Abstract
Background and Objective
Physiologically based pharmacokinetic (PBPK) models for pregnant women have recently been successfully used to predict maternal and umbilical cord pharmacokinetics (PK). Because there is very limited opportunity for conducting clinical and PK investigations for fetal drug exposure, PBPK models may provide further insights. The objectives of this study were to extend a whole-body pregnancy PBPK model by multiple compartments representing fetal organs, and to predict the PK of cefuroxime in the maternal and fetal plasma, the amniotic fluid, and several fetal organs.
Methods
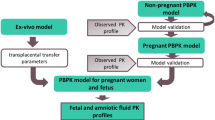
To this end, a previously developed pregnancy PBPK model for cefuroxime was updated using the open-source software Open Systems Pharmacology (PK-Sim®/MoBi®). Multiple compartments were implemented to represent fetal organs including brain, heart, liver, lungs, kidneys, the gastrointestinal tract (GI), muscles, and fat tissue, as well as another compartment lumping organs and tissues not explicitly represented.
Results
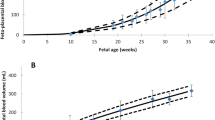
This novel PBPK model successfully predicted cefuroxime concentrations in maternal blood, umbilical cord, amniotic fluid, and several fetal organs including heart, liver, and lungs. Further model validation with additional clinical PK data is needed to build confidence in the model.
Conclusions
Being developed with an open-source software, the presented generic model can be freely re-used and tailored to address specific questions at hand, e.g., to assist the design of clinical studies in the context of drug research or to predict fetal organ concentrations of chemicals in the context of fetal health risk assessment.



Similar content being viewed by others
References
Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188(4):1039–45. https://doi.org/10.1067/mob.2003.223.
Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51 e1–8. https://doi.org/10.1016/j.ajog.2011.02.029.
Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4(2):e004365. https://doi.org/10.1136/bmjopen-2013-004365.
US Food and Drug Administration: Guidance for Industry: Pregnant Women: Scientific and Ethical Considerations for Inclusion in Clinical Trials. 2018. https://www.fdagov/media/112195/download. Accessed 9 Feb 2022.
Endicott S, Haas DM. The current state of therapeutic drug trials in pregnancy. Clin Pharmacol Ther. 2012;92(2):149–50. https://doi.org/10.1038/clpt.2012.81.
Lyerly AD, Little MO, Faden R. The second wave: toward responsible inclusion of pregnant women in research. Int J Fem Approaches Bioeth Fall. 2008;1(2):5–22. https://doi.org/10.1353/ijf.0.0047.
Miller MT, Stromland K. Teratogen update: thalidomide: a review, with a focus on ocular findings and new potential uses. Teratology. 1999;60(5):306–21. https://doi.org/10.1002/(SICI)1096-9926(199911)60:5%3c306::AID-TERA11%3e3.0.CO;2-Y.
Zhang Z, Imperial MZ, Patilea-Vrana GI, Wedagedera J, Gaohua L, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model i: insights into factors that determine fetal drug exposure through simulations and sensitivity analyses. Drug Metab Dispos. 2017;45(8):920–38. https://doi.org/10.1124/dmd.117.075192.
Mian P, Allegaert K, Conings S, et al. Integration of placental transfer in a fetal-maternal physiologically based pharmacokinetic model to characterize acetaminophen exposure and metabolic clearance in the fetus. Clin Pharmacokinet. 2020;59(7):911–25. https://doi.org/10.1007/s40262-020-00861-7.
Abduljalil K, Ning J, Pansari A, Pan X, Jamei M. Prediction of maternal and fetoplacental concentrations of cefazolin, cefuroxime, and amoxicillin during pregnancy using bottom-up physiologically based pharmacokinetic models. Drug Metab Dispos. 2022;50(4):386–400. https://doi.org/10.1124/dmd.121.000711.
Peng J, Ladumor MK, Unadkat JD. Estimation of fetal-to-maternal unbound steady-state plasma concentration ratio of p-glycoprotein and/or breast cancer resistance protein substrate drugs using a maternal-fetal physiologically based pharmacokinetic model. Drug Metab Dispos. 2022;50(5):613–23. https://doi.org/10.1124/dmd.121.000733.
Algharably EA, Di Consiglio E, Testai E, et al. Prediction of in vivo prenatal chlorpyrifos exposure leading to developmental neurotoxicity in humans based on in vitro toxicity data by quantitative in vitro-in vivo extrapolation. Front Pharmacol. 2023;14:1136174. https://doi.org/10.3389/fphar.2023.1136174.
Dallmann A, Ince I, Solodenko J, et al. Physiologically based pharmacokinetic modeling of renally cleared drugs in pregnant women. Clin Pharmacokinet. 2017;56(12):1525–41. https://doi.org/10.1007/s40262-017-0538-0.
Liu XI, Green DJ, van den Anker JN, et al. Mechanistic modeling of placental drug transfer in humans: how do differences in maternal/fetal fraction of unbound drug and placental influx/efflux transfer rates affect fetal pharmacokinetics? Front Pediatr. 2021;9: 723006. https://doi.org/10.3389/fped.2021.723006.
Open Systems Pharmacology; Compounds: Definition and Work Flows: Distribution. https://docsopen-systems-pharmacologyorg/working-with-pk-sim/pk-sim-documentation/pk-sim-compounds-definition-and-work-flow#distribution. Accessed 12 Apr 2023.
Brouwer KL, Aleksunes LM, Brandys B, et al. Human ontogeny of drug transporters: review and recommendations of the Pediatric Transporter Working Group. Clin Pharmacol Ther. 2015;98(3):266–87. https://doi.org/10.1002/cpt.176.
Szeto KX, Le Merdy M, Dupont B, Bolger MB, Lukacova V. PBPK modeling approach to predict the behavior of drugs cleared by kidney in pregnant subjects and fetus. AAPS J. 2021;23(4):89. https://doi.org/10.1208/s12248-021-00603-y.
Abduljalil K, Jamei M, Johnson TN. Fetal Physiologically based pharmacokinetic models: systems information on the growth and composition of fetal organs. Clin Pharmacokinet. 2019;58(2):235–62. https://doi.org/10.1007/s40262-018-0685-y.
US Food and Drug Administration: Guidance for Industry: S5(R3) Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/s5r3-detection-reproductive-and-developmental-toxicity-human-pharmaceuticals. Accessed 3 June 2023
Hvidberg H, Struve C, Krogfelt KA, Christensen N, Rasmussen SN, Frimodt-Moller N. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob Agents Chemother. 2000;44(1):156–63. https://doi.org/10.1128/AAC.44.1.156-163.2000.
Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56(11):1303–30. https://doi.org/10.1007/s40262-017-0539-z.
Dallmann A, Mian P, Van den Anker J, Allegaert K. Clinical pharmacokinetic studies in pregnant women and the relevance of pharmacometric tools. Curr Pharm Des. 2019;25(5):483–95. https://doi.org/10.2174/1381612825666190320135137.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by an FDA Perinatal Health Center Excellence grant (Dionna Green, PI).
Conflict of interest
Dr. André Dallmann is an employee of Bayer AG and uses Open Systems Pharmacology software, tools, and models in his professional role. The remaining authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data are from the literature and are cited in the references.
Code availability
The PBPK model will be available on the OSP GitHub website and can be downloaded freely.
Author contributions
AD and XIL wrote the manuscript. AD, XIL DG, JVA, HA, and GB designed the research. AD and XIL performed the research. AD and XIL analyzed the data.
Additional information
Disclaimer
The views expressed in this paper are those of the authors and do not necessarily represent those of the FDA. No broader FDA policies or perspectives are intended nor should be inferred. The FDA does not recommend any specific PBPK software.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X.I., Green, D.J., van den Anker, J. et al. Development of a Generic Fetal Physiologically Based Pharmacokinetic Model and Prediction of Human Maternal and Fetal Organ Concentrations of Cefuroxime. Clin Pharmacokinet 63, 69–78 (2024). https://doi.org/10.1007/s40262-023-01323-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01323-6