Abstract
Background and Objective
Novel messenger RNA (mRNA)-based therapies, currently in development, are emerging as a promising potential treatment modality for a broad range of life-threatening and life-limiting inherited liver diseases, including methylmalonic acidemia (MMA) and propionic acidemia (PA). However, owing in part to their complexity, they are likely to come at considerable financial cost to healthcare systems. The objective of this research was to synthesize available evidence on the costs and clinical consequences associated with MMA and PA for the purpose of exploratory economic evaluation of novel mRNA-based therapies using an early cost-utility model from the United Kingdom payer perspective.
Methods
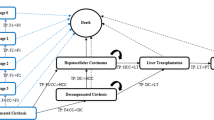
A Markov model was constructed to simulate the costs and outcomes associated with novel mRNA therapies, compared with a combination of dietary management and organ transplantation (standard of care) among hypothetical cohorts of new-born patients with MMA and PA. Key model drivers were identified, and a price threshold analysis was performed to estimate value-based price ranges for future mRNA therapies given willingness-to-pay thresholds for orphan diseases.
Results
mRNA therapy was associated with an additional 5.7 and 1.3 quality-adjusted life-years (QALYs) gained per patient lifetime among patients with MMA and PA, respectively. Key drivers of cost-effectiveness were relative improvement in utility among patients who receive mRNA-based therapy and transplantation, and the cost of mRNA therapy. Assuming a willingness to pay range of £100,000–£300,000 per QALY gained, the model demonstrated mRNA therapy to be cost-effective in MMA and PA at an annual treatment cost of £70,452–£94,575 and £31,313–£36,695, respectively.
Conclusions
Despite the lack of a strong evidence base in MMA and PA, this model provides a useful tool to estimate the cost-effectiveness, and inform value-based pricing, of new mRNA-based therapies. Our analyses also identified areas for research that will have the greatest value in reducing uncertainty in future health economic evaluations of such treatments.






Similar content being viewed by others
References
Fraser JL, Venditti CP. Methylmalonic and propionic acidemias: clinical management update. Curr Opin Pediatr. 2016;28(6):682–93.
Imbard A, Garcia Segarra N, Tardieu M, et al. Long-term liver disease in methylmalonic and propionic acidemias. Mol Genet Metab. 2018;123(4):433–40.
Deodato F, Boenzi S, Santorelli FM, et al. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142c(2):104–12.
Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308(15):857–61.
Hörster F, Baumgartner MR, Viardot C, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut−, cblA, cblB). Pediatr Res. 2007;62(2):225–30.
Richter T, Nestler-Parr S, Babela R, et al. Rare disease terminology and definitions—a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value in Health. 2015;18(6):906–14.
Baumgartner MR, Hörster F, Dionisi-Vici C, et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;2(9):130.
An D, Frassetto A, Jacquinet E, et al. Long-term efficacy and safety of mRNA therapy in two murine models of methylmalonic acidemia. EBioMedicine. 2019;45:519–28.
Häberle J, Chakrapani A, Ah Mew N, et al. Hyperammonaemia in classic organic acidaemias: a review of the literature and two case histories. Orphanet J Rare Dis. 2018;13(1):219–219.
Critelli K, McKiernan P, Vockley J, et al. Liver transplantation for propionic acidemia and methylmalonic acidemia: perioperative management and clinical outcomes. Liver Transplant. 2018;24(9):1260–70.
Niemi AK, Kim IK, Krueger CE, et al. Treatment of methylmalonic acidemia by liver or combined liver-kidney transplantation. J Pediatr. 2015;166(6):1455-61.e1.
Zimak J, Shang Y, Zhao W. mRNA rescues neonatal acidemia while mice report no aftereffects. EBioMedicine. 2019;46:23–4.
Jiang L, Park JS, Yin L, et al. Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia. Nat Commun. 2020;11(1):5339.
Berraondo P, Martini PGV, Avila MA, et al. Messenger RNA therapy for rare genetic metabolic diseases. Gut. 2019;68(7):1323–30.
Chandler RJ, Di Pasquale G, Sloan JL, et al. Systemic gene therapy for methylmalonic acidemia using the novel adeno-associated viral vector 44.9. MolTherapy Methods Clin Dev. 2022;27:61–72.
An D, Schneller JL, Frassetto A, et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 2017;21(12):3548–58.
Moderna Expands the Field of mRNA Medicine with Positive Clinical Results Across Cancer, Rare Disease, and Infectious Disease (Press Release). Moderna, Inc. September 2023. Available at: https://investors.modernatx.com/news/news-details/2023. Accessed Jan 2024. 2023.
Witzigmann D, Kulkarni JA, Leung J, et al. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev. 2020;159:344–63.
Joint Formulary Committee. British National Formulary (online) London: BMJ Group and Pharmaceutical Press. Available at: http://www.medicinescomplete.com. Accessed 25 Jan 2024.
National Institute for Health and Care Excellence (NICE). Changes to NICE drug appraisals: what you need to know. Available at: https://www.nice.org.uk/news/feature/changes-to-nice-drug-appraisals-what-you-need-to-know. Accessed 2 Apr 2021.
National Cost Collection for the NHS. National Schedule of NHS Costs 2018/19. Available at: https://www.england.nhs.uk/national-cost-collection/. Accessed 7 April 2020.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/the-reference-case. Accessed 7 April 2020.
Li M, Dick A, Montenovo M, et al. Cost-effectiveness of liver transplantation in methylmalonic and propionic acidemias. Liver Transplant. 2015;21(9):1208–18.
Perito ER, Rhee S, Roberts JP, et al. Pediatric liver transplantation for urea cycle disorders and organic acidemias: United Network for Organ Sharing data for 2002–2012. Liver Transplant. 2014;20(1):89–99.
Quintero J, Molera C, Juamperez J, et al. the role of liver transplantation in propionic acidemia. Liver Transplant. 2018;24(12):1736–45.
Chu TH, Chien YH, Lin HY, et al. Methylmalonic acidemia/propionic acidemia - the biochemical presentation and comparing the outcome between liver transplantation versus non-liver transplantation groups. Orphanet J Rare Dis. 2019;14(1):73.
Splinter K, Niemi AK, Cox R, et al. impaired health-related quality of life in children and families affected by methylmalonic acidemia. J Genet Couns. 2016;25(5):936–44.
Mazariegos G, Shneider B, Burton B, et al. Liver transplantation for pediatric metabolic disease. Mol Genet Metab. 2014;111(4):418–27.
de Baulny HO, Benoist JF, Rigal O, et al. Methylmalonic and propionic acidaemias: management and outcome. J Inherit Metab Dis. 2005;28(3):415–23.
Grünert SC, Müllerleile S, De Silva L, et al. Propionic acidemia: clinical course and outcome in 55 pediatric and adolescent patients. Orphanet J Rare Dis. 2013;10(8):6.
Chapman KA, Gropman A, MacLeod E, et al. Acute management of propionic acidemia. Mol Genet Metab. 2012;105(1):16–25.
Zwickler T, Haege G, Riderer A, et al. Metabolic decompensation in methylmalonic aciduria: which biochemical parameters are discriminative? J Inherit Metab Dis. 2012;35(5):797–806.
Abacan M, Boneh A. Use of carglumic acid in the treatment of hyperammonaemia during metabolic decompensation of patients with propionic acidaemia. Mol Genet Metab. 2013;109(4):397–401.
Nashabat M, Obaid A, Al Mutairi F, et al. Evaluation of long-term effectiveness of the use of carglumic acid in patients with propionic acidemia (PA) or methylmalonic acidemia (MMA): study protocol for a randomized controlled trial. BMC Pediatr. 2019;19(1):195.
Gebhardt B, Dittrich S, Parbel S, et al. N-carbamylglutamate protects patients with decompensated propionic aciduria from hyperammonaemia. J Inherit Metab Dis. 2005;28(2):241–4.
Jurecki E, Ueda K, Frazier D, et al. Nutrition management guideline for propionic acidemia: an evidence- and consensus-based approach. Mol Genet Metab. 2019;126(4):341–54.
Touati G, Valayannopoulos V, Mention K, et al. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis. 2006;29(2–3):288–98.
Camp KM, Lloyd-Puryear MA, Huntington KL. Nutritional treatment for inborn errors of metabolism: indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol Genet Metab. 2012;107(1–2):3–9.
Kölker S, Garcia-Cazorla A, Valayannopoulos V, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis. 2015;38(6):1041–57.
Kölker S, Valayannopoulos V, Burlina AB, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis. 2015;38(6):1059–74.
Sloan JL, Manoli I, Venditti CP. Liver or combined liver-kidney transplantation for patients with isolated methylmalonic acidemia: who and when? J Pediatr. 2015;166(6):1346–50.
Thiboonboon K, Leelahavarong P, Wattanasirichaigoon D, et al. An economic evaluation of neonatal screening for inborn errors of metabolism using tandem mass spectrometry in Thailand. PLoS ONE. 2015;10(8): e0134782.
National Institute for Health and Care Excellence (NICE) health technology evaluations: the manual. Processes and methods [PMG36]. Available at: https://www.nice.org.uk/process/pmg36/chapter/committee-recommendations. Accessed 4 Apr 2024.
Jones-Hughes T, Snowsill T, Haasova M, et al. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. Health Technol Assess. 2016;20(62):1–594.
Rafique M. Propionic acidaemia: demographic characteristics and complications. J Pediatr Endocrinol Metab. 2013;26(5–6):497–501.
Richards J, Gunson B, Johnson J, et al. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18(4):461–6.
Department of Health. Reference Costs 2017-18. 2018. Available at: https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/. Accessed 25 Jan 2024.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by Fortrea Development Limited (previously Covance Inc. and Labcorp Drug Development).
Conflict of Interests
AS, MAW and GJ are employees of Fortrea Development Limited. PEB, MJ and IA were employees of Fortrea Development Limited during the conduct of the study.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data
Data used in this analysis were taken from published or publicly available sources.
Code Availability
Not applicable.
Author Contributions
AS, IA, PEB and MJ conceptualized the model. PEB, IA and MJ developed the initial model. AS, MAW and GJ adapted the model. All authors contributed to results interpretation and writing of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bretos-Azcona, P.E., Wallace, M., Jootun, M. et al. An Early Cost-Utility Model of mRNA-Based Therapies for the Treatment of Methylmalonic and Propionic Acidemia in the United Kingdom. Clin Drug Investig (2024). https://doi.org/10.1007/s40261-024-01363-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s40261-024-01363-1