Abstract
Background
Warfarin acts by inhibiting the reduction of vitamin K (VK) to its active form, thereby decreasing the production of VK-dependent coagulation proteins. The aim of this research is to develop a joint model for the VK-dependent clotting factors II, VII, IX and X, and the anticoagulation proteins, proteins C and S, during warfarin initiation.
Methods
Data from 18 patients with atrial fibrillation who had warfarin therapy initiated were available for analysis. Nine blood samples were collected from each subject at baseline, and at 1–5, 8, 15 and 29 days after warfarin initiation and assayed for factors II, VII, IX and X, and proteins C and S. Warfarin concentration–time data were not available. The coagulation proteins data were modelled in a stepwise manner using NONMEM® Version 7.2. In the first stage, each of the coagulation proteins was modelled independently using a kinetic-pharmacodynamic model. In the subsequent step, the six kinetic-pharmacodynamic models were combined into a single joint model.
Results
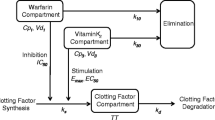
One patient was administered VK and was excluded from the analysis. Each kinetic-pharmacodynamic model consisted of two parts: (1) a common one-compartment pharmacokinetic model with first-order absorption and elimination for warfarin; and (2) an inhibitory E max model linked to a turnover model for coagulation proteins. In the joint model, an unexpected pharmacodynamic lag was identified and the estimated degradation half-life of VK-dependent coagulation proteins were in agreement with previously published values. The model provided an adequate fit to the observed data.
Conclusion
The joint model represents the first work to quantify the influence of warfarin on all six VK-dependent coagulation proteins simultaneously. Future work will expand the model to predict the influence of exogenously administered VK on the time course of clotting factor concentrations after warfarin overdose and during perioperative warfarin reversal procedures.







Similar content being viewed by others
References
Gong IY, Schwarz UI, Crown N, et al. Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation. PLoS One. 2011;6(11):e27808. doi:10.1371/journal.pone.0027808.
Hamberg AK, Dahl ML, Barban M, et al. A PK-PD model for predicting the impact of age, CYP2C9, and VKORC1 genotype on individualization of warfarin therapy. Clin Pharmacol Ther. 2007;81(4):529–38. doi:10.1038/sj.clpt.6100084.
Hamberg AK, Wadelius M, Lindh JD, et al. A pharmacometric model describing the relationship between warfarin dose and INR response with respect to variations in CYP2C9, VKORC1, and age. Clin Pharmacol Ther. 2010;87(6):727–34. doi:10.1038/clpt.2010.37.
Holford NH. Clinical pharmacokinetics and pharmacodynamics of warfarin: understanding the dose-effect relationship. Clin Pharmacokinet. 1986;11(6):483–504. doi:10.2165/00003088-198611060-00005.
Sasaki T, Tabuchi H, Higuchi S, Ieiri I. Warfarin-dosing algorithm based on a population pharmacokinetic/pharmacodynamic model combined with Bayesian forecasting. Pharmacogenomics. 2009;10(8):1257–66. doi:10.2217/pgs.09.65.
Wright DF, Duffull SB. Development of a bayesian forecasting method for warfarin dose individualization. Pharm Res. 2011;28(5):1100–11. doi:10.1007/s11095-011-0369-x.
Wright DF, Duffull SB. A Bayesian dose-individualization method for warfarin. Clin Pharmacokinet. 2013;52(1):59–68. doi:10.1007/s40262-012-0017-6.
Xue L, Holford NH, Miao L (eds). Warfarin PKPD: theory, body composition and genotype. In: Annual Meeting of the Population Approach Group in Europe. Lisbon; 2016 (7–10 June).
McCollum D, Tait RC, Conkie J, et al. The effect of initiation of oral anticoagulation on protein Z and coagulation activation. Br J Haematol. 2004;125(Suppl. 1):1.
Tait RC, Sefcick A. A warfarin induction regimen for out-patient anticoagulation in patients with atrial fibrillation. Br J Haematol. 1998;101(3):450–4.
Mackie I, Cooper P, Lawrie A, et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Int J Lab Hematol. 2013;35(1):1–13. doi:10.1111/ijlh.12004.
O’Reilly RA, Aggeler PM, Leong LS. Studies on the coumarin anticoagulant drugs: the pharmacodynamics of warfarin in man. J Clin Invest. 1963;42(10):1542–51.
Yuen E, Gueorguieva I, Wise S, et al. Ethnic differences in the population pharmacokinetics and pharmacodynamics of warfarin. J Pharmacokinet Pharmacodyn. 2010;37(1):3–24. doi:10.1007/s10928-009-9138-4.
Pitsiu M, Parker EM, Aarons L, Rowland M. A Bayesian method based on clotting factor activity for the prediction of maintenance warfarin dosage regimens. Ther Drug Monit. 2003;25(1):36–40.
Wajima T, Isbister GK, Duffull SB. A comprehensive model for the humoral coagulation network in humans. Clin Pharmacol Ther. 2009;86(3):290–8. doi:10.1038/clpt.2009.87.
Shivva V, Korell J, Tucker IG, Duffull SB. An approach for identifiability of population pharmacokinetic-pharmacodynamic models. CPT Pharmacomet Syst Pharmacol. 2013;2(6):e49. doi:10.1038/psp.2013.25.
Shivva V, Korell J, Tucker IG, Duffull SB. Parameterisation affects identifiability of population models. J Pharmacokinet Pharmacodyn. 2014;41(1):81–6. doi:10.1007/s10928-013-9347-8.
Holford NH, Kirkpatrick C, Duffull SB (eds). NONMEM termination status is not an important indicator of the quality of bootstrap parameter estimates. In: Annual Meeting of the Population Approach Group in Europe. Brugge; 2006.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51. doi:10.1208/s12248-011-9255-z.
Henin E, You B, VanCutsem E, et al. A dynamic model of hand-and-foot syndrome in patients receiving capecitabine. Clin Pharmacol Ther. 2009;85(4):418–25. doi:10.1038/clpt.2008.220.
Jacqmin P, Snoeck E, van Schaick EA, et al. Modelling response time profiles in the absence of drug concentrations: definition and performance evaluation of the K-PD model. J Pharmacokinet Pharmacodyn. 2007;34(1):57–85. doi:10.1007/s10928-006-9035-z.
Tod M. Evaluation of drugs in pediatrics using K-PD models: perspectives. Fundam Clin Pharmacol. 2008;22(6):589–94. doi:10.1111/j.1472-8206.2008.00649.x.
Chan E, McLachlan AJ, Pegg M, et al. Disposition of warfarin enantiomers and metabolites in patients during multiple dosing with rac-warfarin. Br J Clin Pharmacol. 1994;37(6):563–9.
D’Angelo A, Della Valle P, Crippa L, et al. Relationship between international normalized ratio values, vitamin K-dependent clotting factor levels and in vivo prothrombin activation during the early and steady phases of oral anticoagulant treatment. Haematologica. 2002;87(10):1074–80.
O’Reilly RA, Aggeler PM. Studies on coumarin anticoagulant drugs: initiation of warfarin therapy without a loading dose. Circulation. 1968;38(1):169–77.
Bowie EJ, Thompson JH Jr, Didisheim P, Owen CA Jr. Disappearance rates of coagulation factors: transfusion studies in factor-deficient patients. Transfusion. 1967;7(3):174–84.
Pitsiu M, Parker EM, Aarons L, Rowland M. Population pharmacokinetics and pharmacodynamics of warfarin in healthy young adults. Eur J Pharm Sci. 1993;1(3):151–7.
Hjort PF, Egeberg O, Mikkelsen S. Turnover of prothrombin, factor VII and factor IX in a patient with hemophilia A. Scand J Clin Lab Invest. 1961;13(4):668–72.
Peyvandi F, Garagiola I, Seregni S. Future of coagulation factor replacement therapy. J Thromb Haemost. 2013;11(Suppl. 1):84–98. doi:10.1111/jth.12270.
D’Angelo A, Vigano-D’Angelo S, Esmon CT, Comp PC. Acquired deficiencies of protein S: protein S activity during oral anticoagulation, in liver disease, and in disseminated intravascular coagulation. J Clin Invest. 1988;81(5):1445–54.
Riess H, Binsack T, Hiller E. Protein C antigen in prothrombin complex concentrates: content, recovery and half life. Blut. 1985;50(5):303–6.
Bertola JP, Mazoyer E, Bergmann JF, et al. Early prediction of the sensitivity of warfarin in elderly patients by the fall in factor VIIc and protein C at the induction of treatment. Thromb Res. 2003;109(5–6):287–91.
Vainieri H, Wingard LB Jr. Effect of warfarin on the kinetics of the vitamin K-dependent clotting factors in rats. J Pharmacol Exp Ther. 1977;201(2):507–17.
Chan E, McLachlan A, O’Reilly R, Rowland M. Stereochemical aspects of warfarin drug interactions: use of a combined pharmacokinetic-pharmacodynamic model. Clin Pharmacol Ther. 1994;56(3):286–94.
Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 Suppl.):8S–21S.
Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1–7.
Shao J, Wu CFJ. A general theory for jackknife variance estimation. Ann Stat. 1989;17(3):1176–97. doi:10.1214/aos/1176347263.
Bose A, Chatterjee S. Comparison of bootstrap and jackknife variance estimators in linear regression: second order results. Stat Sin. 2002;12(2):575–98.
Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37(1):36–48. doi:10.2307/2685844.
Bonate PL, Steimer JL. Pharmacokinetic-pharmacodynamic modeling and simulation. New York: Springer; 2006.
Wright DF. Model-based drug dosing. Dunedin: University of Otago; 2013.
Acknowledgements
Dr. David Foster, University of South Australia and Dr. Stefanie Hennig, University of Queensland offered their multicore facilities for parallel computing and helped execute some of the NONMEM® runs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Qing Xi Ooi, Daniel F.B. Wright, R. Campbell Tait, Geoffrey K. Isbister and Stephen B. Duffull have no conflicts of interest that are directly relevant to the contents of this study.
Consent to Participate
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
Qing Xi Ooi received the University of Otago doctoral scholarship. Geoffrey K. Isbister is funded by a National Health and Medical Research Council Senior Research Fellowship, ID 1061041. No funding was received for this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ooi, Q.X., Wright, D.F.B., Tait, R.C. et al. A Joint Model for Vitamin K-Dependent Clotting Factors and Anticoagulation Proteins. Clin Pharmacokinet 56, 1555–1566 (2017). https://doi.org/10.1007/s40262-017-0541-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0541-5