Abstract
Background and Objective
Long-acting injectable antipsychotics have shown benefits over oral medications with reduced hospitalization rates and improved health-related quality of life. RBP-7000 (PERSERIS®) is a monthly risperidone formulation (90 or 120 mg) for the treatment of schizophrenia administered by subcutaneous abdominal injection. The objective of this study was to assess a higher dose of 180 mg RBP-7000 and an alternate injection site.
Methods
Following stabilization on 6 mg/day (3 mg twice daily) oral risperidone, clinically stable schizophrenic participants received 3 monthly doses of 180 mg RBP-7000 in the abdomen followed by a fourth monthly dose of 180 mg RBP-7000 in the upper arm (each dose administered as two 90-mg injections). The primary endpoint was the steady-state average plasma concentration (Cavg(ss)) of risperidone and total active moiety after oral and RBP-7000 administration. Secondary endpoints included measures of clinical efficacy (Positive and Negative Syndrome Scale, Clinical Global Impression Scale for Severity of Illness), safety, and local injection-site tolerability to assess the switch from oral risperidone and compare injection sites.
Results
In all, 23 participants received at least one dose of RBP-7000, 16 received all four doses, and 15 completed the study. Monthly doses of 180 mg RBP-7000 provided similar Cavg(ss) of total active moiety compared with 6 mg/day oral risperidone. The pharmacokinetics of RBP-7000 were similar after injection in the abdomen versus upper arm. Clinical efficacy measures remained stable throughout the study. All RBP-7000 injections were well tolerated with no unexpected safety findings.
Conclusions
The results support the use of 180 mg RBP-7000 in schizophrenic patients stable on 6 mg/day oral risperidone and a second injection site in the upper arm.
Trial Registration
ClinicalTrials.gov identifier: NCT03978832.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study assessed a higher dose (180 mg) and an alternate injection site for RBP-7000 monthly risperidone injection in patients with schizophrenia stabilized on 6 mg/day oral risperidone. Plasma exposure data in combination with clinical efficacy measures support the conclusion that RBP-7000 180-mg dose approximates 6 mg/day oral risperidone. Administration of RBP-7000 at the back of the upper arm showed comparable exposure to administration in the abdomen with no clinically meaningful differences in safety or efficacy measures. |
1 Introduction
Schizophrenia is a severe, chronic, and disabling mental disorder affecting an estimated 21 million people worldwide, with a global age-standardized point prevalence of 0.28% [1, 2]. For most individuals with schizophrenia, maintenance treatment with antipsychotic drugs is necessary to reduce the risk of relapse or recurrence of an acute exacerbation [3]. Risperidone is an atypical antipsychotic medication with potent antagonist effects at the dopamine type-2 (D2) and serotonin type 2A (5-HT2A) receptors [4, 5]. The clinical effect of risperidone results from the combined action of risperidone and its major metabolite, 9-hydroxyrisperidone, and the sum of risperidone and 9-hydroxyrisperidone constitutes the total active moiety [4]. Although risperidone and other antipsychotics were initially developed as oral medications, high rates of nonadherence led to the development of long-acting injectables (LAIs) [6]. Studies have shown that patients initiated on LAI antipsychotics experience significantly reduced hospitalization rates [7,8,9] and improved health-related quality of life [10, 11].
RBP-7000 (PERSERIS®) is an extended-release subcutaneous (SC) injectable suspension of risperidone (90 and 120 mg) approved for the treatment of schizophrenia in adults in the USA and Canada. RBP-7000 is administered once monthly and provides therapeutic plasma concentrations from the first injection without the need for supplemental oral risperidone or loading dose [12]. RBP-7000 uses the well-established ATRIGEL® delivery system, which consists of a biodegradable poly (DL-lactide-co-glycolide) polymer and a biocompatible solvent, N-methyl-2-pyrrolidone. When injected, the delivery system solidifies upon contact with body fluids and provides a sustained release of risperidone over the monthly dosing interval. Clinical efficacy, safety, and tolerability of RBP-7000 injections for the treatment of acute schizophrenia have been demonstrated in Phase 3 trials consisting of an 8-week, double-blind, placebo-controlled, randomized study [13,14,15] and a 52-week, open-label, long-term safety study [16]. In both studies, participants reported high satisfaction with and preference for RBP-7000 as compared with their most recent pre-study antipsychotic medication [17, 18]. Also, participants’ overall well-being and health-related quality of life were significantly greater after treatment with RBP-7000 compared with placebo [17] and were stable over time with long-term treatment [18].
Based on the average plasma concentration (Cavg) of total active moiety, it was shown that 90 mg RBP-7000 corresponds to 3 mg/day oral risperidone and 120 mg RBP-7000 corresponds to 4 mg/day oral risperidone [12]. The purpose of the present study was to assess the pharmacokinetics, safety, tolerability, and efficacy of monthly injections of a higher dose of 180 mg RBP-7000 that would provide total active moiety exposure (Cavg) equivalent to 6 mg/day of oral risperidone. Another objective of the study was to assess an alternate injection site (the back of the upper arm). RBP-7000 is currently approved for SC injection in the abdominal region, and another injection site would provide additional flexibility for treatment administration given the chronic nature of schizophrenia and different patient preferences. Although the extent of absorption does not generally differ among SC injection sites, the rate of absorption may vary [19, 20], and the pharmacokinetics of RBP-7000 were evaluated following SC injection in the upper arm compared with the abdomen.
2 Methods
2.1 Study Design
This open-label Phase 4 study was conducted between June 2019 and May 2020. We recruited patients with clinically stable schizophrenia [assessed using Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria] who were between 18 and 65 years old and were on a stable dose of 5 or 6 mg/day of oral risperidone. Any daily or twice-daily dosing combination (i.e., 3/3, 4/2, 3/2) was acceptable. Since risperidone is mainly metabolized by cytochrome P450 (CYP) 2D6, which is subject to genetic polymorphism, only extensive CYP2D6 metabolizers were allowed to participate in the study based on CYP2D6 genotyping results from blood sampling at screening. Details on study inclusion/exclusion criteria are provided in Online Resource 1.
It was planned for 25 subjects to receive study drug treatment. The sample size was based on earlier clinical experience and pharmacokinetic knowledge and was expected to provide an adequate number of subjects (at least 15 evaluable) to assess the pharmacokinetic parameters of RBP-7000 as well as oral risperidone. All participants provided written informed consent before screening procedures. The study was approved by the local ethics committee and was registered at ClinicalTrials.gov (identifier: NCT03978832).
Eligible participants who met the screening criteria were first stabilized on 6 mg/day of oral risperidone given as 3 mg twice daily (BID)] 12 h apart for 5 days (Day −5 to− Day −1). Those who completed the stabilization period received up to 4 doses of 180 mg RBP-7000 every 28 days (on Days 1, 29, 57, and 85); each 180 mg dose was administered as 2, 90-mg SC injections. The first 3 doses were administered in the abdominal region (the 2 injections of 90 mg were administered in different quadrants of the abdomen, and injection sites were rotated between doses to minimize irritation). The fourth dose of 180 mg was administered in the back of the upper arm (participants received 1 injection of 90 mg in each arm).
The safety data from five participants following the first dose of 180 mg RBP-7000 were reviewed prior to continuation of the remaining study. At the end-of-study (EOS) visit, participants were evaluated and prescribed an appropriate maintenance antipsychotic at a dosage determined by the physician. The study design is depicted in Online Resource 2.
2.2 Assessments
Blood samples for the determination of risperidone and 9-hydroxyrisperidone plasma concentrations were collected during oral risperidone stabilization period (Day −5–Day −2: prior to the morning dose; Day −1: prior to and at 0.5, 1, 2, 4, 6, and 12 h after the morning dose) and after each SC injection of RBP-7000 [Doses 1 and 2: pre-dose and 2, 4, 6, 24, 48, 168, 240, 336, 408, 504, and 576 h post-dose; Doses 3 and 4: same schedule with additional samples at 12, 120, 192, 216, 288, and 672 h (Dose 4 only)]. The additional blood samples taken after Doses 3 and 4 of RBP-7000 were collected for a more accurate calculation of RBP-7000 pharmacokinetic parameters at steady-state. Approximately 6 mL of blood was collected at each time point into pre-labelled vacutainer tubes containing ethylenediaminetetraacetic acid (K2-EDTA). Blood sampling was performed by appropriately qualified and trained study personnel, either by individual venipuncture or through an inserted indwelling catheter with a saline lock in the subject’s forearm to minimize subject’s risk and discomfort.
Adverse events (AEs) were monitored and reported by the investigator or designee throughout the study until the follow-up telephone call on Day 120. All clinically significant symptoms were reported as AEs, and all AEs and corresponding treatment were recorded in the database. The World Health Organization’s Uppsala Monitoring Centre (WHO-UMC) causality assessment was used to define the relationship of an AE to the administration of RBP-7000. Clinical assessments for safety included the Abnormal Involuntary Movement Scale (AIMS) for tardive dyskinesia [21], the Simpson-Angus Scale (SAS) [22], the Barnes Akathisia Rating Scale (BARS) [23], and the Columbia-Suicide Severity Rating Scale (C-SSRS) [24]. AIMS, SAS, and BARS evaluations were conducted at screening, on Day −1, and on Days 2, 8, 15, 22, 29, 50, 57, 85, and 113. C-SSRS assessments were performed at screening, on Day −1, and on Days 8, 15, 36, 57, 64, 78, 94, and 113. Additional safety and tolerability evaluations included injection site pain [using a patient-reported 100 mm Visual Analog Scale (VAS)] and injection site tolerability gradings measured after each injection, as well as vital signs, changes in clinical laboratory results, physical examinations, and 12-lead electrocardiograms (ECG).
Efficacy evaluations were conducted at screening, on Day −1, and on Days 2, 8, 15, 22, 29, 50, 57, 85, and 113 following administration of RBP-7000. Efficacy was assessed using the Positive and Negative Syndrome Scale (PANSS) [25] and the Clinical Global Impression Scale for Severity of Illness (CGI-S) [21].
2.3 Bioanalytical Assay
Plasma concentrations of risperidone and 9-hydroxyrisperidone were determined using a validated method of liquid chromatography with tandem mass spectrometry (LC–MS/MS). The analytical methodology was based on the following procedure: 0.05 mL of human plasma containing internal standards (d4-risperidone, d4-9-hydroxyrisperidone) was first acidified and then extracted using solid-phase extraction. Analysis required evaporation of elute solvent before being reconstituted. An aliquot of the extract was injected onto a Sciex API 5500 LC–MS/MS equipped with a high-performance liquid chromatography column. Quantitation was performed using 1/x2 weighted linear least squares regression analysis generated from fortified plasma calibration standards prepared immediately prior to each run. The method was validated for specificity, linearity, lower limit of quantitation, precision, accuracy, recovery, and stability for a range of 0.1–100 ng/mL for both analytes. The overall precision for risperidone and 9-hydroxyrisperidone was greater than 11.7%; the overall accuracy was within ± 4.4%. The recoveries of both analytes and internal standards were greater than 91%. The established short-term and long-term stability covered the maximum sample storage time.
The total active moiety plasma concentration was calculated as the sum of risperidone and 9-hydroxyrisperidone plasma concentrations after correction for their molecular weights (410 for risperidone and 426 for 9-hydroxyrisperidone) according to the following formula: [active moiety] = [risperidone] + [9‐hydroxyrisperidone] × (410/426).
2.4 Pharmacokinetics and Statistical Analyses
Non-compartmental pharmacokinetic analyses were performed on the pharmacokinetic population, defined as participants who received oral risperidone or at least 1 dose of RBP-7000 and who provided an adequate number of blood samples for determination of pharmacokinetic parameters (N = 24). The primary endpoint for the study was the steady-state average plasma concentration (Cavg(ss)) of risperidone and total active moiety measured after administration of oral risperidone (Day −1) and RBP-7000 (Dose 3). Secondary pharmacokinetic parameters included maximum plasma concentration at steady-state (Cmax(ss)), minimum plasma concentration at steady-state (Cmin(ss)), and percent fluctuation calculated as (Cmax(ss) – Cmin(ss))/Cavg(ss) × 100. Steady-state attainment after oral and SC dosing was evaluated using descriptive summary statistics of plasma concentrations. For the primary analysis (comparison of RBP-7000 Dose 3 versus oral risperidone), only the data from those participants who received all 3 doses of RBP-7000 and provided an adequate number of blood samples for the determination of Cavg(ss) were considered (N = 16). Mean plasma concentration-time profiles and pharmacokinetic parameters determined after the fourth RBP-7000 dose (alternate site, back of upper arm) were compared with those obtained after the third RBP-7000 dose (abdominal site) for risperidone, 9-hydroxyrisperidone, and total active moiety. Pharmacokinetic parameter comparisons were done using geometric mean ratios (GMRs) with 90% confidence intervals (CIs) derived from random effects models, with the logarithm of the pharmacokinetic parameter value as the dependent variable, dose number as the independent variable, and subject as a random effect.
Additional secondary endpoints were safety and tolerability of the RBP-7000 injections, which were ascertained in participants who had received at least one dose of RBP-7000 (safety population; N = 23). Secondary endpoints for efficacy were the change from baseline in PANSS subscale scores and CGI-S score. These endpoints were summarized for the efficacy population (N = 23) defined as participants who received at least 1 dose of RBP-7000 and had at least one post-dose efficacy observation.
All statistical analyses were performed using SAS® software version 9.4 (SAS Institute, Inc., Cary, NC, USA). WinNonlin Phoenix version 6.3 (Pharsight Corporation) was used for pharmacokinetic parameter calculation.
3 Results
3.1 Study Participants
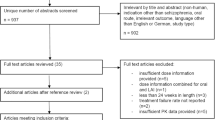
The disposition of study participants is shown in Fig. 1. In all, 25 of the 69 screened participants received oral risperidone during the stabilization period, 23 received at least 1 dose of RBP-7000, 16 received all 4 doses of RBP-7000, and 15 completed the study. One participant received all 4 SC doses but was lost to follow-up prior to EOS procedures.
Demographic and baseline characteristics for the safety and pharmacokinetic populations were similar based on descriptive statistics (Table 1). The majority of participants were male, and age ranged from 26 to 65 years. Nearly three-quarters were Black or African American, with whites comprising the next highest race (approximately 21%); 13% were of Hispanic or Latino ethnicity. Body mass index (BMI) ranged from 19.6 to 34.8 kg/m2.
3.2 Pharmacokinetics
The mean pharmacokinetic profile for the total active moiety throughout the study is presented in Fig. 2A. Data show that steady-state for RBP-7000 was achieved by the end of the second dosing interval, while plasma concentrations close to steady-state were attained after the first dose of RBP-7000. Steady-state pharmacokinetic parameters of risperidone, 9-hydroxyrisperidone, and total active moiety are summarized in Table 2 for oral risperidone (Day −1) and RBP-7000 for Dose 3 (abdominal site) and Dose 4 (upper arm). Table 2 also presents GMRs with their 90% CIs for comparison of pharmacokinetic parameters between oral risperidone and RBP-7000 on one hand and between the two injection sites on the other hand.
A Total active moiety plasma concentrations (mean + SD) throughout the study (from Day −5 to Day 113) in semi-logarithmic scale; B Change from baseline (mean ± SD) in Positive and Negative Symptom Scale (PANSS) total score; and C Change from baseline (mean ± SD) in Clinical Global Impression Scale for Severity of Illness (CGI-S) score. EOS, end of study
Results show that similar Cavg(ss) of the total active moiety was achieved after 3 monthly doses of 180 mg RBP-7000 compared with 6 mg/day of oral risperidone (3 mg BID), with geometric means of 44.05 ng/mL and 43.73 ng/mL, respectively (GMR [90% CI]: 1.01 [0.91, 1.12]). For risperidone, Cavg(ss) was higher following the third dose of RBP-7000 compared with oral risperidone (GMR [90% CI]: 1.98 [1.54, 2.55]). This was largely due to two participants whose risperidone Cavg(ss) values were higher but their corresponding 9-hydroxyrisperidone levels were lower, resulting in similar Cavg(ss) values for the total active moiety.
On the basis of geometric means, risperidone and total active moiety Cmax(ss) appeared slightly higher after RBP-7000 administration compared with oral risperidone, although the 90% CI for GMR included 1. Risperidone Cmin(ss) was higher after RBP-7000 administration (GMR [90% CI]: 4.02 [3.03, 5.33]), while total active moiety Cmin(ss) was higher after oral administration (GMR [90% CI]: 0.70 [0.62, 0.80]). The percent fluctuation for RBP-7000 was lower than for risperidone (135% versus 252% for oral dosing), but slightly higher for total active moiety (106% versus 68% for oral dosing) based on geometric mean data.
Administration of RBP-7000 at an alternate injection site (back of upper arm) provided comparable pharmacokinetic profile (Fig. 3) and similar plasma exposure (Cavg(ss)) of total active moiety compared with injection in the abdominal region (Table 2). Geometric means for Cavg(ss) were 43.73 ng/mL following injection in the upper arm compared with 44.05 ng/mL following injection in the abdomen (GMR [90% CI]: 0.99 [0.92, 1.07]). Other pharmacokinetic parameters were similar between the two injection sites with GMRs close to 1 (1.09 for Cmax(ss) and 1.00 for Cmin(ss)).
3.3 Safety and Tolerability
Overall, 69.6% (16 of 23) of participants had 1 or more treatment-emergent adverse event (TEAE) during the study (Online Resource 3) and 26.1% (6 of 23) of participants had TEAEs assessed by the investigator as related to study drug (Online Resource 4). The most common TEAEs were upper respiratory tract infection [number of events (n) = 3], blood prolactin increased (n = 2), decreased appetite (n = 2), dyskinesia (n = 2), fall (n = 2), and headache (n = 2). The most frequently reported system organ class included nervous system disorders (five participants, 21.7%); four participants had five TEAEs associated with extrapyramidal symptoms [akathisia (n = 1), dyskinesia (n = 2), Parkinsonian gait (n = 1), tremor (n = 1)]. Most TEAEs during the study were of mild severity (25 events in 11 participants, 47.8%) or moderate severity (5 events in 4 participants, 17.4%). There was one participant with a severe TEAE (tooth extraction) and one study treatment discontinuation due to a TEAE (Parkinson’s gait). There were no participants with serious adverse events (SAEs) reported during the study; however, there was one SAE that occurred after study completion (Day 133). This SAE was an increase in liver enzymes, which was considered moderate in severity and unrelated to study treatment. There were no deaths in the study.
In general, there were no clinically meaningful differences in the pattern of injection site gradings following the first dose compared with subsequent doses of RBP-7000. Injection site pain, tenderness, erythema/redness, and induration/swelling grades were graded as none in the vast majority of cases. In the few cases that reported a reaction, these cases were graded as mild in severity. Injection site burning/stinging decreased within 1 h after each dose in most participants. Participant reports of injection site pain on a VAS were generally on the lower end of the scale, with no clinically meaningful differences between doses (Online Resource 5). After each dose, injection site pain scores decreased within 1 h in most participants. Overall, injection-site tolerability assessments revealed no clinically meaningful differences between the two injection sites, and all injections were considered well tolerated.
Analyses of vital signs, AIMS, SAS, BARS, C-SSRS, and ECG assessments and clinical laboratory evaluations did not reveal any clinically relevant effect of RBP-7000 treatment.
3.4 Efficacy
Mean (± SD) changes from baseline for PANSS total score at each data collection point are shown in Fig. 2B. Overall, the PANSS mean and individual total scores remained stable throughout the study, as did all PANSS subscale (positive, negative, and general psychopathology) mean scores. Figure 2C displays the mean (± SD) change from baseline for the CGI-S observed scores at each timepoint. As seen with the PANSS data, CGI-S scores remained stable throughout the study; mean observed values were identical at baseline (3.3 ± 0.45) and EOS (3.3 ± 0.73). There were no clinically meaningful differences in mean scores by dose or between the two injection sites (Dose 3 and Dose 4). Tables summarizing observed scores and changes from baseline by visit are provided in Online Resource 6 for PANSS total score and Online Resource 7 for CGI-S.
4 Discussion
As expected from the established correspondence between 90 mg RBP-7000 and 3 mg oral risperidone and between 120 mg RBP-7000 and 4 mg oral risperidone, 180 mg RBP-7000 administered as 2 SC injections of 90 mg provided similar exposure of the total active moiety compared with 6 mg/day oral risperidone. The achievement of steady-state plasma concentrations by the end of the second dosing interval for RBP-7000 and the near attainment of steady-state levels after the first dose are consistent with previous knowledge [12, 26]. Slightly higher fluctuations in total active moiety plasma concentration were noted for RBP-7000 compared with oral risperidone, which was given twice daily (3 mg BID). These higher fluctuations did not translate into changes in clinical efficacy measures, and the consistency in plasma exposure over the 113 days of the study was supported by the stability of PANSS scores (total and for each subscale) and CGI-S scores, both individually and on average, over that time period.
The clinical relevance for a seamless switch from oral antipsychotics to LAI antipsychotics is important for several reasons. In a meta-analysis of 42 studies and over 100,000 participants diagnosed with schizophrenia, Kishimoto and colleagues found LAIs were superior to oral antipsychotics in reducing hospitalization rates and treatment discontinuation, even though patients on LAIs had greater illness severity than those prescribed oral antipsychotics [27]. The clinical and economic benefits of LAIs were confirmed in a recent meta-analysis of 25 studies providing real-world evidence for a reduction in hospitalizations and emergency room admissions as well as improved medication adherence compared with oral antipsychotics [9]. Also, previous studies have shown that switching from oral risperidone to LAI risperidone improves health-related quality of life (HRQoL) in patients with schizophrenia [10, 11]. In the 52-week multicenter Phase 3 trial of 120 mg RBP-7000, over two-thirds of patients reported high satisfaction with RBP-7000 throughout the study, with HRQoL scores close to the general population [18].
The present study tested a higher dose of 180 mg RBP-7000 to provide an additional option for patients who may be at risk of relapse. A previous meta-analysis of 26 randomized clinical trials (evaluating fixed doses of atypical antipsychotics, haloperidol, or fluphenazine) explored dose–response relationships for relapse and other outcomes, including rehospitalization, overall symptoms, all-cause discontinuation, and dropout due to AEs [28]. Results showed that dose–response curves were hyperbolic, with rapid improvement at low doses (40% decrease in relapse at 2.5 mg/day risperidone equivalents) up to 5–6 mg/day risperidone equivalents. Beyond that dose, there were no substantial gains in efficacy, while dropouts due to AEs continued to increase. Overall, these findings support the select dose range of 90–180 mg for RBP-7000 covering risperidone equivalent doses of 3–6 mg/day.
Administration of 180 mg as 2 SC injections of 90 mg in the study was shown to be safe and well tolerated. There were no unexpected safety findings, with an AE profile consistent with those previously reported for RBP-7000 at the doses of 90 mg and 120 mg [13, 16] as well as other risperidone products, including 6 mg oral risperidone, with which plasma exposure equivalence was established [5, 29]. We observed no clinically relevant effects of 180 mg RBP-7000 on injection site assessments, clinical safety measures (AIMS, SAS, BARS, and C-SSRS), and other clinical vital signs, which is also consistent with previous clinical studies [13, 16].
A secondary objective of the study was the evaluation of administration of RBP-7000 180 mg at an alternate injection site—the back of the upper arm. For some individuals who may not be comfortable with repeated injections in the abdomen or public exposure of their abdominal region, the potential for SC injection in the upper arm would be an important option. We found similar pharmacokinetic profiles and injection site tolerability between the third dose (abdominal site) and fourth dose (back of upper arm) of 180 mg RBP-7000. There were no clinically meaningful differences in efficacy and safety between the two injection sites, which is supported by similar total active moiety exposure.
A potential limitation of the study is the relatively small sample size, although it is fit to meet the pharmacokinetic objectives of the study.
4.1 Conclusions
Monthly injections of 180 mg RBP-7000 administered as 2 SC injections of 90 mg provided similar average plasma concentration of the total active moiety compared with 6 mg/day oral risperidone given as 3 mg BID. These data combined with the stable clinical efficacy measures support the conclusion that RBP-7000 180 mg dose approximates 6 mg/day oral risperidone. The administration of 180 mg RBP-7000 at the back of the upper arm showed comparable pharmacokinetic profiles to RBP-7000 administration in the abdomen with no clinically meaningful differences in safety or efficacy measures, supporting its use as an alternate injection site. Overall, the safety profile was consistent with the established safety profile of RBP-7000 (90 and 120 mg dosage strengths) and other risperidone products. On the basis of these findings, 180 mg RBP-7000 should be considered an option for patients with schizophrenia who would benefit from switching from 5 to 6 mg/day oral risperidone.
References
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. https://doi.org/10.1016/S0140-6736(17)32154-2.
Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–203. https://doi.org/10.1093/schbul/sby058.
Kane JM. Pharmacologic treatment of schizophrenia. Biol Psychiatry. 1999;46(10):1396–408.
Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163.
RISPERDAL®. Package insert. Janssen Pharmaceuticals, Inc, Titusville, New Jersey; 2007.
Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7:1171–80. https://doi.org/10.2147/PPA.S53795.
Chue P, Llorca P, Duchesne I, Leal A, Rosillon D, Mehnert A. Hospitalization rates in patients during long-term treatment with long-acting risperidone injection. J Appl Res. 2005;5(2):266–74.
Crivera C, DeSouza C, Kozma CM, Dirani RD, Mao L, Macfadden W. Resource utilization in patients with schizophrenia who initiated risperidone long-acting therapy: results from the Schizophrenia Outcomes Utilization Relapse and Clinical Evaluation (SOURCE). BMC Psychiatry. 2011;11:168. https://doi.org/10.1186/1471-244X-11-168.
Lin D, Thompson-Leduc P, Ghelerter I, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–81. https://doi.org/10.1007/s40263-021-00815-y.
Nasrallah HA, Duchesne I, Mehnert A, Janagap C, Eerdekens M. Health-related quality of life in patients with schizophrenia during treatment with long-acting, injectable risperidone. J Clin Psychiatry. 2004;65(4):531–6. https://doi.org/10.4088/jcp.v65n0412.
Macfadden W, DeSouza C, Crivera C, et al. Assessment of effectiveness measures in patients with schizophrenia initiated on risperidone long-acting therapy: the SOURCE study results. BMC Psychiatry. 2011;11:167. https://doi.org/10.1186/1471-244X-11-167.
PERSERIS®. Package insert. Indivior Inc, North Chesterfield, Virginia; 2019.
Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter Phase 3 study. J Clin Psychopharmacol. 2016;36(2):130–40. https://doi.org/10.1097/JCP.0000000000000479.
Ivaturi V, Gopalakrishnan M, Gobburu JVS, et al. Exposure-response analysis after subcutaneous administration of RBP-7000, a once-a-month long-acting ATRIGEL formulation of risperidone. Br J Clin Pharmacol. 2017;83(7):1476–98. https://doi.org/10.1111/bcp.13246.
Le Moigne A, Csernansky J, Leadbetter RA, et al. PANSS individual item and Marder dimension analyses from a pivotal trial of RBP-7000 (monthly extended-release risperidone) in schizophrenia patients. J Clin Psychiatry. 2021;82(5):21m13906. https://doi.org/10.4088/JCP.21m13906.
Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the Phase 3 program. J Clin Psychopharmacol. 2019;39(5):428–33. https://doi.org/10.1097/JCP.0000000000001076.
Isitt JJ, Nadipelli VR, Kouassi A, Fava M, Heidbreder C. Health-related quality of life in acute schizophrenia patients treated with RBP-7000 once monthly risperidone: an 8-week, randomized, double-blind, placebo-controlled, multicenter Phase 3 study. Schizophr Res. 2016;174(1–3):126–31. https://doi.org/10.1016/j.schres.2016.03.020.
Dhanda R, Varghese D, Nadipelli VR, et al. Patient-reported outcomes in schizophrenia patients treated with once-monthly extended-release risperidone in a long-term clinical study. Patient Prefer Adherence. 2019;13:1037–50. https://doi.org/10.2147/PPA.S202173.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14:559–70.
Zou P, Wang F, Wang J, Lu Y, Tran D, Seo SK. Impact of injection sites on clinical pharmacokinetics of subcutaneously administered peptides and proteins. J Control Release. 2021;336:310–21.
Guy W. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health, Rockville, Maryland; 1976; p. 76-338.
Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. https://doi.org/10.1111/j.1600-0447.1970.tb02066.x.
Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. https://doi.org/10.1192/bjp.154.5.672.
Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035–43. https://doi.org/10.1176/ajp.2007.164.7.1035.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. https://doi.org/10.1093/schbul/13.2.261.
Laffont CM, Gomeni R, Zheng B, Heidbreder C, Fudala PJ, Nasser AF. Population pharmacokinetics and prediction of dopamine D2 receptor occupancy after multiple doses of RBP-7000, a new sustained-release formulation of risperidone, in schizophrenia patients on stable oral risperidone treatment. Clin Pharmacokinet. 2014;53(6):533–43. https://doi.org/10.1007/s40262-014-0132-7.
Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603–19. https://doi.org/10.1093/schbul/sbx090.
Leucht S, Bauer S, Siafis S, et al. Examination of dosing of antipsychotic drugs for relapse prevention in patients with stable schizophrenia: a meta-analysis. JAMA Psychiat. 2021;78(11):1238–48. https://doi.org/10.1001/jamapsychiatry.2021.2130.
Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13–39. https://doi.org/10.2147/nedt.2007.3.1.13.
Acknowledgements
The authors thank Karen D. Mittleman, PhD, for medical writing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Indivior Inc., North Chesterfield, VA.
Conflicts of Interest
SNS, JMP, and CLM are employees of Indivior. JK was an Indivior employee at the time of the study. DPW reports grants from AbbVie, Acadia, Alkermes, Allergan, Avanir, Biogen Boehringer Ingelheim, Cerevel, CoMentis, Intra-Cellular Therapies, Indivior, Janssen, Johnson & Johnson PRD, Lundbeck, Lupin, Merck, Novartis, Noven, Otsuka, Prothena, Pfizer, Roche, Sunovion, and Takeda and personal fees from Janssen, Otsuka, Boehringer Ingelheim, Biogen Merck, and Lyndra.
Availability of Data and Materials
Data are available from the authors upon reasonable request. All requests for raw and analyzed data will be promptly reviewed by the sponsor delegate to verify if the request is subject to any confidentiality obligations. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Any data that can be shared will be released via a data use agreement.
Ethics Approval
The study was approved by the local ethics committee on 18 June 2019 and was registered at ClinicalTrials.gov (identifier: NCT03978832).
Author Contributions
SNS, JMP, JK, and CLM contributed to the study design and data analysis. DPW conducted the study. All authors were involved in the interpretation of the data and critically reviewed the manuscript.
Consent to Participate
All participants provided written informed consent before screening procedures.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Jahnavi Kharidia: Formerly employed by Indivior.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Walling, D.P., Shinde, S.N., Pogoda, J.M. et al. An Open-Label Study to Assess Monthly Risperidone Injections (180 mg) Following Switch from Daily Oral Risperidone (6 mg) in Stable Schizophrenic Patients. Clin Drug Investig 44, 251–260 (2024). https://doi.org/10.1007/s40261-024-01347-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01347-1