet Gynecol. 1987;157:50–3.
Boulot P, Cattaneo A, Taib J, Peray P, Lefort G, Hedon B, et al. Hematologic values of fetal blood obtained by means of cordocentesis. Fetal Diagn Ther. 1993;8:309–16.
Forestier F, Daffos F, Catherine N, Renard M, Andreux JP. Developmental hematopoiesis in normal human fetal blood. Blood. 1991;77:2360–3.
Forestier F, Daffos F, Galacteros F, Bardakjian J, Rainaut M, Beuzard Y. Hematological values of 163 normal fetuses between 18 and 30 weeks of gestation. Pediatr Res. 1986;20:342–6.
Millar DS, Davis LR, Rodeck CH, Nicolaides KH, Mibashan RS. Normal blood cell values in the early mid-trimester fetus. Prenat Diagn. 1985;5:367–73.
Jauniaux E, Pahal G, Gervy C, Gulbis B. Blood biochemistry and endocrinology in the human fetus between 11 and 17 weeks of gestation. Reprod Biomed Online. 2000;1:38–44.
Cartlidge PH, Rutter N. Serum albumin concentrations and oedema in the newborn. Arch Dis Child. 1986;61:657–60.
Forestier F, Daffos F, Rainaut M, Bruneau M, Trivin F. Blood chemistry of normal human fetuses at midtrimester of pregnancy. Pediatr Res. 1987;21:579–83.
Gitlin D, Boesman M. Serum alpha-fetoprotein, albumin, and gamma-G-globulin in the human conceptus. J Clin Invest. 1966;45:1826–38.
Lee JN, Chen SS, Richens A, Menabawey M, Chard T. Serum protein binding of diazepam in maternal and foetal serum during pregnancy. Br J Clin Pharmacol. 1982;14:551–4.
Nau H, Krauer B. Serum protein binding of valproic acid in fetus-mother pairs throughout pregnancy: correlation with oxytocin administration and albumin and free fatty acid concentrations. J Clin Pharmacol. 1986;26:215–21.
Acknowledgements
We thank Ms. Eleanor Savill and Ms. Jessica Waite for their assistance with collecting the references and preparing the manuscript and Ruth Clayton for her helpful comments and proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct of this study or the preparation of this article.
Conflict of interest
Khaled Abduljalil, Masoud Jamei, and Trevor N. Johnson are full-time employees of Certara UK Limited. The activities of Certara are supported by a consortium of pharmaceutical companies. The Simcyp Simulators are freely available, following completion of the relevant workshops, to approved members of academic institutions and other non-for-profit organizations for research and teaching purposes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abduljalil, K., Jamei, M. & Johnson, T.N. Fetal Physiologically Based Pharmacokinetic Models: Systems Information on Fetal Blood Components and Binding Proteins. Clin Pharmacokinet 59, 629–642 (2020). https://doi.org/10.1007/s40262-019-00836-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00836-3