Abstract
Ixazomib, the first oral proteasome inhibitor, is approved in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma (MM) who have received at least one prior therapy. Ixazomib is a selective, potent, and reversible inhibitor of the 20S proteasome, and preferentially binds to and inhibits the β5 chymotrypsin-like proteolytic site. Ixazomib absorption is rapid, with a median time to reach maximum plasma concentration of approximately 1 h post-dose. Ixazomib pharmacokinetics (PK) are adequately described by a three-compartment model (terminal half-life of 9.5 days) with first-order linear absorption (oral bioavailability of 58%). Plasma exposures of ixazomib increase in a dose-proportional manner. A high-fat meal decreases both the rate and extent of ixazomib absorption, supporting administration on an empty stomach. Population PK analyses demonstrated that no dose adjustment is required based on age, body size/weight, race, sex, mild-to-moderate renal impairment, or mild hepatic impairment. Results from dedicated studies indicate that a reduced starting dose (from 4 to 3 mg) is appropriate for patients with severe renal impairment, end-stage renal disease requiring dialysis, or moderate-to-severe hepatic impairment. Non-cytochrome P450 (CYP)-mediated metabolism appears to be the major clearance mechanism for ixazomib. Drug–drug interaction studies have shown no meaningful effects of strong inhibitors of CYP3A on ixazomib PK; however, the strong inducer rifampin caused a clinically relevant reduction in ixazomib exposure, supporting the recommendation to avoid concomitant administration of ixazomib with strong CYP3A inducers. Exposure–response analyses of data from the phase III TOURMALINE-MM1 registrational study demonstrate a favorable benefit–risk profile for the approved dose and regimen of weekly ixazomib 4 mg on days 1, 8, and 15 of each 28-day cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Comprehensive evaluation of the pharmacokinetics (PK) of ixazomib and relationships to clinical safety and efficacy played an important role in determining ixazomib posology and the recommended phase III dose, and in globalization using a common dose. |
PK evaluations of food effect, drug–drug interactions, and effects of renal and hepatic impairment supported successful initial submissions for regulatory approval and formed an important component of product labeling to guide appropriate dosing. |
1 Introduction
The ubiquitin–proteasome system is responsible for protein degradation within human cells and plays a key role in a number of biologic processes [1]. The proteasome is involved in the regulation of multiple signaling pathways and in the degradation of misfolded and unwanted proteins. Due to the proliferative nature of multiple myeloma (MM) cells and their dependence on signaling pathways regulated by the proteasome, they are particularly susceptible to proteasome inhibition [1]. MM cells also typically produce excessive amounts of paraprotein [1], and proteasome inhibition prevents degradation of this excess of unwanted proteins, causing endoplasmic reticulum stress and triggering the terminal unfolded protein response [2]. Consequently, proteasome inhibitors (PIs) play a key role in the management of MM [3, 4]. PIs have contributed to the substantial improvements in long-term outcomes for MM patients seen over the past 15 years [5] and are now a backbone of MM therapy. The PIs bortezomib and carfilzomib require parenteral administration and are associated with specific adverse effects, notably peripheral neuropathy (PN) for bortezomib [6,7,8,9] and cardiovascular toxicities for carfilzomib [10,11,12]. With an increased focus on long-term, continuous therapy in MM [13,14,15,16,17], there has been a need for a PI that combines more convenient oral administration with a tolerable safety profile.
Ixazomib, the first oral PI, is approved in combination with lenalidomide and dexamethasone (Rd) for the treatment of patients with MM who have received at least one prior therapy [18, 19]. Approval was based on the results of the randomized, double-blind, placebo-controlled, phase III TOURMALINE-MM1 study, in which 722 patients with relapsed/refractory MM (RRMM) after one to three prior therapies received Rd plus either ixazomib (ixazomib-Rd) or placebo (placebo-Rd) [20]. Ixazomib-Rd significantly improved progression-free survival (PFS) compared with placebo-Rd (median 20.6 vs. 14.7 months, hazard ratio [HR] 0.74, p = 0.01), and a consistent PFS benefit was seen in key patient subgroups. Ixazomib-Rd was also associated with limited additional toxicity compared with placebo-Rd.
The clinical development of ixazomib was rapid, taking only 6 years from the date of initial enrollment of patients to the first phase I studies in RRMM [21, 22] to approval by the US FDA in November 2015 [18]. A critical component of this accelerated development was the prespecified, comprehensive characterization of the clinical pharmacology of this oral agent, incorporating preclinical findings, disease-specific medical considerations, and evolving clinical data. Notably, ixazomib obtained FDA approval without receiving any clinical pharmacology-related postmarketing requirements (PMRs) or commitments (PMCs), an uncommon occurrence for oncology new molecular entities [23]. Furthermore, the clinical pharmacology strategy for ixazomib embraced a model-informed approach, thereby increasing efficiency, reducing the design of registration-enabling clinical pharmacology studies, and minimizing the need for redundant clinical investigations [24]. Here, we review the clinical pharmacology of ixazomib, highlighting the breadth of clinical pharmacology evaluation that was conducted for ixazomib and its translation to prescribing guidance.
2 Molecular Structure and Physicochemical Properties of Ixazomib
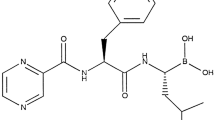
Ixazomib is a modified peptide boronic acid. It is formulated as a stable citrate ester, ixazomib citrate [25], which rapidly hydrolyzes to the biologically active boronic acid form, ixazomib, under physiological conditions (i.e. upon exposure to aqueous solutions or plasma) [25]. The chemical structures of ixazomib citrate and ixazomib are shown in Fig. 1. Ixazomib is highly soluble and has low permeability, suggesting it is a Biopharmaceutics Classification System (BCS) class 3 drug. The chemical structure of ixazomib contains one chiral center, with the drug substance determined to be ≥ 99.0% R-enantiomer. No chiral inversion of ixazomib occurs in plasma following administration (data on file).
The structure of ixazomib citrate (top), which rapidly hydrolyzes under physiological conditions to the active compound, ixazomib (bottom). Ixazomib citrate has the chemical name 2-[(1R)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]-5-oxo-1,3,2-dioxaborolane-4,4-diacetic acid, with an empirical formula of C20H23BCl2N2O9 and a molecular weight of 517.12 g/mol; ixazomib has an empirical formula of C14H19BCl2N2O4 and a molecular weight of 361.03 g/mol
3 Mechanism of Action and Pharmacodynamics of Ixazomib
3.1 In Vitro Studies
In vitro studies have demonstrated that ixazomib is a selective, potent, and reversible inhibitor of the 20S proteasome that preferentially binds to and inhibits the β5 chymotrypsin-like proteolytic site with a half maximal inhibitory concentration (IC50) value of 3.4 nmol/L [25]. The potency of ixazomib for the β1 caspase-like and β2 trypsin-like proteolytic sites is approximately 10-fold and 1000-fold less than for the β5 site, respectively, with IC50 values of 31 and 3500 nmol/L. This selectivity and potency is similar to that seen with bortezomib, and both agents induce time- and dose-dependent increases in ubiquitinated proteins in vitro [25, 26]. However, ixazomib differs from bortezomib in terms of its binding kinetics, having a substantially shorter proteasome dissociation half-life (t½); t½ was approximately sixfold quicker with ixazomib (18 min) versus bortezomib (110 min) [25].
3.2 Blood 20S Proteasome Inhibition
In vivo preclinical studies comparing ixazomib with bortezomib have shown that maximal 20S proteasome inhibition in blood is similar between the two agents; however, bortezomib had a more sustained pharmacodynamic effect than ixazomib, which may be related to the differences in proteasome-binding kinetics [25].
Pharmacodynamic analyses of data from clinical studies have indicated that ixazomib inhibits whole blood 20S proteasome activity in a dose-dependent manner [27, 28]. Pharmacodynamic profiling in a phase I study of intravenous ixazomib showed that proteasome inhibition in whole blood was immediate, and that proteasome activity recovered to predose levels within 24 h of single-dose administration in patients treated at or below the maximum tolerated dose (MTD) [28]. This blood 20S proteasome inhibition profile differs from that seen with the slowly reversible agent bortezomib [29] and the irreversible inhibitor carfilzomib [30]; however, it is important to note that although blood 20S proteasome inhibition is a marker of target inhibition in blood, it is not a marker for downstream pathway modulation, nor is it a marker of target inhibition in tumors and does not directly correlate with antitumor activity [27]. Accordingly, in the absence of established quantitative pharmacologic linkage to antitumor activity, blood 20S proteasome inhibition was primarily used as a proximal pharmacodynamic biomarker during development, and was not utilized to guide decisions on dose/schedule selection, based on fundamental scientific principles underlying application of pharmacodynamic biomarkers in translational oncology [24, 31]. As such, dose/schedule selection was informed by prospective evaluation of the safety and clinical activity of once-weekly and twice-weekly dosing schedules in MM patients [21, 22, 32,33,34].
3.3 Effects on Tumor ATF3, a Pharmacodynamic Marker of the Unfolded Protein Response
In addition to evaluating whole blood pharmacodynamics, the effects of ixazomib in tumor tissue have been studied by analyzing levels of tumor activating transcription factor-3 (ATF-3), a marker of the unfolded protein response and endoplasmic reticulum stress. Therefore, ATF-3 levels represent a downstream pharmacodynamic marker for pathway modulation following proteasome inhibition [28]. In a phase I study of patients with advanced solid tumors, ixazomib administration was shown to upregulate intratumoral ATF-3 levels [28]. Analysis of pre- and post-dose tumor biopsies from patients indicated target engagement/pathway modulation within the tumor, with six of seven paired samples showing a statistically significant increase in post-dose levels of ATF-3 [28]. These data provided the first pharmacodynamic evidence of proteasome pathway inhibition by ixazomib in humans; however, these pharmacodynamic responses to ixazomib did not translate into clinical responses in solid tumors [28].
4 Pharmacokinetics of Ixazomib
The pharmacokinetics (PK) of ixazomib have been extensively characterized in clinical studies. In addition to non-compartmental PK analyses of data collected in phase I dose-finding trials [21, 22, 27, 28, 33,34,35], two population PK analyses have been conducted to support the switch from body surface area (BSA)-based dosing to fixed dosing, and to determine the impact of various intrinsic or extrinsic factors on ixazomib PK [36, 37]. This section summarizes the absorption, distribution, metabolism, and elimination properties of ixazomib.
4.1 Absorption
4.1.1 Dose-Linearity and Absolute Bioavailability
Ixazomib absorption is rapid following oral administration, with a median time to reach maximum plasma concentration (Tmax) of approximately 1 h post-dose [21, 22, 33, 35]. An initial population PK analysis using data from four phase I studies of ixazomib administered via intravenous and oral dosing, and a subsequent population PK analysis incorporating data from 10 clinical studies, including results from the phase III TOURMALINE-MM1 study, demonstrated that ixazomib PK were adequately described by a three-compartment model with first-order linear absorption [36, 37]. Plasma exposure of ixazomib increases dose-proportionally [21, 22, 35], based on non-compartmental analyses. This conclusion of dose-linear PK was corroborated by subsequent population PK analysis, in which no readily apparent relationship was observed between oral dose (0.2–10.6 mg) and apparent oral clearance [36]. An absolute oral bioavailability of 58% was determined for ixazomib based on the population PK analysis [36].
4.1.2 Bridging Across Early Development Formulations
The capsule formulation for ixazomib was modified following early phase I clinical studies to include talc and magnesium stearate as excipients. The updated capsule formulation was used in all other subsequent and ongoing studies, including in the pivotal TOURMALINE-MM1 study [20], and is also the current commercially available formulation. A randomized two-way crossover study was performed to assess the single-dose PK of ixazomib following administration of each capsule formulation. Results from this study demonstrated that the disposition of ixazomib was similar after administration of either capsule formulation with geometric least squares mean ratios for maximum plasma concentration (Cmax; 1.16) and total systemic exposure (AUC216; 1.04) that were close to 1 [38]. Accordingly, the results of this relative bioavailability study provided justification for the pooling of data generated across studies using these formulations in integrated analyses of population PK, safety, and efficacy as part of the overall benefit–risk evaluation of ixazomib [38].
4.1.3 Food Effect
Administration with food can alter the absorption and metabolism of an oral drug [39]. In accordance with US regulatory guidance [40], the effect of a high-calorie, high-fat meal on ixazomib PK was evaluated in a phase I study in patients with advanced solid tumors or lymphoma [41]. Patients received a 4 mg oral dose of ixazomib on day 1, with or without food, followed by another dose on day 15 under the alternate food intake condition. Under fed conditions, the Tmax of ixazomib was delayed by approximately 3 h compared with administration in the fasted state (4.0 vs. 1.02 h), and AUC and Cmax were reduced by 28 and 69%, respectively (Fig. 2) [41]. These findings indicate that administration after consumption of a high-fat meal decreases the rate and extent of ixazomib absorption, with a modest negative food effect on AUC. Nevertheless, this 28% reduction in total systemic exposure has potential clinical relevance and cannot be disregarded because ixazomib exposures at the starting dose level of 4 mg would be effectively decreased to those observed with a 3 mg dose, i.e. the first reduced dose level. As such, and consistent with recommendations in the TOURMALINE-MM1 pivotal trial for drug administration on an empty stomach, it is recommended that ixazomib be taken on an empty stomach, at least 1 h before or at least 2 h after food [18, 19]. The observed negative food effect on ixazomib bioavailability, coupled with a delay in Tmax, is entirely consistent with expectations for a BCS class 3 drug [42].

Reproduced from Gupta et al., with permission, J Clin Pharmacol, published by Wiley Periodicals, Inc. on behalf of the American College of Clinical Pharmacology [41]
Mean plasma concentration–time profiles of ixazomib under fasted and fed conditions. The inset shows the first 24 h after dosing. Error bars indicate standard deviation.
4.2 Distribution
The steady-state volume of distribution of ixazomib is large and was estimated to be 543 L based on the population PK model [36]. In vitro plasma protein binding assays were utilized in two clinical studies in patients with varying degrees of renal function and hepatic function [43, 44]. These evaluations demonstrated that ixazomib is highly bound to plasma proteins (99%), with the extent of protein binding being unaffected by renal or hepatic function status [43, 44].
As red blood cells (RBCs) contain high concentrations of proteasomes, ixazomib exhibits extensive, concentration-dependent RBC partitioning [25]. This was demonstrated in patients with relapsed/refractory amyloid light-chain (AL) amyloidosis receiving single-agent ixazomib, where the overall blood-to-plasma AUC ratio was approximately 10 among patients who received weekly ixazomib 4 mg [35]. Reflecting the concentration-dependence in RBC partitioning, this ratio changed over time post-dose, with the Cmax blood-to-plasma ratios on days 1 and 15 being 2.38 and 2.89, and the AUC168 blood-to-plasma ratios being 12.7 and 9.86, respectively [35]. As highlighted in Sect. 3, in vivo xenograft studies suggested that, due to its more rapidly reversible proteasome binding kinetics, ixazomib distributes much more readily from the blood to tumor tissue than bortezomib, resulting in a greater tumor-to-blood area under the effect–time curve (AUE) ratio and prolonged pharmacodynamic effects [25]; however, it should be noted that due to its better tolerability, the ixazomib dose was approximately tenfold higher than the bortezomib dose in these studies, which may have led to greater target saturation in blood, and thus greater exposure in tumor tissue. Distribution of ixazomib into tumor tissue was demonstrated in a phase I study in patients with advanced non-hematologic malignancies, in which ixazomib was present in post-dose biopsies from all 10 patients evaluated [28]; however, a quantitative analysis of tumor distribution kinetics was not possible due to the collection of tumor samples for PK analysis at a single post-dose timepoint per patient under non-steady-state conditions. This was a result of the inherent practical limitations in collecting tumor samples from invasive biopsy procedures.
Ixazomib is a low-affinity substrate of P-glycoprotein (P-gp), but is not a substrate of breast cancer resistance protein (BCRP), multidrug resistance-associated protein 2 (MRP2), or hepatic organic anion transporting polypeptides (OATPs), based on in vitro studies [18, 19]. In vitro studies have also determined that ixazomib is not an inhibitor of P-gp, BCRP, MRP2, OATP1B1, OATP1B3, organic cation transporter (OCT) 2, OAT1, OAT3, multidrug and toxin extrusion proteins (MATE) 1, or MATE2-K [18, 19]. Consequently, the risk of transporter-mediated drug–drug interactions (DDIs) with ixazomib was predicted to be low [18, 19, 45].
4.3 Metabolism
The biotransformation pathways of ixazomib have been examined in a phase I study utilizing 14C-ixazomib in patients with advanced solid tumors or lymphoma [46]. The major biotransformation pathways included hydrolysis, deboronation, and N-dealkylation (Fig. 3). None of the major metabolites retained the boronic acid moiety of ixazomib and are thus unlikely to be pharmacologically active [46].
Biotransformation pathways of ixazomib. The figure shows the proposed metabolic pathways of ixazomib in humans, with the asterisk showing the position of the 14C-label utilized in the ADME study. M numbers refer to one of eight putative metabolites; FH, P, and U numbers refer to metabolite numbers identified in fecal homogenate, plasma, and urine. Reproduced from Pusalkar et al., Drug Metabolism and Pharmacokinetics [46]. Copyright 2018, with permission from Elsevier/the Japanese Society for the Study of Xenobiotics. ADME absorption, distribution, metabolism, and excretion
In vitro studies have evaluated the contribution of seven cytochrome P450 (CYP) isozymes to ixazomib metabolism using microsomes containing recombinantly expressed human CYP enzymes [18, 19, 47]. At clinically relevant concentrations, no single CYP isozyme predominantly contributed to ixazomib metabolism; however, at concentrations higher than those observed clinically, ixazomib was metabolized by multiple CYP isozymes, with estimated relative contributions being the highest for CYP3A4 (42%), CYP1A2 (26%), and CYP2B6 (16%) [18, 19, 47]. Based on these findings, non-CYP-mediated clearance appears to be the major contributor to ixazomib clearance, and only minimal CYP-mediated DDIs with CYP inhibitors would be expected.
4.4 Elimination
As noted in Sect. 4.1, ixazomib demonstrated multiphasic disposition kinetics that were best described by a three-compartment model with linear distribution and elimination kinetics [36, 37]. Based on a population PK analysis, systemic clearance was approximately 1.9 L/h, with inter individual variability of 44%, and the geometric mean terminal disposition phase half-life of ixazomib was 9.5 days [36]. Clinical studies of weekly oral dosing indicated an accumulation ratio of approximately 2.0 after multiple dosing [21, 27, 35].
Excretion of ixazomib was investigated in the mass balance component of the phase I study utilizing 14C-ixazomib [48]. Blood, urine, and fecal samples were collected from seven patients with advanced solid tumors or lymphoma, with accelerator mass spectrometry used to measure the amount of total radioactivity excreted. In the five PK-evaluable patients, the mean total cumulative recovery of ixazomib-related radioactivity in urine and feces combined was 83.9%, with recovery predominantly (62.1%) in the urine [48]. Notably, only 3.23% of the administered dose was recovered in the urine as unchanged ixazomib during the 168-h post-dose collection interval, indicating that renal clearance of ixazomib was minimal (geometric mean of 0.119 L/h) [48]. The log-linear decline of plasma concentrations of total radioactivity (i.e. drug-related material) paralleled that of parent drug concentrations, supporting the inference of formation rate-limited clearance of ixazomib metabolites.
4.5 Intrinsic Factors
4.5.1 Age, Body Surface Area, and Sex
The two population PK analyses that were conducted for ixazomib [36, 37] were important for determining the impact of multiple intrinsic factors on the PK of ixazomib. The initial population PK analysis utilized pooled data from 226 patients enrolled across four phase I trials in RRMM, relapsed/refractory lymphoma, and advanced solid tumors. The focus of the initial population PK analysis was to investigate the feasibility of switching from BSA-based dosing for ixazomib to fixed dosing, which would considerably simplify ixazomib administration in future studies [37]. Although a small effect of BSA (range 1.3–2.6 m2) was observed on the volume of distribution of the second peripheral compartment, there was no relationship between BSA and ixazomib clearance. Furthermore, simulations estimated that ixazomib exposure was similar following oral dosing with a BSA-based dose of 2.23 mg/m2 or a fixed dose of 4 mg [37]. Following this initial population PK analysis, a decision was made to switch from BSA-based dosing to fixed-dose administration, with a 4 mg weekly dose selected for use in the TOURMALINE-MM1 [20] and TOURMALINE-MM2 (NCT01850524) phase III trials. Similar findings were seen in the subsequent population PK analysis [36]. BSA on the volume of the second peripheral compartment was the only covariate included in the final model. None of the additional covariates tested were found to impact systemic clearance. These observations support the use of fixed dosing over BSA-based dosing for ixazomib, and are included in the US and European labeling for ixazomib [18, 19].
The initial population PK analysis also showed that neither age nor sex had any clinically meaningful effect on ixazomib PK [37]. This finding was corroborated by the subsequent population PK analysis, which included data from 755 ixazomib-treated patients [36], including patients enrolled in the phase III TOURMALINE-MM1 trial [20]. The covariates of age (Fig. 4) and sex (Fig. 5) were found to not have an impact on ixazomib PK, and thus no dose adjustments are required [36].
Impact of intrinsic factors on ixazomib PK, as determined from an integrated population PK analysis, including (a) age, (b) total bilirubin, (c) body surface area, (d) creatinine clearance, (e) weight, (f) hematocrit, and (g) albumin. Red and black dots indicate the median and 5th and 95th percentile of individual covariate values, respectively. Numbers (brackets) show the percentage change in AUC∞ at the 5th and 95th percentile relative to the value at the median, based on the shown linear regression (and 95% CI). Reproduced from Gupta et al., with permission from Springer Nature: Adis International, Clinical Pharmacokinetics (https://link.springer.com/journal/40262). © 2017 [36]. ALB serum albumin, AUC area under the concentration–time curve, BILI total bilirubin, BSA body surface area, CI confidence interval, CrCl creatinine clearance, HCT hematocrit, PK pharmacokinetics, WGT weight
Impact of categorical covariates on ixazomib PK, as determined from an integrated population PK analysis, including (a) sex, (b) race, (c) exposure to lenalidomide/dexamethasone, and (d) smoking status. Red and black dots indicate the mean exposure in the most prevalent category and in other categories, respectively. Numbers (brackets) in the top of the plots show the percentage change in AUC∞ (with 95% CI) in other categories relative to the most prevalent category, while numbers at the bottom show patients in each category. Reproduced from Gupta et al., with permission from Springer Nature: Adis International, Clinical Pharmacokinetics (https://link.springer.com/journal/40262). © 2017 [36]. AUC area under the concentration–time curve, CI confidence interval, len/dex lenalidomide/dexamethasone, PK pharmacokinetic
4.5.2 Race
Race (White vs. non-White) was another covariate that demonstrated no clinically meaningful effect on ixazomib PK in the initial population PK analysis [37]. Nevertheless, because the PK of a drug can be affected by race/ethnicity [49,50,51,52], two dedicated phase I studies further evaluated the PK of ixazomib in representative East Asian [53] and Japanese [54] patient populations in order to assess the suitability of Asia-inclusive globalization of the pivotal clinical program at a common global dose. The data from these studies were then included in the subsequent, more comprehensive population PK analysis (Fig. 5) [36].
One dose-finding phase I study investigated the PK of weekly oral ixazomib in combination with Rd in East Asian RRMM patients [53]. Of the 43 patients enrolled, 47% were Chinese, 37% were Korean, and 16% were of another Asian ethnicity. The PK of ixazomib, when administered in combination with Rd, appeared similar across the different East Asian racial subgroups. In this East Asian patient population, an ixazomib dose of 4 mg once weekly, in combination with Rd, was confirmed as the recommended phase II/III dose [53].
A planned comparison of dose-normalized AUC data for East Asian patients with pooled historical data from Western patients with RRMM or AL amyloidosis [21, 33, 35] revealed higher systemic exposures in East Asian patients (28% on day 1, 49% on day 15); however, these increases were considered to be modest in the context of the associated PK variability (percentage coefficient of variation [%CV] for AUC168; on day 1: 78% in East Asian patients, 95% in Western patients; on day 15: 48 and 44%, respectively) [53]. Furthermore, exposure in Asian patients at the global phase III dose of 4 mg was not expected to exceed that at the MTD in Western patients (2.97 mg/m2 [33], which equates to 5.5 mg), suggesting that dose adjustments are not required in this population [53]. In the China Continuation of the global phase III TOURMALINE-MM1 study, analysis of PK data using the second population PK model [36] showed that mean ixazomib exposure (AUC) was 80% higher in Chinese patients versus White patients [55]. The reasons for this observation are not known; however, importantly, the higher exposures did not appear to translate into a greater incidence of adverse events, and the ixazomib-Rd regimen demonstrated a favorable benefit-risk profile in Chinese patients with RRMM in this continuation study [55].
The PK of weekly oral ixazomib after administration of a 4 mg dose were also investigated, alone or in combination with Rd, in 14 Japanese RRMM patients [54]. Although geometric mean Cmax and AUC168 values were slightly lower in the ixazomib-Rd cohort, the high interpatient variability makes drawing definitive conclusions about ixazomib PK between the single-agent and combination cohorts in this study difficult. Notably, there was no clinically meaningful effect of Rd treatment on the PK of ixazomib as assessed by population PK analysis (Fig. 5) [36].
Overall, the two studies in East Asian [53] and Japanese [54] patients indicated no clinically relevant effect of Asian race on the PK of ixazomib, which was further supported by the results of the second population PK analysis (Fig. 5) [36]. The results of this integrated population PK analysis showed that Black patients and patients of other races had similar systemic exposures of ixazomib relative to White patients, whereas East Asian patients had a mean AUC∞ that was 35% higher compared with White patients. However, the range of individual patients’ systemic exposures in East Asian and White patients substantially overlapped, and, when considered in conjunction with the safety and efficacy findings, it was determined that both Japanese and East Asian patients could be included in global phase III trials without the need for dose adjustment, i.e. at the 4 mg starting dose of ixazomib [36].
4.5.3 Renal Function
Renal impairment, which is a major complication of MM [56], can alter the PK of anticancer drugs, even if the kidney is not involved in drug elimination. This may lead to increased toxicity, particularly if the degree of renal impairment is severe [57,58,59]. As noted in Sect. 4.4, renal clearance of unchanged ixazomib was minimal based on the findings from the mass balance study [48]. Furthermore, the initial population PK analysis demonstrated no impact of mild-to-moderate renal impairment, defined as a creatinine clearance (CrCl) of 30–89 mL/min, on ixazomib PK [37]. Specifically, this analysis included patients with CrCl values ranging from 21.9 to 213.7 mL/min, and did not identify CrCl as a clinically meaningful covariate on ixazomib PK parameters [37]. Based on this analysis, patients with mild-to-moderate renal impairment were allowed to participate in phase III trials of ixazomib without the need for an initial dose adjustment [37]. This conclusion was further supported by the findings of the subsequent, more extensive population PK analysis, which evaluated patients with CrCl values ranging from 25.8 to 297 mL/min [36]. This analysis also showed that mild-to-moderate renal impairment had no impact on the systemic clearance of ixazomib, and that there was no correlation between CrCl and ixazomib systemic exposure (Fig. 4) [36].
However, patients with MM can present with or develop more severe renal impairment (CrCl < 30 mL/min) or end-stage renal disease (ESRD). These patient populations were excluded from clinical trials during development. Thus, a dedicated phase I/Ib PK and safety study was conducted to characterize the single-dose PK of ixazomib in patients with either normal renal function (CrCl ≥ 90 mL/min), severe renal impairment (CrCl < 30 mL/min), or ESRD requiring hemodialysis [43]. After a single 3 mg oral dose, ixazomib was rapidly absorbed across all renal function groups, with a median Tmax of 1.0-1.25 h. Severe renal impairment and ESRD requiring hemodialysis had a similar effect on ixazomib PK. As a result, data from these two renal function groups were pooled and compared with the normal renal function group (Fig. 6a). Based on geometric least squares mean ratios, unbound and total systemic exposures of ixazomib (AUC) were 38 and 39% higher, respectively, in patients with severe renal impairment or ESRD requiring hemodialysis versus patients with normal renal function. Therefore, a reduced starting dose of 3 mg was recommended for MM patients with severe renal impairment or ESRD requiring dialysis because a 3 mg dose would be expected to provide systemic exposures comparable with those achieved in patients with normal renal function after receiving the standard 4 mg dose. Additionally, total ixazomib concentrations were similar in pre- and post-dialyzer samples collected hourly from ESRD patients during a 4 h hemodialysis session, indicating that ixazomib can be administered without regard to the timing of dialysis [43]. The findings of the population PK analyses and dedicated renal impairment study, as well as the associated dosing recommendations, are reflected in the US prescribing information [18] and EU Summary of Product Characteristics [19].
Unbound systemic exposures of ixazomib (AUClast) in patients with (a) normal renal function versus severe renal impairment/ESRD, or (b) normal hepatic function versus moderate-to-severe hepatic impairment. The box lines denote the 25th, 50th, and 75th percentile, and whiskers represent the 10th and 90th percentile for each category. Reproduced with permission from (a) Gupta et al., Br J Haematol, published by John Wiley & Sons Ltd [43], and (b) Gupta et al., Br J Clin Pharmacol, published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society [44]. AUC area under the concentration–time curve, ESRD end-stage renal disease
4.5.4 Hepatic Function
Population PK analyses [36, 37] and a dedicated phase I PK study [44] were conducted to evaluate the effect of hepatic impairment on ixazomib PK. Patients with MM present with or develop hepatic impairment much less frequently than renal impairment [60, 61]. Nevertheless, as metabolism appears to be the major mechanism of ixazomib clearance [47, 48], reduced liver function may result in increased systemic exposures of ixazomib. In addition, patients with moderate or severe hepatic impairment were excluded from clinical studies conducted during development. Analyses were therefore undertaken to evaluate the impact of various degrees of hepatic impairment on the PK of ixazomib.
In the initial population PK analysis [37], total bilirubin over the range of 1.7–39.3 µmol/L (0.01–2.30 mg/dL) showed no association with ixazomib clearance or systemic exposure when examined as a covariate. Similarly, the second population PK analysis confirmed that none of the parameters generally associated with liver function status, i.e. total bilirubin (1.71–39.3 µmol/L [0.01–2.30 mg/dL]), aspartate aminotransferase (4–127 U/L), and serum albumin (12–55 g/L) (Fig. 4), had any clinically meaningful impact on systemic exposures of ixazomib, thereby indicating that no dose adjustments are necessary for patients with mild hepatic impairment [36].
To investigate the effects of more severe liver disease on ixazomib PK, a phase I hepatic impairment study was conducted in patients with advanced solid tumors who had moderate or severe hepatic impairment as defined by the National Cancer Institute Organ Dysfunction Working Group criteria [44]. Lower doses of ixazomib were administered to patients with hepatic impairment to account for the anticipated higher exposures and to ensure patient safety. Patients with normal hepatic function, moderate hepatic impairment, or severe hepatic impairment received 4, 2.3, or 1.5 mg doses of ixazomib, respectively. After single-dose administration, ixazomib was rapidly absorbed in all three hepatic function groups, with a median Tmax of approximately 1–1.5 h post-dose. Moderate and severe hepatic impairment had a similar effect on ixazomib PK, supporting the pooling of data from these two groups for the purposes of making comparisons to the normal hepatic function group (Fig. 6b). Based on geometric least squares mean ratios, unbound and total dose-normalized systemic exposures of ixazomib (AUC) were 27 and 20% higher, respectively, in patients with moderate or severe hepatic impairment versus patients with normal hepatic function [44]. As a result, a reduced weekly starting dose of ixazomib 3 mg is recommended for patients with moderate-to-severe hepatic impairment. For these hepatically impaired patients, the 3 mg dose would be expected to provide systemic exposures comparable to those achieved with the 4 mg dose in patients with normal hepatic function. The aforementioned findings from the population PK analyses and dedicated hepatic impairment study, along with the corresponding posology recommendations, are reflected in the US prescribing information [18] and EU Summary of Product Characteristics [19].
4.6 Extrinsic Factors
4.6.1 Drug–Drug Interactions
Coadministration of ixazomib with other anti-myeloma agents has been investigated in a number of clinical studies, with the findings demonstrating the feasibility of combining ixazomib in a range of regimens [33, 34, 62, 63]. Two dose-finding, phase I/II trials investigated the PK of once- and twice-weekly oral ixazomib in combination with Rd in patients with newly diagnosed MM (NDMM) [33, 34]. In both studies, ixazomib PK data were consistent with observations following once- and twice-weekly administration as a single-agent in patients with RRMM [21, 22], indicating no PK interaction with Rd. Consistently, the second population PK analysis also revealed no impact of Rd coadministration on ixazomib exposure (Fig. 5) [36].
Ixazomib has also been studied in combination with melphalan and prednisone (MP) in a phase I/II trial in elderly, transplant-ineligible NDMM patients [62]. The PK of weekly oral ixazomib at the recommended phase II dose in combination with MP were comparable with reported single-agent PK data after weekly administration [21], indicating no clinically meaningful effect of MP on ixazomib PK [62]. Similar conclusions were reached for cyclophosphamide and dexamethasone (Cd) based on an analysis of the PK of ixazomib in combination with these two agents in a phase II study [63]. Ixazomib systemic exposures in combination with Cd were similar to those seen with single-agent ixazomib [21], thereby suggesting no PK interaction during coadministration with Cd.
DDI studies have been conducted to evaluate the effect of coadministration of strong CYP3A inhibitors or strong CYP3A inducers on ixazomib PK. As noted in Sect. 4.3, in vitro studies indicate that, at clinically relevant concentrations, ixazomib is metabolized predominantly by non-CYP enzymes/proteins [18, 19]. At supratherapeutic concentrations, multiple CYP isoforms were capable of metabolizing ixazomib, with the highest estimated relative contributions being noted for CYP3A4 (42%) and CYP1A2 (26%) [18, 19, 47]. Based on this observation, a multi-arm DDI study was undertaken to investigate the effect of the strong CYP3A inhibitors ketoconazole and clarithromycin, and the strong CYP3A inducer rifampin, on the single-dose PK of ixazomib in patients with advanced solid tumors or lymphoma [47].
Neither ketoconazole (400 mg once daily) nor clarithromycin (500 mg twice daily) produced clinically relevant effects on systemic exposures of ixazomib [47]. For example, in the DDI study with clarithromycin, patients received clarithromycin 500 mg twice daily for 16 days, with a single 2.5 mg dose of ixazomib on day 6. Ixazomib PK parameters in the presence of clarithromycin were compared with PK parameters measured after a single 2.5 mg single-agent dose of ixazomib in a separate arm of the study (Fig. 7a). The geometric least squares mean ratio for ixazomib AUC264 (with versus without clarithromycin) was 1.11. Based on these DDI study results and simulations from a physiologically based PK (PBPK) model that predicted similar AUC ratios for two other strong CYP3A inhibitors (ritonavir and itraconazole), it was concluded that no dose adjustment is necessary when ixazomib is coadministered with CYP3A inhibitors [47].

Reproduced with permission from Gupta et al., J Clin Pharmacol, published by Wiley Periodicals, Inc. on behalf of American College of Clinical Pharmacology [47]
Mean ixazomib plasma concentration–time profiles with and without coadministration of (a) clarithromycin or (b) rifampin. Insets show the first 24 h after dosing. Error bars indicate standard error.
However, in the rifampin DDI study, a clinically significant reduction in ixazomib Cmax and AUC were observed during coadministration with rifampin (Fig. 7b) [47]. Geometric least squares mean ratios (with versus without rifampin) for ixazomib AUClast and Cmax were 0.26 and 0.46, respectively, reflecting approximate decreases of 74 and 54% in these parameters in the presence of rifampin [47]. A potential explanation for these findings is that although the contribution of CYP-mediated metabolism to the baseline clearance of ixazomib is minimal, the contribution of CYP3A and other rifampin-inducible enzymes to ixazomib clearance in the induced state is likely higher. This is likely due to the high capacity/efficiency of CYP3A induction by rifampin, as well as the pleiotropic induction effects of rifampin that may result in an increased contribution of pregnane X receptor-inducible enzymes and possibly P-gp-mediated efflux to the total clearance of ixazomib [47]. These rifampin DDI study findings resulted in the recommendation to avoid concomitant administration of ixazomib with strong CYP3A inducers [18, 19].
The potential for DDIs between ixazomib and inhibitors of CYP1A2, such as ciprofloxacin, and the effect of smoking status (considering that smoking induces CYP1A2 activity) was studied as part of the second population PK analysis [36]. Reflecting the smaller contribution of CYP1A2 versus CYP3A4 to ixazomib metabolism in in vitro studies at supratherapeutic concentrations, coadministration of CYP1A2-modulatory drugs had no impact on ixazomib clearance in the population PK model (although it should be noted that only 1.4% of patients received coadministered CYP1A2-modulatory drugs) [36]. Similarly, smoking status had no effect on ixazomib clearance or exposure (Fig. 5). Thus, no dose adjustments are required during coadministration with CYP1A2 inhibitors or for smokers [36].
5 Exposure–Response Relationship
5.1 Concentration–QTc Relationship
It is imperative to assess the effect of anticancer drugs on cardiac parameters, including the QTc interval, due to the potential risk of cardiac toxicity [64,65,66]. This is particularly relevant for PIs as carfilzomib treatment has been linked with cardiac failure and ischemia [10, 11, 67]. For ixazomib, a three-part, integrated, non-clinical and clinical risk assessment of its effect on the QTc interval was undertaken. This assessment utilized cardiac safety data from in vitro studies, non-clinical in vivo evaluations, and PK/pharmacodynamic model-based analysis of electrocardiogram data from phase I clinical studies, and was successfully undertaken as an alternative to a dedicated QTc study [68].
Human ether-a-go–go-related gene (hERG) assays, which evaluate the main pharmacodynamic mechanism underlying prolongation of the ventricular action potential and QT interval extension in humans, indicated that the in vitro binding potency for ixazomib to the hERG channel was weak. Additionally, an in vivo telemetry analysis in dogs showed no evidence of treatment-related QT/QTc prolongation, or clinically relevant changes in other electrocardiogram parameters, heart rate, or blood pressure at ixazomib doses up to 4.2 mg/m2. Finally, time-matched triplicate ECG and PK data from four phase I clinical studies [21, 22, 27, 28] were utilized in a population model-based concentration–QTc analysis [68]. Even at an ixazomib plasma concentration of 200 ng/mL (approximately four times the geometric mean Cmax at the approved 4 mg dose), ixazomib appeared to have no clinically meaningful effect on QTc, with predicted mean drug-induced changes in QTc that were well below 5 ms (Fig. 8), the regulatory threshold specified by the International Conference on Harmonization (ICH) E14 guidelines [65, 66]. Additionally, no relationship was seen between ixazomib concentration and the RR interval [68]. Taken together, the results of the cardiac risk assessment showed no clinically meaningful effects of ixazomib on QTc or heart rate at clinically relevant exposures [68]. The US FDA and the European Medicines Agency accepted this analysis in lieu of a dedicated QT study, and these results are integrated in the ixazomib US prescribing information and European Summary of Product Characteristics [18, 19].

Reproduced from Gupta et al., with permission from Springer Nature: Springer, Cancer Chemotherapy and Pharmacology (https://link.springer.com/journal/280). © 2015 [68]
Effect of ixazomib plasma concentration on QTc prolongation, where ΔΔQTc represents the mean drug-induced change in QTc with increasing concentration. Top panel shows heart rate-corrected QT interval using Fridericia’s method (QTcF), and the bottom panel shows heart rate-corrected QT interval using the population-based correction method (QTcP).
5.2 Exposure–Response Relationship of Single-Agent Ixazomib in Relapsed/Refractory Multiple Myeloma (RRMM)
A dedicated exposure–response analysis was undertaken of single-agent ixazomib in RRMM [69]. Logistic regression was utilized to investigate relationships between ixazomib plasma exposure and safety using phase I data from 44 patients with RRMM treated with single-agent ixazomib [69]. Significant relationships were identified between ixazomib exposure and grade 3 or higher neutropenia, grade 3 or higher thrombocytopenia, grade 2 or higher rash, grade 2 or higher fatigue, and grade 2 or higher diarrhea, as well as the clinical benefit rate. No relationship was apparent between ixazomib exposure and grade 2 or higher PN; however, the sample size was small, and only six patients had grade 2 or higher PN events, two in the lower-exposure group and four in the higher-exposure group (dichotomized by median exposure). These exposure–response relationships indicated a favorable benefit/risk profile at doses of 3 and 4 mg weekly, and, based on these data, it was recommended that patients receiving ixazomib in the two phase III maintenance trials (TOURMALINE-MM3 and TOURMALINE-MM4) should receive an initial weekly dose of 3 mg, with a subsequent increase to 4 mg if the 3 mg dose is adequately tolerated after four cycles, to provide maximum clinical benefit balanced with acceptable tolerability [69].
5.3 Exposure–Safety Relationships of Ixazomib-Rd in the RRMM Phase III Trial
Exposure–safety analyses were conducted to better understand the relationship between the phase III ixazomib dose and safety in the TOURMALINE-MM1 study [20]. PK data from patients in the ixazomib-Rd arm of TOURMALINE-MM1 were included in an exposure–safety analysis to determine the relationship between ixazomib exposure (with patients divided into exposure quartiles) and the risk of clinically relevant hematologic and non-hematologic adverse events [32]. Statistically significant associations were demonstrated using logistic regression modeling between ixazomib exposure and the risk of grade 3 or higher anemia and thrombocytopenia, and grade 2 or higher diarrhea, fatigue, nausea, PN, and rash (Fig. 9) [32]. Related to these findings, higher ixazomib exposure was associated with a lower relative dose intensity of lenalidomide, likely due to the increased need for toxicity-related dose reductions [32].
Logistic regression analysis of the relationship between ixazomib exposure (by quartiles) and the probability of clinically relevant grade 3 or higher hematologic and grade 2 or higher non-hematologic adverse events in the ixazomib-Rd arm of TOURMALINE-MM1. Reproduced from Gupta et al., with permission from Springer Nature: Springer, Targeted Oncology (https://link.springer.com/journal/11523). © 2017 [32]. CI confidence interval, Rd lenalidomide/dexamethasone
5.4 Exposure–Efficacy Relationships of Ixazomib-Rd in the RRMM Phase III Trial
Exposure–efficacy analyses (of PFS and response rates) were also conducted using population PK model-derived individual estimates of ixazomib PK parameters in patients enrolled in the ixazomib-Rd arm of TOURMALINE-MM1, with patients again divided into ixazomib exposure quartiles [32]. There was no relationship between ixazomib exposure and PFS, and the median PFS was longer for each ixazomib exposure quartile in the ixazomib-Rd arm versus the placebo-Rd arm (Fig. 10) [32]. Consistent with these observations, ixazomib systemic exposure was not identified as a predictor of PFS in Cox proportional hazards modeling, confirming the consistent treatment benefit across the range of exposures achieved at the 4 mg starting dose in combination with Rd in patients with RRMM. Similarly, based on logistic regression analysis, there were no relationships between ixazomib exposure and response rates, and the overall response rate was higher for each ixazomib exposure quartile in the ixazomib-Rd arm versus the placebo-Rd arm.
Analysis of the relationship between ixazomib exposure (by quartiles) and PFS at the initial report of TOURMALINE-MM1 in the ixazomib-Rd (IRd) arm, and comparison with overall PFS in the placebo-Rd arm. Reproduced from Gupta et al., with permission from Springer Nature: Springer, Targeted Oncology (https://link.springer.com/journal/11523). © 2017 [32]. PFS progression-free survival, HR hazard ratio, CI confidence interval, Rd lenalidomide/dexamethasone
6 Conclusions
The PK of ixazomib, underlying sources of population variability, and relationships to clinical safety and efficacy outcomes have been comprehensively evaluated in multiple clinical and dedicated clinical pharmacology studies, as well as through model-based analyses using population PK and exposure–response models. The findings from these studies and analyses have played an important role in determining ixazomib posology, including confirming the feasibility of using fixed dosing in the adult population, and elucidating the recommended phase III dose and the recommended reduced starting dose for patients with severe renal impairment, ESRD requiring dialysis, or moderate-to-severe hepatic impairment. A food effect study established the importance of taking ixazomib on an empty stomach at least 1 h before or 2 h after food, based on the reduced ixazomib exposure and Cmax observed when administered in the fed state, and DDI studies have shown the need to avoid concomitant administration of ixazomib with strong CYP3A inducers such as rifampin. Importantly, data from clinical studies have demonstrated that other common anti-myeloma agents do not impact the PK of ixazomib. The results from these PK evaluations supported the successful initial submissions for regulatory approval of ixazomib, and form an important component of product labeling by guiding appropriate dosing and administration practices. Taken together, this body of clinical pharmacology knowledge on ixazomib enables the feasibility of long-term dosing with all-oral ixazomib-containing regimens in MM across clinical contexts of use.
References
Lub S, Maes K, Menu E, De Bruyne E, Vanderkerken K, Van Valckenborgh E. Novel strategies to target the ubiquitin proteasome system in multiple myeloma. Oncotarget. 2016;7(6):6521–37.
Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–16.
Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 Suppl 4:iv52–iv61.
Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Chandler JC, et al. Multiple myeloma, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(2):230–69.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–8.
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–20.
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40.
Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86(1):23–31.
Richardson PG, Delforge M, Beksac M, Wen P, Jongen JL, Sezer O, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26(4):595–608.
Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Ziogas D, Eleutherakis-Papaiakovou E, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449–54.
Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98(11):1753–61.
Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol. 2018;4(3):e174519.
Guglielmelli T, Palumbo A. Multiple myeloma: is a shift toward continuous therapy needed to move forward? Expert Rev Hematol. 2015;8(3):253–6.
Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015;33(30):3459–66.
Facon T. Maintenance therapy for multiple myeloma in the era of novel agents. Hematol Am Soc Hematol Educ Program. 2015;2015:279–85.
Ludwig H, Zojer N. Fixed duration vs continuous therapy in multiple myeloma. Hematol Am Soc Hematol Educ Program. 2017;2017(1):212–22.
Musto P, Montefusco V. Are maintenance and continuous therapies indicated for every patient with multiple myeloma? Expert Rev Hematol. 2016;9(8):743–51.
Millennium Pharmaceuticals Inc. NINLARO® (ixazomib) capsules, for oral use. United States Prescribing Information. 2016. https://www.ninlaro.com/prescribing-information.pdf. Accessed 1 June 2018.
Takeda Pharma A/S. NINLARO European product assessment report—product information. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003844/WC500217620.pdf. Accessed 1 June 2018.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–34.
Kumar SK, Bensinger WI, Zimmerman TM, Reeder CB, Berenson JR, Berg D, et al. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124(7):1047–55.
Richardson PG, Baz R, Wang M, Jakubowiak AJ, Laubach JP, Harvey RD, et al. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124(7):1038–46.
Faucette S, Wagh S, Trivedi A, Venkatakrishnan K, Gupta N. Reverse translation of US Food and Drug Administration reviews of oncology new molecular entities approved in 2011–2017: lessons learned for anticancer drug development. Clin Transl Sci. 2018;11(2):123–46.
Gupta N, Hanley MJ, Diderichsen PM, Yang H, Ke A, Teng Z et al. Model-informed drug development for ixazomib, an oral proteasome inhibitor. Clin Pharmacol Ther. Epub 15 Feb 2018. https://doi.org/10.1002/cpt.1047.
Kupperman E, Lee EC, Cao Y, Bannerman B, Fitzgerald M, Berger A, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970–80.
Chauhan D, Tian Z, Zhou B, Kuhn D, Orlowski R, Raje N, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 2011;17(16):5311–21.
Assouline SE, Chang J, Cheson BD, Rifkin R, Hamburg S, Reyes R, et al. Phase 1 dose-escalation study of IV ixazomib, an investigational proteasome inhibitor, in patients with relapsed/refractory lymphoma. Blood Cancer J. 2014;4:e251.
Smith DC, Kalebic T, Infante JR, Siu LL, Sullivan D, Vlahovic G, et al. Phase 1 study of ixazomib, an investigational proteasome inhibitor, in advanced non-hematologic malignancies. Invest New Drugs. 2015;33(3):652–63.
Reece DE, Sullivan D, Lonial S, Mohrbacher AF, Chatta G, Shustik C, et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol. 2011;67(1):57–67.
Lee SJ, Levitsky K, Parlati F, Bennett MK, Arastu-Kapur S, Kellerman L, et al. Clinical activity of carfilzomib correlates with inhibition of multiple proteasome subunits: application of a novel pharmacodynamic assay. Br J Haematol. 2016;173(6):884–95.
Venkatakrishnan K, Ecsedy JA. Enhancing value of clinical pharmacodynamics in oncology drug development: an alliance between quantitative pharmacology and translational science. Clin Pharmacol Ther. 2017;101(1):99–113.
Gupta N, Yang H, Hanley MJ, Zhang S, Liu R, Kumar S, et al. Dose and schedule selection of the oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma: clinical and model-based analyses. Target Oncol. 2017;12(5):643–54.
Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15(13):1503–12.
Richardson PG, Hofmeister CC, Rosenbaum CA, Htut M, Vesole DH, Berdeja JG, et al. Twice-weekly ixazomib in combination with lenalidomide-dexamethasone in patients with newly diagnosed multiple myeloma. Br J Haematol. 2018;182(2):231–44.
Sanchorawala V, Palladini G, Kukreti V, Zonder JA, Cohen AD, Seldin DC, et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130(5):597–605.
Gupta N, Diderichsen PM, Hanley MJ, Berg D, van de Velde H, Harvey RD, et al. Population pharmacokinetic analysis of ixazomib, an oral proteasome inhibitor, including data from the phase III TOURMALINE-MM1 study to inform labelling. Clin Pharmacokinet. 2017;56(11):1355–68.
Gupta N, Zhao Y, Hui AM, Esseltine DL, Venkatakrishnan K. Switching from body surface area-based to fixed dosing for the investigational proteasome inhibitor ixazomib: a population pharmacokinetic analysis. Br J Clin Pharmacol. 2015;79(5):789–800.
Hanley MJ, Gupta N, Venkatakrishnan K, Bessudo A, Sharma S, O’Neil BH, et al. A phase 1 study to assess the relative bioavailability of two capsule formulations of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. J Clin Pharmacol. 2018;58(1):114–21.
Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–56.
United States Food and Drug Administration. Guidance for Industry. Bioavailability and bioequivalence studies for orally administered drug products—general considerations. Revision 1. 2003. https://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf. Accessed 1 June 2018.
Gupta N, Hanley MJ, Venkatakrishnan K, Wang B, Sharma S, Bessudo A, et al. The effect of a high-fat meal on the pharmacokinetics of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. J Clin Pharmacol. 2016;56(10):1288–95.
Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60(6):717–33.
Gupta N, Hanley MJ, Harvey RD, Badros A, Lipe B, Kukreti V, et al. A pharmacokinetics and safety phase 1/1b study of oral ixazomib in patients with multiple myeloma and severe renal impairment or end-stage renal disease requiring haemodialysis. Br J Haematol. 2016;174(5):748–59.
Gupta N, Hanley MJ, Venkatakrishnan K, Perez R, Norris RE, Nemunaitis J, et al. Pharmacokinetics of ixazomib, an oral proteasome inhibitor, in solid tumour patients with moderate or severe hepatic impairment. Br J Clin Pharmacol. 2016;82(3):728–38.
Clemens J, Welti L, Schafer J, Seckinger A, Burhenne J, Theile D, et al. Bortezomib, carfilzomib and ixazomib do not mediate relevant transporter-based drug-drug interactions. Oncol Lett. 2017;14(3):3185–92.
Pusalkar S, Plesescu M, Gupta N, Hanley M, Venkatakrishnan K, Wu J-T, et al. Human biotransformation pathways of the oral proteasome inhibitor ixazomib: metabolite profiling of [14C]-ixazomib in plasma and excreta of patients with advanced solid tumors. Drug Metab Pharmacokinet. 2018;33(1 Suppl):S73–4.
Gupta N, Hanley MJ, Venkatakrishnan K, Bessudo A, Rasco DW, Sharma S, et al. Effects of strong CYP3A inhibition and induction on the pharmacokinetics of ixazomib, an oral proteasome inhibitor: results of drug-drug interaction studies in patients with advanced solid tumors or lymphoma and a physiologically based pharmacokinetic analysis. J Clin Pharmacol. 2018;58(2):180–92.
Gupta N, Zhang S, Pusalkar S, Plesescu M, Chowdhury S, Hanley MJ, et al. A phase I study to assess the mass balance, excretion, and pharmacokinetics of [(14)C]-ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2018;36(3):407–15.
Johnson JA. Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther. 2000;38(2):53–60.
Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84(3):417–23.
Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97(3):263–73.
Venkatakrishnan K, Burgess C, Gupta N, Suri A, Takubo T, Zhou X, et al. Toward optimum benefit-risk and reduced access lag for cancer drugs in Asia: a global development framework guided by clinical pharmacology principles. Clin Transl Sci. 2016;9(1):9–22.
Gupta N, Goh YT, Min CK, Lee JH, Kim K, Wong RS, et al. Pharmacokinetics and safety of ixazomib plus lenalidomide-dexamethasone in Asian patients with relapsed/refractory myeloma: a phase 1 study. J Hematol Oncol. 2015;8:103.
Suzuki K, Handa H, Chou T, Ishizawa K, Takubo T, Kase Y. Phase 1 study of ixazomib alone or combined with lenalidomide-dexamethasone in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol. 2017;105(4):445–52.
Hou J, Jin J, Xu Y, Wu D, Ke X, Zhou D, et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China continuation study. J Hematol Oncol. 2017;10(1):137.
Yadav P, Cook M, Cockwell P. Current trends of renal impairment in multiple myeloma. Kidney Dis (Basel). 2016;1(4):241–57.
Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008;83(6):898–903.
Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther. 2006;109(1–2):1–11.
Zhang Y, Zhang L, Abraham S, Apparaju S, Wu TC, Strong JM, et al. Assessment of the impact of renal impairment on systemic exposure of new molecular entities: evaluation of recent new drug applications. Clin Pharmacol Ther. 2009;85(3):305–11.
Bhandari MS, Mazumder A, Vesole DH. Liver involvement in multiple myeloma. Clin Lymphoma Myeloma. 2007;7(8):538–40.
Stansfield LC, Gonsalves WI, Buadi FK. The use of novel agents in multiple myeloma patients with hepatic impairment. Future Oncol. 2015;11(3):501–10.
San Miguel JF, Gutierrez MAE, Špicka I, Mateos M-V, Song K, Craig MD et al. The oral proteasome inhibitor ixazomib in combination with melphalan-prednisone (MP) for patients with newly diagnosed multiple myeloma (NDMM): phase 1/2 dose-escalation study (NCT01335685). Haematologica. 2017;102(s2):111–2 (abstract P339, and poster presented at the 22nd Congress of the European Hematology Association, Madrid, Spain, 22–25 June 2017).
Kumar S, Grzasko N, Delimpasi S, Jędrzejczak WW, Grosicki S, Kyrtsonis M-C et al. Phase 2 study of the all-oral combination of ixazomib plus cyclophosphamide and low-dose dexamethasone (ICd) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). Blood. 2016;128(22):3327 (abstract and poster presented at the 58th American Society of Hematology Annual Meeting and Exposition, San Diego, CA, USA, 3–6 December 2016).
Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use E14 guideline. J Clin Pharmacol. 2006;46(5):498–507.
European Medicines Agency (EMA). ICH topic E14. The clinical evaluation of QT/QTc Interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002879.pdf. Accessed 1 June 2018.
United States Food and Drug Administration. Guidance for industry. E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073153.pdf. Accessed 1 June 2018.
Onyx Pharmaceuticals Inc. Kyprolis® (carfilzomib) for injection. United States Prescribing Information. 2018. https://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/kyprolis/kyprolis_pi.pdf. Accessed 1 June 2018.
Gupta N, Huh Y, Hutmacher MM, Ottinger S, Hui AM, Venkatakrishnan K. Integrated nonclinical and clinical risk assessment of the investigational proteasome inhibitor ixazomib on the QTc interval in cancer patients. Cancer Chemother Pharmacol. 2015;76(3):507–16.
Gupta N, Labotka R, Liu G, Hui AM, Venkatakrishnan K. Exposure-safety-efficacy analysis of single-agent ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma: dose selection for a phase 3 maintenance study. Invest New Drugs. 2016;34(3):338–46.
Acknowledgements
The authors would like to thank Jane Saunders and Steve Hill of FireKite (an Ashfield Company, part of UDG Healthcare PLC) for medical writing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Writing assistance was funded by Millennium Pharmaceuticals, Inc. All editorial procedures complied with Good Publication Practice-3 (GPP3) guidelines (Battisti WP, et al. Ann Intern Med. 2015;163(6):461–464).
Conflicts of interest
Neeraj Gupta, Michael J. Hanley, Cindy Xia, Richard Labotka, and Karthik Venkatakrishnan are employees of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. R. Donald Harvey discloses research funding to his institution from Takeda.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gupta, N., Hanley, M.J., Xia, C. et al. Clinical Pharmacology of Ixazomib: The First Oral Proteasome Inhibitor. Clin Pharmacokinet 58, 431–449 (2019). https://doi.org/10.1007/s40262-018-0702-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-018-0702-1