Abstract
Background and Objective
PM00104 (Zalypsis®) is a novel marine-derived compound that has shown antineoplastic activity against a number of human tumour cell lines. Myelosuppression was found to be a PM00104 dose-limiting toxicity during phase I studies. The objective of this study was to characterize the time course of neutropenia after intravenous PM00104 administration in cancer patients.
Methods
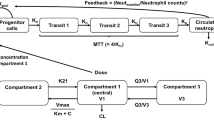
Absolute neutrophil counts (ANCs) and pharmacokinetic data from 144 patients receiving PM00104 doses ranging from 0.053 to 5 mg/m2 were used to estimate the system-related (baseline ANC [Circ0], mean transit time [MTT], feedback on proliferation [γ] and maturation [δ]) and drug-specific (first-order elimination rate constant from effect compartment [ke0] [α and β]) parameters of a modified Friberg’s model. The concentrations in the effect compartment (Ce) were assumed to reduce the proliferation rate of the progenitor cells according to the function \( \alpha \times {\text{C}}_{\text{e}}{}^{{{\upbeta}}}.\) Model evaluation and simulations were undertaken to evaluate the effect of dose intensity, dose density and the intravenous infusion duration on severe neutropenia incidence.
Results
The typical values (between-subject variability [%]) of the Circ0, MTT, γ, δ, ke0, α and β were estimated to be 5.66 × 109 cells/L (13 %), 149 h (29 %), 0.136, 0.191, 0.00639 h−1 (32 %), 0.332 L/µg (24 %) and 1.47, respectively. Age, bodyweight, sex, serum albumin, total protein, liver metastases, number of previous chemotherapy lines and performance status were not associated with model parameters. The model evaluation evidenced an accurate prediction of the neutropenia grade 3 and/or 4 incidence. Simulations indicated that PM00104 dose and dosing interval, but not infusion duration, were the main determinants of the neutropenia severity and duration.
Conclusions
The time course of neutropenia following PM00104 was well characterized by the model developed. The model-predicted time course of the ANCs and its variability confirmed that neutropenia is reversible, of short duration and non-cumulative.





Similar content being viewed by others
References
Rinehart KL, Holt TG, Fregeau NL, et al. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J Org Chem. 1990;55:4512–5.
Fontana A, Cavaliere P, Wahidulla S, et al. A new antitumor isoquinoline alkaloid from the marine nudibranch Jorunna funebris. Tetrahedron. 2000;56:7305–8.
Oku N, Matsunaga S, van Soest RW, et al. Renieramycin J, a highly cytotoxic tetrahydroisoquinoline alkaloid, from a marine sponge Neopetrosia sp. J Nat Prod. 2003;66:1136–9.
Leal JF, García-Hernández V, Moneo V, et al. Molecular pharmacology and antitumor activity of Zalypsis in several human cancer cell lines. Biochem Pharmacol. 2009;78:162–70.
Ocio EM, Maiso P, Chen X, et al. Zalypsis: a novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood. 2009;113:3781–91.
Guirouilh-Barbat J, Antony S, Pommier Y. Zalypsis (PM00104) is a potent inducer of gamma-H2AX foci and reveals the importance of the C ring of trabectedin for transcription-coupled repair inhibition. Mol Cancer Ther. 2009;8:2007–14.
Massard C, Margetts J, Amellal N, et al. Phase I study of PM00104 (Zalypsis®) administered as a 1-hour weekly infusion resting every fourth week in patients with advanced solid tumors. Invest New Drugs. Epub 12 June 2012.
Friberg LE, Henningsson A, Maas H, et al. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20:4713–21.
Leger F, Loos WJ, Bugat R, et al. Mechanism-based models for topotecan-induced neutropenia. Clin Pharmcol Ther. 2004;76:567–78.
Latz JE, Karlsson MO, Rusthoven JJ, et al. A semimechanistic-physiologic population pharmacokinetic/pharmacodynamic model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol. 2005;57:412–26.
Sandström M, Lindman H, Nygren P, et al. Model describing the relationship between pharmacokinetics and hematologic toxicity of epirubicin-docetaxel regimen in breast cancer patients. J Clin Oncol. 2005;23:413–21.
van Kesteren C, Zandvliet AS, Karlsson MO, et al. Semi-physiological model describing the hematological toxicity of the anti-cancer agent indisulam. Invest New Drugs. 2005;23:225–34.
Sandstrom M, Lindman H, Nygren P, et al. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol. 2006;58:143–56.
Ramón-López A, Nalda-Molina R, Valenzuela B, et al. Semi-mechanistic model for neutropenia after high dose of chemotherapy in breast cancer patients. Pharm Res. 2009;26:1952–62.
Hing J, Perez-Ruixo JJ, Stuyckens K, et al. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of trabectedin (ET-743, Yondelis®) induced neutropenia. Clin Pharmacol Ther. 2008;83:130–43.
Quartino AL, Friberg LF, Karlsson MO. A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs. 2012;30:833–45.
Pérez-Ruixo C, Valenzuela B, Fernández Teruel C, González-Sales M, Miguel-Lillo B, Soto-Matos A, et al. Population pharmacokinetics of PM00104 (Zalypsis®) in cancer patients. Cancer Chemother Pharmacol. 2012;69:15–24.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit: a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30:387–404.
Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–40.
Dale DC, Liles WC, Llewellyn C, et al. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) on neutrophil kinetics and function in normal human volunteers. Am J Hematol. 1998;57:7–15.
Girard P. Data transformation and parameter transformations in NONMEM. http://www.page-meeting.org/page/page2002/PascalGirardPage2002.pdf. Accessed 20 Aug 2012.
Savic RM, Karlsson MO. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20.
Kloft C, Wallin J, Henningsson A, et al. Population pharmacokinetic-pharmacodynamic model for neutropenia with patient subgroup identification: comparison across anticancer drugs. Clin Cancer Res. 2006;12:5481–90.
Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic-pharmacodynamic models. I: models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–28.
Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993.
Brendel K, Comets E, Laffont C, et al. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23:2036–49.
Joerger M, Huitema AD, Richel DJ, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res. 2007;13:6410–8.
Kathman SJ, Williams DH, Hodge JP, et al. A Bayesian population PK-PD model of ispinesib-induced myelosuppression. Clin Pharmacol Ther. 2007;81:88–94.
Panetta JC, Schaiquevich P, Santana VM, et al. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res. 2008;14:318–25.
Troconiz IF, Garrido MJ, Segura C, et al. Phase I dose-finding study and a pharmacokinetic/pharmacodynamic analysis of the neutropenic response of intravenous diflomotecan in patients with advanced malignant tumours. Cancer Chemother Pharmacol. 2006;57:727–35.
Lord BI, Bronchud MH, Owens S, et al. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA. 1989;86:9499–503.
Schmitz S, Franke H, Brusis J, et al. Quantification of the cell kinetic effects of G-CSF using a model of human granulopoiesis. Exp Hematol. 1993;21:755–60.
Yap TA, Cortes-Funes H, Shaw H, Rodriguez R, Olmos D, Lal R, et al. First-in-man phase I trial of two schedules of the novel synthetic tetrahydroisoquinoline alkaloid PM00104 (Zalypsis) in patients with advanced solid tumours. Br J Cancer. 2012;106:1379–85.
PharmaMar SA. A phase I multicenter, open-label, dose-escalating study of PM00104 administered as 1 and 3 hours intravenous infusion every 3 weeks in subjects with advanced solid tumors or lymphoma. [EUDRACT identifier: 2004-002585-38]. European Union Drug Regulating Authorities Clinical Trials. https://eudract.ema.europa.eu/. Accessed 4 Sep 2012.
PharmaMar SA. A phase I study of Zalypsis (PM00104) in subjects with advanced malignant solid tumors or lymphoma [ClinicalTrials.gov identifier NCT00359294]. US National Institutes of Health, ClinicalTrials.gov. http://www.clinicaltrials.gov. Accessed 4 Sep 2012.
PharmaMar SA. A phase I multicenter, open-label, dose-escalating clinical and pharmacokinetic study of PM00104 administered every 3 weeks, intravenously, over 24 hours to subjects with advanced malignant solid tumors or lymphoma. [EUDRACT identifier: 2005-002588-10]. European Union Drug Regulating Authorities Clinical Trials. https://eudract.ema.europa.eu/. Accessed 4 Sep 2012.
PharmaMar SA. A phase I multicenter, open-label, dose-escalating clinical and pharmacokinetic study of PM00104 administered weekly resting every fourth week, intravenously, over 1 hour, to subjects with advanced malignant solid tumors or lymphoma. [EUDRACT identifier: 2005-002587-28]. European Union Drug Regulating Authorities Clinical Trials. https://eudract.ema.europa.eu/. Accessed 4 Sep 2012.
Acknowledgments
The authors would like to thank the patients, investigators and their medical, nursing and laboratory staff who participated in the clinical trials included in the present study. In particular, we recognize the effort from the laboratory staff of the ICON DS (Manchester, UK) who participated in the bioanalytical analysis of the PM00104 samples. The views expressed in this article are the personal views of the authors reflecting their scientific knowledge of this topic and should not be understood or quoted as being made on behalf of the companies at which the authors currently work.
Conflict of interest
Carlos Fernández Teruel, Bernardo Miguel-Lillo and Arturo Soto-Matos are employees of Pharma Mar SA, which supported this study. Mario González-Sales and Carlos Pérez-Ruixo are from Consulting Projects for Research, which acts as a consultant for Pharma Mar SA, and received consultation fees for contributing to the current analysis. Belén Valenzuela and Juan Jose Pérez-Ruixo were members of the Pharmaceutical Sciences Department, University Miguel Hernández, Alicante, Spain, at the time that this project was started. Belén Valenzuela and Juan Jose Pérez-Ruixo declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Sales, M., Valenzuela, B., Pérez-Ruixo, C. et al. Population Pharmacokinetic–Pharmacodynamic Analysis of Neutropenia in Cancer Patients Receiving PM00104 (Zalypsis®). Clin Pharmacokinet 51, 751–764 (2012). https://doi.org/10.1007/s40262-012-0011-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0011-z