Abstract
Background
The efficacy of once-weekly (OW) glucagon-like peptide-1 receptor agonists (GLP-1RAs) has been established in several trials in people with type 2 diabetes mellitus (T2DM); however, real-world evidence on their effectiveness is limited. This study evaluated the effectiveness of OW GLP-1RA regarding glycemic and weight outcomes, and relative to DPP-4i in a comparator analysis.
Methods
This observational cohort study evaluated glycated hemoglobin (HbA1c) and weight outcomes in people with T2DM with two or more prescription claims for the same OW GLP-1RA using a pre-post study design (including for a semaglutide OW T2DM subgroup, hereafter referred to as semaglutide). Comparator analysis for the same outcome was performed for OW GLP-1RAs versus DPP-4i and semaglutide subgroup versus DPP-4i. A linked patient population from the IQVIA PharMetrics® Plus database and the Ambulatory Electronic Medical Records (AEMR) database was analyzed using data from January 2017 to April 2022. HbA1c and weight were assessed at baseline and at the end of the 12-month post-index period. Inverse probability of treatment weighting (IPTW) was used to adjust for imbalances in baseline patient characteristics in the comparator analysis.
Results
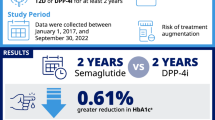
In the pre-post analysis, a greater numerical reduction in HbA1c and weight was observed for the semaglutide subgroup (N = 354) relative to the OW GLP-1RA cohort (N = 921). In the semaglutide subgroup, 52.5% and 34.2% of patients achieved HbA1c of < 7.0% and ≥ 5% weight loss, respectively. For the comparator analysis, the OW GLP-1RAs (N = 651) were significantly more effective (p < 0.001) in reducing HbA1c (− 1.5% vs. − 1.0%) and weight (− 3.2 kg vs. − 1.0 kg) than the DPP-4is (N = 431). Similarly, the semaglutide cohort (N = 251) also displayed more effectiveness (p < 0.001) in reducing HbA1c (− 1.7% vs. − 0.9%) and weight (− 4.1 kg vs. − 1.3 kg) than the respective DPP-4i cohort (N = 417). Patients initiating OW GLP-1RAs, including the semaglutide cohort, were at least twice as likely to achieve HbA1c and weight outcomes as well as composite outcomes compared with those initiating DPP-4is.
Conclusion
The study reinforces that OW GLP-1RAs are more effective in glycemic control and weight reduction compared with DPP-4is in people with T2DM in the real-world setting. These findings align with the recommendation in the current guidelines for utilizing glucose-lowering treatment regimens that support weight-management goals in people with T2DM.
Plain Language Summary
In type 2 diabetes mellitus (T2DM), glucagon-like peptide-1 receptor agonists (GLP-1RAs) are used for managing blood sugar levels and major adverse cardiovascular event risk reduction. In clinical trials, once-weekly (OW) GLP-1RAs showed better control of blood sugar levels and body weight than those administered daily, as well as another class of daily T2DM medications called dipeptidyl peptidase-4 inhibitors (DPP-4is). However, there is limited evidence of OW GLP-1RAs-based routine care to confirm these findings. This study gathered prescription and outcomes data for people with T2DM (January 2017–April 2022) from two linked US databases. Body weight measurements and glycated hemoglobin (HbA1c) test results (measuring average blood sugar levels) were used to evaluate the effectiveness of OW GLP-1RAs (exenatide, dulaglutide, and semaglutide) via a pre-post analysis, and compare OW GLP-1RAs with DPP-4is. We found that treatment with semaglutide lowered body weight and blood sugar levels to a greater extent than OW GLP-1RAs in the pre-post analysis. In the comparator analysis, people receiving OW GLP-1RAs, including semaglutide, were at least twice as likely to achieve reduced HbA1c levels and body weight compared with those receiving DPP-4is. People receiving OW GLP-1RAs were three times more likely than those on DPP-4is to achieve the recommended target of HbA1c < 7.0% and weight loss ≥ 5%, while treatment with semaglutide increased this likelihood by > 4.6 times. This study shows clear benefits of OW GLP-1RAs, building on current evidence for integration of this treatment into overall management of T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

The study adds to the current evidence that OW GLP-1RAs are more effective in glycemic control and weight reduction compared with DPP-4is in people with T2DM in the real-world setting. |
Patients initiating OW GLP-1RAs, including the semaglutide cohort, were at least twice as likely to achieve HbA1c and weight outcomes as well as composite outcomes compared with those initiating DPP-4is. |
The findings from this study provide clinicians and diabetes educators with additional evidence to support the use of OW GLP-1RAs for glycemic control, weight, and overall T2DM management. |
1 Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a drug class that encompass multiple treatments that mimic the glucoregulatory effects of endogenous GLP-1, to reduce glycated hemoglobin (HbA1c) and body weight, as well as benefit people with atherosclerotic cardiovascular disease (ASCVD) [1, 2]. Importantly GLP-1RAs have demonstrated cardiovascular benefits and reduced risk of major adverse cardiovascular events such as non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death [3, 4]. Hence, GLP-1RAs are recommended by clinical guidelines as first-line treatment for people with type 2 diabetes mellitus (T2DM) and established ASCVD or those at high risk for ASCVD, irrespective of the HbA1c level. GLP-1RAs are also recommended over insulin in people with T2DM, when possible [2].
The older-generation, short-acting GLP-1RAs requiring once- or twice-daily subcutaneous administrations were superseded by newer-generation, long-acting GLP-1RAs requiring once-weekly (OW) subcutaneous administration, thus reducing the injection burden and improving adherence [1, 5]. Several OW GLP-1RA agents have been approved by the US Food and Drug Administration (FDA) since 2012: exenatide OW (Bydureon®), dulaglutide OW (Trulicity®), and semaglutide OW T2DM (Ozempic®; hereafter referred to as semaglutide) [6,7,8]. In addition, an oral daily formulation of semaglutide (Rybelsus®) and a novel dual-targeted treatment, tirzepatide (Mounjaro™, glucose-dependent insulinotropic polypeptide/GLP-1RA), have also been developed and approved for the treatment of T2DM [9, 10].
Randomized controlled trials (RCTs) such as DURATION-1 and -5 (exenatide OW compared with exenatide twice daily) and AWARD-1 (dulaglutide OW compared with exenatide twice daily) demonstrated the superior efficacy of OW GLP-1RAs over daily subcutaneous regimens of GLP-1RAs [11,12,13]. The findings were echoed in the retrospective observational STAY study, which showed that people with T2DM receiving OW GLP-1RAs experienced higher reductions in HbA1c at 6 and 12 months compared with those receiving daily subcutaneous GLP-1RAs. OW treatments were also associated with significantly higher persistence and adherence than daily treatments [14]. Furthermore, among all GLP-1RAs (short- and long-acting), semaglutide has consistently exhibited superiority in achieving HbA1c and weight outcomes across various studies [15,16,17]. A network meta-analysis of 26 trials involving patients with inadequate glycemic control with one or two oral antihyperglycemic agents reported greater reductions in HbA1c and weight with semaglutide compared with other GLP-1RAs [17]. Semaglutide also demonstrated superior glycemic control and weight-loss efficacy compared with exenatide OW and dulaglutide OW in the SUSTAIN-3 and -7 clinical trials, respectively [15, 16].
GLP-1RAs have also demonstrated superiority over another class of commonly used incretin-based therapies, dipeptidyl peptidase-4 inhibitors (DPP-4is) [2, 18, 19]. A comparative effectiveness (GRADE) study found that GLP-1RAs (i.e., liraglutide) were significantly more effective in achieving and maintaining target levels of HbA1c compared with DPP-4is (i.e., sitagliptin) as a second-line treatment add-on to metformin for T2DM [20]. Nevertheless, more real-world evidence is warranted to compare newer-generation OW GLP-1RAs, especially semaglutide, with DPP-4is on glycemic control and weight reduction among people with T2DM.
To fill the knowledge gap, this study utilized a claims database linked to an electronic medical records (EMR) database to evaluate the HbA1c and weight outcomes associated with OW GLP-1RA using a pre-post study design. The same outcomes were also assessed to compare OW GLP-1RA with DPP-4i in a comparator analysis. A subgroup analysis on the semaglutide cohort was also conducted.
2 Methods
2.1 Study Design and Data Source
This retrospective, observational, cohort database study evaluated HbA1c and weight outcomes among people with T2DM initiated on OW GLP-1RAs (exenatide, dulaglutide, and semaglutide), using a pre-post study design (Online Supplemental Material (OSM), Fig. S1). Initiators of DPP-4is were identified for the comparator analysis. A linked patient population from the IQVIA PharMetrics® Plus database and the IQVIA Ambulatory EMR (AEMR) database was analyzed using data from January 2017 to April 2022. IQVIA PharMetrics® Plus database includes more than 190 million unique patients, representative of the commercially insured US population under 65 years of age. It provides a longitudinal view of inpatient and outpatient services, data on prescription and office-/outpatient-administered drugs, costs, and detailed enrollment information. The AEMR database comprises electronic medical records for over 82 million patients sourced from over 800 ambulatory practices covering > 100,000 physicians. Key data collected include vitals and lab results. All data used in this study are compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) to protect patient privacy.
2.2 Study Population
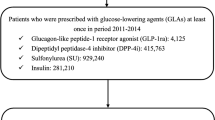
People (aged ≥ 18 years) with T2DM with two or more prescription claims for the same OW GLP-1RA (exenatide, dulaglutide, or semaglutide) were identified from PharMetrics® Plus database between 1 January 2018 and 30 April 2021 (this selection window was chosen because the newest agent, semaglutide, was approved at the end of 2017 in the USA; however, this period prevented inclusion of semaglutide 2 mg in the study). Oral semaglutide or tirzepatide were not able to be included in this study because an adequate sample size or follow-up period to study was not obtained due to the study period. The index date was the date of the first observed claim during the selection window. The second claim for the same OW GLP-1RA had to occur after the index date through the end of the 12-month post-index period. Other inclusion criteria were continuous enrollment during the 12-month pre- and post-index periods, linkage to the AEMR database, and one or more T2DM diagnosis in the 12-month pre-index period or the index date. Patients were also required to have available HbA1c and weight values at baseline and at the end of the 12-month post-index period in the AEMR database, with baseline HbA1c ≥ 7.0%. Patients were excluded if they had a prescription claim for any GLP-1RA (single agent or combination product for the treatment of T2DM or obesity) during the 12-month pre-index period; also excluded were those with diagnosis for type 1 diabetes mellitus, gestational diabetes, or comorbid conditions related to unintentional weight change, or with pharmacy claims for anti-obesity medication during the 12-month pre- and post-index periods. The full eligibility criteria are provided in Fig. 1a. Of the OW GLP-1RA cohort, the subgroup initiating semaglutide was also identified.
Patient attrition in: a pre- and post-index cohorts and comparator cohorts; b DPP-4i comparator cohort. aThe date of the first observed claim was termed the index date. AEMR Ambulatory Electronic Medical Records, DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, ID identity, IPTW inverse probability of treatment weighting; OW once-weekly, T2DM type 2 diabetes mellitus
For the comparator analysis, a cohort initiating DPP-4i was selected with two or more prescription claims for the same DPP-4i during the selection window. Similar eligibility criteria used for the OW GLP-1RA cohort were applied, with further exclusions for prescription claims for any DPP-4i in the 12-month pre-index period or GLP-1RA in the 12-month pre-index period or index date (Fig. 1b). The subsets of the OW GLP-1RA cohort and the semaglutide subgroup without any DPP-4i in the 12-month pre-index period or the index date were utilized for the comparator analysis (Fig. 1a).
2.3 Study Outcomes
HbA1c and weight were assessed at baseline (within 180 days pre-index to 30 days post-index, considering the value closest (absolute) to the index date) and at the end of the 12-month post-index period (360 days post-index ± 90 days, taking the value closest to day 360). Body mass index (BMI) was assessed among those with available baseline and post-index BMI. Changes in HbA1c, weight, and BMI from baseline to the end of the 12-month post-index period were calculated by subtracting the baseline value from the post-index value. Outcomes also included post-index achievement of ≥ 5%, ≥ 10%, or ≥ 15% weight reduction. Composite outcomes considered the aforementioned weight-reduction targets combined with achieving post-index HbA1c < 7.0%.
2.4 Covariates
The Andersen’s behavioral model was used to guide covariate selection [21,22,23]. Predisposing factors included age, gender, and race/ethnicity (as available from AEMR). Enabling factors included payer type, health-plan type, geographic region, and physician specialty associated with the index date. Need factors comprised the Charlson Comorbidity Index, Diabetes Complications Severity Index, relevant comorbidities, blood pressure (as available from AEMR), and index year, as well as other medications (including antidiabetic medications (ADMs) and other relevant medications) measured over the 12-month pre-index period.
2.5 Statistical Methods
Descriptive statistics were applied for all relevant study measures and presented using frequency (number of patients (N)) and percentage (%) of total study patients observed in each category for categorical measures, as well as mean, standard deviation, and median for continuous and count variables. Certain continuous variables were also categorized into intervals per relevance. A p-value of < 0.05 was considered statistically significant for all comparisons described below.
2.5.1 Pre-post Analysis
Within-group comparisons of changes in HbA1c, weight, and BMI for the OW GLP-1RA cohort and semaglutide subgroup were conducted using the paired t-test, Wilcoxon signed-rank test, and McNemar’s test. Outcomes were descriptively compared between the OW GLP-1RA cohort and semaglutide subgroup, and based only on numerical differences. Multivariate logistic regression models were also developed to further evaluate the association between patient characteristics and HbA1c and weight outcomes. Covariates considered for inclusion in the models comprised relevant baseline characteristics, guided by the univariate findings, and following review of collinearity.
2.5.2 Comparator Analysis
Inverse probability of treatment weighting (IPTW) was used to adjust for imbalances in baseline patient characteristics between the comparator OW GLP-1RA and DPP-4i cohorts, as well as comparator semaglutide and DPP-4i cohorts. IPTW was used to estimate the average treatment effect (expected causal effect of the treatment across all individuals in the population) utilizing the entire sample. First, standardized mean difference (SMD) was used to assess imbalances in baseline characteristics for each pairwise comparison and an absolute value of ≥ 0.10 indicated imbalance for a variable. For pairwise comparisons, the propensity score for each individual (the predicted probability of being in the respective cohort) was estimated based on observed covariates and used to calculate each subject’s weight. Baseline characteristics that were imbalanced pre-IPTW and/or considered to be clinically relevant were included in the IPTW logistic regression models used to derive the weights. A binary flag was also included to account for the COVID-19-related time frame (last month of follow-up before March 2020). A stabilized IPTW approach was utilized, thus reducing the type I error by preserving the sample sizes in pseudo-datasets rather than doubling the sample size as in traditional IPTW [24]. Additionally, weight values greater than five were truncated to five, given the potential bias of outliers. SMD was used to assess remaining imbalance in baseline characteristics post-IPTW.
Post-IPTW pairwise-dependent comparisons of HbA1c, weight, and BMI between the comparator OW GLP-1RA and DPP-4i cohorts, and comparator semaglutide and DPP-4i cohorts, were assessed by weighted chi-square tests for categorical variables and weighted t-tests (mean) or Wilcoxon rank-sum test (median) for continuous variables. Multivariate logistic regression models were developed to further evaluate the association between therapy cohort and HbA1c and weight outcomes, while adjusting for the IPTW weight and any remaining confounders (i.e., baseline characteristics that were imbalanced post-IPTW).
3 Results
3.1 Pre-post Analysis
3.1.1 Baseline Demographics and Clinical Characteristics
The study population included a total of 921 patients in the OW GLP-1RA cohort, with 354 patients belonging to the semaglutide subgroup. Figure 1 details the attrition of the study sample. Baseline demographics and clinical characteristics were mostly similar between the cohorts, with a mean age of 54.5 years for the OW GLP-1RA cohort and 53.8 years for the semaglutide subgroup (OSM, Tables S1a, b). At index, 38.4% of patients from the OW GLP-1RA cohort initiated treatment with semaglutide, 54.1% with dulaglutide OW, and 7.5% with exenatide OW. The mean baseline HbA1c values for the OW GLP-1RA cohort and semaglutide subgroup were 8.9% and 8.8%, and the mean weights were 107.1 kg and 108.7 kg, respectively.
3.1.2 Glycated Hemoglobin (HbA1c), Weight, and Body Mass Index (BMI) Outcomes
There was a significant reduction in mean HbA1c in the OW GLP-1RA cohort (− 1.4%, p < 0.001) and semaglutide subgroup (− 1.6%, p < 0.001) (OSM, Table S2). Importantly, a numerically greater proportion of patients achieved HbA1c < 7.0 % post-index in the semaglutide subgroup (52.5%) than in the OW GLP-1RA cohort (41.4%).
Similar outcomes were observed for weight and BMI. The mean change in weight was − 3.3 kg (p < 0.001) in the OW GLP-1RA cohort and − 4.3 kg (p < 0.001) in the semaglutide subgroup (OSM, Table S3a). The proportion of patients experiencing a ≥ 5% decrease in weight post-index was numerically greater in the semaglutide subgroup (34.2%) than in the OW GLP-1RA cohort (27.7%). For patients with available baseline and post-index values, BMI was significantly reduced (p < 0.001) in patients in the OW GLP-1RA and semaglutide subgroup, and the proportion of people with obesity also decreased across all patients (OSM, Table S3b).
3.1.3 Composite Outcomes
The semaglutide subgroup had a numerically greater proportion of patients (24.9%) achieving the composite post-index HbA1c (< 7.0%) and weight outcomes (≥ 5% weight loss) than the OW GLP-1RA cohort (17.2%) (OSM, Fig. S2). A similar trend was observed for composite post-index HbA1c < 7.0% and ≥ 10% weight loss.
3.1.4 Association Between Baseline Characteristics and Study Outcomes
Logistic regression analysis revealed that for the OW GLP-1RA cohort, patients initiating semaglutide had a more than two times (138.6%, p = 0.005) higher chance of achieving post-index HbA1c < 7.0% compared with those initiating exenatide OW after controlling for other covariates (OSM, Table S4a). However, the odds of achieving HbA1c < 7.0% with semaglutide were lower with baseline use of sulfonylureas or insulin and higher baseline HbA1c of ≥ 8.0% (OSM, Table S4b).
3.2 Comparator Analyses
3.2.1 Baseline Demographics and Clinical Characteristics Pre- and Post-Inverse Probability of Treatment Weighting (IPTW)
In the first comparator analysis, the comparator OW GLP-1RA and DPP-4i cohorts comprised 651 and 431 patients, post-IPTW adjustment, respectively (Fig. 1a, b). In the second comparator analysis, the comparator semaglutide and DPP-4i cohorts comprised 251 and 417 patients post-IPTW adjustment, respectively (Fig. 1a, b). Baseline demographics were well balanced for both comparator cohorts post-IPTW (OSM, Tables S5a and S5b). The pre-IPTW imbalances in baseline clinical characteristics were rectified post-IPTW but new imbalances emerged that were controlled in subsequent regression (OSM, Tables S6a and S6b).
3.2.2 HbA1c Outcomes Post-IPTW
The OW GLP-1RA and the semaglutide cohorts achieved significantly lower mean post-index HbA1c than the respective DPP-4i cohorts (7.4% vs. 8.0%, p < 0.001; and 7.2% vs. 7.9%, p < 0.001, respectively; OSM, Table S7). The mean change in HbA1c was significantly more pronounced for both the OW GLP-1RA and semaglutide cohorts versus the respective DPP-4i cohorts (− 1.5% vs. − 1.0%, p < 0.001; and − 1.7% vs. − 0.9%, p < 0.001, respectively; Fig. 2a). Considerably higher proportions of patients achieved HbA1c goals of < 7.0% in the OW GLP-1RA and semaglutide cohorts, relative to the respective DPP-4i cohorts (43.4% vs. 26.7%, p < 0.001; and 53.8% vs. 29.9%, p < 0.001, respectively; Fig. 2b).
a Mean and median change in HbA1c from baseline to post-index in comparator cohorts. b Proportion of patients achieving post-index HbA1c of < 7.0% and < 6.5% in comparator cohorts. DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, OW once-weekly
3.2.3 Weight Outcomes Post-IPTW
For both the OW GLP-1RA and semaglutide cohorts, mean change in post-index weight loss was significantly superior to the respective DPP-4i cohorts (−3.2 kg vs. − 1.0 kg, p < 0.001; and − 4.1 kg vs. − 1.3 kg, p < 0.001, respectively; Fig. 3a). A significantly higher proportion of patients in both OW GLP-1RA and semaglutide cohorts achieved a weight loss of ≥ 5% compared with the respective DPP-4i cohorts (26.7% vs. 14.8%, p < 0.001; and 32.9% vs. 17.1%, p < 0.001, respectively; Fig. 3b). Detailed weighted outcomes are presented in OSM, Table S8.
Both OW GLP-1RA and semaglutide cohorts led to greater reductions in BMI versus the respective DPP-4i cohorts (− 1.1 kg/m2 vs. − 0.4 kg/m2, p < 0.001; and − 1.4 kg/m2 vs. − 0.5 kg/m2, p < 0.001, respectively; Fig. 4). Of those with available baseline and post-index BMI, the proportion of people with obesity (BMI ≥ 30.0 kg/m2) decreased from 78.9% at baseline to 76.0% post-index in the OW GLP-1RA cohort, whereas the DPP-4i cohort had a marginal increase from 75.9 to 77.2% (OSM, Table S9). Further, in the semaglutide cohort, the proportion of people with obesity decreased from 80.8% at baseline to 76.0% post-index, but in the DPP-4i cohort, the proportion of people with obesity remained comparable (77.3% and 77.0%, respectively).
3.2.4 Composite Outcomes Post-IPTW
A significantly higher proportion of patients in the OW GLP-1RA and semaglutide cohorts achieved the composite outcome goals, relative to the respective DPP-4i cohorts in the post-index period (HbA1c < 7.0% and ≥ 5% weight loss: 17.4% (OW GLP-1RA cohort) vs. 6.4% (respective DPP-4i cohort), p < 0.001; and 25.3% (semaglutide cohort) vs. 7.3% (respective DPP-4i cohort), p < 0.001; HbA1c < 7.0% and ≥ 10% weight loss: 7.6% (OW GLP-1RA cohort) vs. 2.8% (respective DPP-4i cohort), p < 0.001; and 10.0% (semaglutide cohort) vs. 3.0% (respective DPP-4i cohort), p < 0.001, respectively (Fig. 5)).
3.2.5 Association Between Treatment Cohorts and Outcomes After Adjusting for the IPTW Weight and Any Remaining Confounders
Weighted logistic regression models showed that patients treated with OW GLP-1RAs or semaglutide were at least two and three times more likely, respectively, to attain target HbA1c < 7.0% compared with DPP-4i. The likelihood of achieving ≥ 5% weight loss was also at least two times higher and nearly three times higher for ≥ 10% weight loss for the respective cohorts versus DPP-4i (Fig. 6a, b). For both OW GLP-1RA and semaglutide cohorts, there was more than three times and two and a half times higher odds, respectively, of meeting the composite outcome of HbA1c < 7.0% with either ≥ 5% weight loss or ≥ 10% weight loss versus the respective DPP-4i cohorts.
a Odds ratios from multivariate logistic regression models for HbA1c and weight outcomes for comparator OW GLP-1RA versus DPP-4i (IPTW). b Odds ratios from multivariate logistic regression models for HbA1c and weight outcomes for comparator semaglutide versus DPP-4i (IPTW). DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, IPTW inverse probability of treatment weighting, OW once-weekly
4 Discussion
This observational study involving a large, commercially insured US population strengthens the evidence that OW GLP-1RAs, including semaglutide, are effective in reducing HbA1c, weight, BMI, and composite outcomes among people with T2DM in the real world. To our knowledge, this is one of the first studies to assess a broad range of diabetes-related, real-world clinical outcomes among US adults with T2DM treated with OW GLP-1RA. A notable strength of this study was that linked patient data from both claims and EMR data were used, which supported the extraction of longitudinal follow-up data with clinical outcomes of interest. Application of stringent inclusion and exclusion criteria led to the selection of the most relevant covariates. Furthermore, the utilization of appropriate study design and statistical methods minimized the potential for confounding errors and selection bias, confirming the robustness of the study.
The pre-post analyses indicated that reduction in HbA1c, weight, and BMI were numerically greater for the semaglutide subgroup relative to the OW GLP-1RA cohort. Over half of the semaglutide subgroup achieved post-index HbA1c < 7.0%, and the proportion of patients experiencing a ≥ 5% decrease in weight loss post-index was also higher compared with the OW GLP-1RA cohort. These findings are consistent with previous RCTs [16]. A review of 14 head-to-head phase 3 trials also found an overall reduction in HbA1c with OW GLP-1RAs of − 1.1% to − 1.9% across the trials similar to the current study [25]. Our observations with semaglutide were mirrored by the SUSTAIN-3 trial, which demonstrated superior HbA1c reduction of semaglutide 1 mg over exenatide OW 2 mg (mean change of − 1.5% vs. − 0.9%) and weight loss (mean change of − 5.6 kg vs. − 1.9 kg). The proportion of patients achieving target HbA1c < 7.0% was also greater with semaglutide treatment compared with exenatide OW [15]. Another RCT, SUSTAIN-7, also validated the improved glycemic control of semaglutide 1 mg over dulaglutide OW 1.5 mg (HbA1c mean reduction: − 1.8% vs. − 1.4%) and weight loss (− 6.5 kg vs. − 3.0 kg) [16].
For the comparator analysis, OW GLP-1RAs, including semaglutide, were more effective in reducing HbA1c, weight, and BMI than DPP-4i. Patients initiating OW GLP-1RAs, including semaglutide, were twice as likely to achieve all clinical outcomes of interest (HbA1c and/or weight-loss goals) compared with those initiating DPP-4i. These outcomes from the comparator analysis are aligned with previous RCTs and real-world studies [19, 26,27,28,29]. The DURATION-2 trial demonstrated greater mean HbA1c reduction (− 1.5% vs. − 0.9%) and weight loss (− 2.3 kg vs. − 1.5 kg) in patients treated with exenatide OW compared with sitagliptin (a DPP-4i). Furthermore, a greater proportion of patients displayed weight loss ≥ 5% relative to sitagliptin (28% vs. 10%) [30]. Likewise, the AWARD-5 trial also demonstrated the superiority of dulaglutide OW over sitagliptin in terms of glycemic control and weight loss, wherein the reduction values were similar to those in our study. The mean change in body weight was also greater for 1.5 mg dulaglutide OW (− 3.0 kg) and 0.75 mg dulaglutide OW (− 2.6 kg) compared with sitagliptin (− 1.5 kg) [31]. The SUSTAIN-2 trial, which compared the efficacy of semaglutide OW (1.0 mg) T2DM with sitagliptin as add-on treatment in people with T2DM, demonstrated significant reduction in mean HbA1c (1.6% vs. 0.5%, p < 0.001) and body weight (6.1 kg vs. 1.9 kg, p < 0.001) [32]. Although our study outcomes are aligned with published evidence, differences in the absolute values can be attributed to the differences in study design, study population, and real-world prescribing practices and patient behaviors (e.g., adherence/persistence, optimizing dose, etc.). The results observed in this study were interpreted in the context that patients were required to use at least two prescriptions of index drug but were not required to be either persistent/adherent to the therapies or escalated to the maintenance dose, indicating that the effects are regardless of doses. However, we still see this extent of improvement in the outcomes in a relatively short time period.
The clear benefits demonstrated in this study by the OW GLP-1RA therapy class, including semaglutide, provide clinicians and diabetes educators with additional evidence associating the use of OW GLP-1RAs for glycemic control, weight reduction, and overall T2DM management in people with T2D. Our study findings may also suggest the benefits of preferential use of these agents earlier in the treatment pathway. There is evidently scope for change in clinical practice to improve patient care and health outcomes by improving adherence through increased adoption of OW regimens of the GLP-1RA drug class. Current guidelines recommend a holistic, multifactorial, individualized treatment approach that emphasizes achieving glycemic and weight goals, together with reducing the risk of cardiovascular comorbidities [2, 33]. In line with the recommendations, making weight loss a primary treatment goal along with glycemic control among people with T2DM should be considered, given the favorable outcomes associated with weight management [34]. The evidence from this study as well as previous studies [19, 31, 35] may further support the use of GLP-1RAs over DPP-4is in glycemic control, weight loss, and cardiovascular risk reduction. The potential to switch between GLP-1RAs in clinical practice has also been supported by evidence from a retrospective study that demonstrated significant long-term improvements in glycemic control and weight when people with T2DM switched from other GLP-1RAs to semaglutide [36]. Furthermore, more evidence regarding the effects of OW GLP-1RAs in combination with other ADMs is warranted in future research.
Despite evidence from clinical trials and real-world studies validating the benefits of OW GLP-1RAs, the adoption of this drug class for T2DM treatment has accelerated in recent years but is still suboptimal, especially among the population for whom it is potentially most beneficial (e.g., those with cardiovascular diseases or obesity) [37,38,39]. The accumulation of evidence on the benefits of OW GLP-1RAs may not necessarily lead to the optimal use of these drugs in guideline-recommended populations as it is a multifaceted issue extending beyond this. Contributing factors may include clinical barriers (e.g., lack of practical knowledge on the use of drug, fear of adverse effects or injections), patient access barriers (e.g., affordability), and other factors (e.g., inadequate awareness or lower confidence in use among healthcare professionals outside endocrinologists) [40,41,42]. Addressing these barriers may require collaborative efforts from various stakeholders to implement targeted interventions or policies and ultimately improve patient outcomes.
It should be noted that the study had certain limitations. The results from this type of observational cohort study need to be interpreted with caution and can only establish associations, not cause-and-effect relationships. In addition, the overall study sample was identified from the PharMetrics® Plus database, which is limited to patients with commercially managed insurance; thus, the findings may not be generalizable to uninsured, Medicare, or Medicaid populations. Finally, administrative claims data do not provide as much clinical detail as medical records because they are primarily collected for the purposes of payment. Therefore, there is a potential for miscoding or misclassification. Since AEMR data were used to assess the primary outcomes including changes in HbA1c and weight, our sample may be biased toward capturing patients who undergo routine check-ups. Finally, this study followed patients for only 12 months, which cannot establish long-term effectiveness. Therefore, future research is warranted to assess these outcomes over the long term and among other populations.
5 Conclusion
The study adds to the current evidence that OW GLP-1RAs are more effective in glycemic control and weight reduction compared with DPP-4is in people with T2DM in the real-world setting. These findings align with the recommendation in the current guidelines for utilizing glucose-lowering treatment regimens that support weight-management goals in people with T2DM.
References
Brunton SA, Wysham CH. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad Med. 2020;132(sup2):3–14.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140-S57.
Grewal S, Zaman N, Borgatta L, Nudy M, Foy AJ, Peterson B. Usefulness of glucagon-like peptide-1 receptor agonists to reduce adverse cardiovascular disease events in patients with type 2 diabetes mellitus. Am J Cardiol. 2021;154:48–53.
Hupfeld C, Mudaliar S. Navigating the “MACE” in cardiovascular outcomes trials and decoding the relevance of atherosclerotic cardiovascular disease benefits versus heart failure benefits. Diabetes Obes Metab. 2019;21(8):1780–9.
Gallwitz B, Giorgino F. Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Front Endocrinol (Lausanne). 2021;12: 645507.
Eli Lilly and Company. FDA Approves Trulicity™ (dulaglutide), Lilly's Once-Weekly Therapy for Adults with Type 2 Diabetes. https://investor.lilly.com/news-releases/news-release-details/fda-approves-trulicitytm-dulaglutide-lillys-once-weekly-therapy. Accessed 8 Dec 2023.
Novo Nordisk. Novo Nordisk Company Announcement: Ozempic® (semaglutide) approved in the US. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=712. Accessed 8 Dec 2023.
U.S. Food and Drug Administration. Approval Letter, BYDUREON Extended-Release for Injectable Suspension. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022200Orig1s000Approv.pdf. Accessed 8 Dec 2023.
Eli Lilly and Company. News Release-FDA approves Lilly's Mounjaro™ (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for the treatment of adults with type 2 diabetes. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-mounjarotm-tirzepatide-injection-first-and. Accessed 8 Dec 2023.
Novo Nordisk. Company announcement-Rybelsus® (semaglutide tablets), the first GLP-1 in a tablet approved in the US. https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=356. Accessed 8 Dec 2023.
Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–10.
Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33(6):1255–61.
Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37(8):2159–67.
Polonsky WH, Arora R, Faurby M, Fernandes J, Liebl A. Higher rates of persistence and adherence in patients with type 2 diabetes initiating once-weekly vs daily injectable glucagon-like peptide-1 receptor agonists in US Clinical Practice (STAY Study). Diabetes Ther. 2022;13(1):175–87.
Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–66.
Pratley RE, Aroda VR, Lingvay I, Ludemann J, Andreassen C, Navarria A, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–86.
Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving 1–2 oral anti-diabetic drugs. Diabetes Ther. 2018;9(3):1149–67.
Berg JK, Shenouda SK, Heilmann CR, Gray AL, Holcombe JH. Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study. Diabetes Obes Metab. 2011;13(11):982–9.
Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol (Lausanne). 2020;11:178.
Nathan DM, Lachin JM, Balasubramanyam A, Burch HB, Buse JB, Grade Study Research Group, et al. Glycemia reduction in type 2 diabetes—glycemic outcomes. N Engl J Med. 2022;387(12):1063–74.
Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208–20.
Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10.
Andersen R, Newman JF. Societal and Individual Determinants of Medical Care Utilization in the United States. Milbank Q. 2005;83(4). https://doi.org/10.1111/j.1468-0009.2005.00428.x.
Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273–7.
Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320.
Xu L, Yu SQ, Gao L, Huang Y, Wu SS, Yang J, et al. Effects of incretin-based therapies on weight-related indicators among patients with type 2 diabetes: a network meta-analysis. Biomed Environ Sci. 2020;33(1):37–47.
Aroda VR, Ahmann A, Cariou B, Chow F, Davies MJ, Jodar E, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1–7 trials. Diabetes Metab. 2019;45(5):409–18.
Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32(8):776–90.
Minze MG, Klein MS, Jernigan MJ, Wise SL, Fruge K. Once-weekly exenatide: an extended-duration glucagon-like peptide agonist for the treatment of type 2 diabetes mellitus. Pharmacotherapy. 2013;33(6):627–38.
Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–9.
Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58.
Ahren B, Masmiquel L, Kumar H, Sargin M, Karsbol JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–54.
Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394–405.
Campos C, Unger J. Primary care management of type 2 diabetes: a comparison of the efficacy and safety of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Postgrad Med. 2021;133(8):843–53.
Lingvay I, Kirk AR, Lophaven S, Wolden ML, Shubrook JH. Outcomes in GLP-1 RA-experienced patients switching to once-weekly semaglutide in a real-world setting: the retrospective Observational EXPERT Study. Diabetes Ther. 2021;12(3):879–96.
Eberly LA, Yang L, Essien UR, Eneanya ND, Julien HM, Luo J, et al. Racial, ethnic, and socioeconomic inequities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2(12): e214182.
Bae JP, Kadziola ZA, Liu D, Chinthammit C, Boye KS, Mather KJ. An early assessment of the real-world treatment patterns of type 2 diabetes: a comparison to the 2018 ADA/EASD Consensus Report Recommendations. Diabetes Ther. 2022;13(8):1499–510.
Nair R, Mody R, Yu M, Cowburn S, Konig M, Prewitt T. Real-world treatment patterns of glucose-lowering agents among patients with type 2 diabetes mellitus and cardiovascular disease or at risk for cardiovascular disease: an observational, cross-sectional retrospective study. Diabetes Ther. 2022;13(11–12):1921–32.
Vaduganathan M, Patel RB, Singh A, McCarthy CP, Qamar A, Januzzi JL Jr, et al. Prescription of glucagon-like peptide-1 receptor agonists by cardiologists. J Am Coll Cardiol. 2019;73(12):1596–8.
Arnold SV, de Lemos JA, Zheng L, Rosenson RS, Ballantyne CM, Alam S, et al. Use of optimal medical therapy in patients with diabetes and atherosclerotic cardiovascular disease: Insights from a prospective longitudinal cohort study. Diabetes Obes Metab. 2023;25(6):1750–7.
Romera I, Rubio-de Santos M, Artola S, Suarez Fernandez C, Conget I. GLP-1 RAs in Spain: a short narrative review of their use in real clinical practice. Adv Ther. 2023;40(4):1418–29.
Acknowledgements
The authors would like to thank Kasturi Chatterjee, PhD, Adivitiya Bihagara, PhD, Jay Patel, PharmD, and Daria Renshaw, BA, of IQVIA for medical writing and editorial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Novo Nordisk Inc.
Competing Interests
XT, JA, LX, CLG, MG, YP are employed by Novo Nordisk Inc. LX has been employed by Pfizer within the last 3 years. VD and KBC are employees of IQVIA, which received funding from Novo Nordisk Inc. for this study. AK is an employee of Baptist Medical Network MedFirst Primary Care – Quarry, and served as consultant/speaker for Abbott, Astellas, Dexcom, Eli Lilly, Mannkind, and Novo Nordisk Inc.
Data Availability
The original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (see https://www.iqvia.com/contact/sf).
Ethics Approval
Researchers accessed data in compliance with the Health Insurance Portability and Accountability Act (HIPAA). This study was considered exempt from the requirements for research in human subjects in the US because it was a retrospective analysis of commercially available, de-identified secondary data.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
All authors were involved in the concept and design of the study, data interpretation, as well as critically reviewing, editing, and approving the manuscript. VD and KBC were also involved in data analyses.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tan, X., Divino, V., Amamoo, J. et al. Real-World Effectiveness of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists (OW GLP-1RAs) in Comparison with Dipeptidyl Peptidase-4 Inhibitors (DPP-4is) for Glycemic Control and Weight Outcomes in Type 2 Diabetes Mellitus (RELATE). Clin Drug Investig 44, 271–284 (2024). https://doi.org/10.1007/s40261-024-01354-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01354-2