Abstract
Background and Objective
Most evidence suggests that the pharmacokinetics of monoclonal antibodies (mAbs) are not meaningfully altered by patient characteristics, including racial/ethnic differences. Nevertheless, the pharmacokinetic profile of eptinezumab has not been evaluated in a Chinese population. This study was designed to confirm the hypothesis that the pharmacokinetic profile of the anti-calcitonin gene-related peptide mAb, eptinezumab, is similar in healthy Chinese individuals to that of healthy non-Asian individuals and non-Asian patients with migraine.
Methods
Over a study period of 12 weeks, healthy adult Chinese participants (N = 20) were randomized (1:1) to receive a single intravenous dose of eptinezumab 100 mg (n = 10) or 300 mg (n = 10) in a prospective, single-site, open-label parallel-group trial. Blood samples for the evaluation of plasma eptinezumab concentrations were obtained over 84 days, and standard pharmacokinetic parameters were derived.
Results
Mean maximum plasma concentrations (Cmax) of eptinezumab occurred 1.0−1.5 h post start of infusion, were similar between the 100 mg and 300 mg dose groups, and slowly declined in a biphasic manner. Cmax and area under the drug concentration–time curve (AUC) increased in a dose-proportional manner. Volume of distribution and clearance were similar between the 100 mg and 300 mg dose groups, and half-life was 22.5−28.1 days. Eptinezumab was generally well tolerated with no new safety signals identified. Only one participant, randomized to the 100 mg dose group, was positive for eptinezumab anti-drug antibodies, but negative for neutralizing antibodies, with no impact on pharmacokinetics.
Conclusion
The pharmacokinetic profile of eptinezumab in healthy Chinese individuals was generally similar to that reported for non-Asian populations with migraine, and eptinezumab was generally well tolerated. Evaluation of immunogenicity showed no evidence of an impact of anti-drug antibodies or neutralizing antibodies on safety profiles. This supports the globally approved doses of 100 mg and 300 mg as being appropriate for Chinese patients with episodic migraine or chronic migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study shows that eptinezumab, a preventive treatment for migraine, is well tolerated in healthy Chinese individuals, with pharmacokinetic properties similar to those seen in non-Asian populations. |
The pharmacokinetic profile of eptinezumab in healthy Chinese individuals is consistent with those seen in previous clinical trials of eptinezumab in patients with episodic and chronic migraine, with no reports of serious adverse events. |
1 Introduction
Migraine is a chronic debilitating disease with substantial clinical, economic, and personal burdens [1]. The introduction of monoclonal antibodies (mAbs) directed against calcitonin gene-related peptides (CGRPs) has provided a significant clinical advance in the treatment of migraine. Eptinezumab is an anti-CGRP mAb that has demonstrated efficacy in patients with episodic (PROMISE-1) and chronic (PROMISE-2) migraine [2, 3]. In these trials, eptinezumab was well tolerated with rapid and sustained efficacy as measured by a reduction in migraine days. The reduction in migraine frequency was evident as early as the first day after dosing, with consistent efficacy seen across demographic and baseline characteristic subgroups [3, 4]. In the DELIVER trial, eptinezumab also demonstrated a significant migraine preventive effect, reduced migraine frequency as well as severity, and improved quality of life in adults with migraine and 2–4 previous preventive treatment failures [1, 5]. Further, eptinezumab has been shown to shorten the time to headache and symptom resolution when administered during a moderate to severe migraine attack [6]. The pharmacokinetic profile of eptinezumab is characterized by a rapid attainment of maximum plasma concentrations, linear pharmacokinetics, and a terminal half-life of 27 days [7, 8].
Eptinezumab is approved and available in several countries to date; however, the pharmacokinetic profile and safety of eptinezumab in a Chinese population has not been previously published. To date, most clinical evidence suggests that there are no clinically meaningful differences in mAb treatment effects between ethnic populations, and there is no scientific rationale for such a difference [9,10,11,12]. However, until regulatory agencies rule on whether ethnic-based pharmacokinetic studies are necessary, such studies continue to be recommended. As part of the clinical development of eptinezumab in China, this randomized, single-dose trial was conducted to assess the pharmacokinetic parameters, safety, and immunogenicity of eptinezumab following a single intravenous (IV) infusion of eptinezumab in healthy Chinese participants to support the hypothesis that there are no differences in the pharmacokinetics of eptinezumab between Chinese and non-Asian populations.
2 Methods
2.1 Trial Design
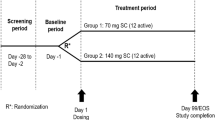
An interventional, prospective, single-site, randomized, open-label, parallel-group, single-dose trial was conducted at Zhongshan Hospital at Fudan University in Beijing, China. Participants were under supervision of the clinic from baseline (Day ‒1) until Day 3 (48 h post-dose) and then completed eight additional out-of-clinic visits. The total trial duration per participant was 12 weeks (from Day ‒1 to Day 84). The trial was conducted in compliance with Good Clinical Practice and in accordance with the ethical principles described in the Declaration of Helsinki [13, 14]. All participants provided written informed consent prior to trial enrollment.
2.2 Participants
Healthy male and female Chinese participants were aged 18–55 years, inclusive, with a body mass index (BMI) of ≥19–≤25 kg/m2. The aim was to enroll approximately equal numbers of male and female participants in this study, which took place at one site in China. Participants had to be healthy; thus, they were excluded if they had orthostatic hypotension; prolonged corrected QT (QTc) interval; clinically significant immunological, cardiovascular, respiratory, metabolic, renal, hepatic, gastrointestinal, endocrinological, hematological, dermatological, venereal, neurologic, or psychiatric disease; or history of cancer (other than basal cell or Stage 1 squamous cell carcinoma of the skin or adequately treated cervical intraepithelial neoplasia). Participants were also excluded if they used nicotine products, had significant alcohol consumption, had major surgery within 6 months, or tested positive for hepatitis B surface antigen (HBsAg), anti- hepatitis C virus (HCV), anti-Treponema pallidum (TP), or substance abuse. Participants were required to remain sexually abstinent, to engage exclusively in same-sex relationships, or to use two methods of contraception. All study procedures were approved by the Medical Ethics Committees of Fudan University, Zhongshan Hospital (approval number: 2020-110; approval date: 19 October 2020).
2.3 Treatment, Assessments, and Endpoints
Participants (N = 20) were randomized 1:1 to receive a single IV infusion of either eptinezumab 100 mg (n = 10) or 300 mg (n = 10) concentrate for solution for infusion, 100 mg/mL added to 100 mL of 0.9% saline and infused over 30 min. The random allocation sequence was generated by a standard procedure in Statistical Analysis Software (SAS), operated by the sponsor’s randomization administrator. All doses were administered under the supervision of suitably qualified study site staff. For quantification of eptinezumab, 2 mL of blood was drawn on Day 1 (pre-dose, at the end of infusion, and 0.5, 1, 1.5, 2, 3, 4, 8, and 12 h after the end of the infusion); Day 2 (24 h), Day 3 (48 h), Day 5 (96 h), Day 12 (264 h), Day 21 (480 h), Day 28 (648 h), Day 42 (984 h), Day 54 (1272 h), Day 70 (1656 h), and the completion visit on Day 84 (1992 h) after the end of the infusion. An additional 3 mL of blood was drawn on Day 1 (pre-dose) and at the completion visit (day 84) or withdrawal visit for analysis of anti-drug antibodies.
Plasma samples were analyzed for eptinezumab using a validated assay [15, 16]. Sample plasma/serum samples were collected and prepared using standard separation techniques reported elsewhere [17]. Samples were stored frozen prior to analysis. The pharmacokinetic analysis was conducted using WinNonLin®, Version 8.1. On the basis of the individual plasma concentration data, pharmacokinetic parameters were determined using standard non-compartmental methods. Individual plasma concentrations below the lower limit of quantification were imputed with a value of zero from the time of pre-dose sampling (t = 0) up to the time of maximal concentration (tmax). Beyond the tmax, individual plasma concentrations below the lower limit of quantification were not used in the derivations. Serum samples were analyzed for anti-eptinezumab antibodies using a validated bioanalytical method. The bioanalytical methods were validated in accordance with the US Food and Drug Administration (FDA) Guidance for Industry (Center for Drug Evaluation and Research) and with Chinese guidance [18, 19].
Adverse events (AEs) were collected on the basis of investigator observation and during open questioning at the screening visit, the baseline visit (Day ‒1), at each day during the confinement to the clinic, and at each of the remaining out-of-clinic visits. AEs were coded according to MedDRA (Version 24.0) terms and assessed for severity and relationship to study medication. Results from relevant tests and examinations [e.g., laboratory tests, vital signs, weight changes, electrocardiograms (ECGs)] were also collected and were recorded as AEs if considered clinically significant by the investigator.
2.4 Bioanalytical Assay
For measurement of free eptinezumab in dipotassium ethylenediaminetetraacetic acid (K2 EDTA) plasma, a quantitative, sandwich enzyme-linked immunosorbent assay (ELISA) technique was developed and validated [15, 16]. Human alpha-calcitonin gene-related peptide (α-CGRP) was immobilized through passive adsorption on a microtiter plate to capture eptinezumab that was present in calibration standards, controls, and test samples. After incubation, unbound material was removed in a wash step, and bound eptinezumab was detected using a commercial mouse anti-human immunoglobulin G (IgG), horseradish peroxidase (HRP)-labeled mAb. After a second wash step, a peroxidase substrate, 3, 3′, 5, 5′ tetramethylbenzidine (TMB), added to the wells reacted with the HRP conjugate to produce a colorimetric signal that was proportional to the amount of eptinezumab bound by the capture antigen. The concentration of eptinezumab in test samples was derived from a calibration curve. The validated assay detection range was 50.0–16,000 ng/mL (dilution linearity up to 1:3125). Assay minimum required dilution was 1:20. The assay passed the validation criteria for all regulated validation parameters [15, 16].
For measurement of anti-eptinezumab antibodies in serum, three tiers of electrochemiluminescence (ECL) ligand-binding assays (screening assay, confirmatory assay, and titer assay) were developed and validated [18, 19]. In the screening assay, test samples were pre-treated (eptinezumab and anti-eptinezumab antibody complexes were separated) by an acid dissociation technique. Samples were then added to a mixture of biotin-labelled eptinezumab and SULFO-TAG-labelled eptinezumab and allowed to complex with ADA. The labelled drug–ADA complexes were transferred to a streptavidin plate where they were captured. The plate was washed to remove any non-specific bound complexes, and read buffer (2X) was added to each well of the plate before the plate was read on the Meso Scale® Discovery (MSD) Quickplex.Z. In the confirmatory assay, eptinezumab was added to serum samples and controls in excess prior to the acid dissociation step. In the titer assay, the test sample was diluted past the screening assay sensitivity limit to generate a semi-quantitative dilution factor expressed as a titer value. The validated sensitivities of the screening and confirmatory assays were 9.91 ng/mL and 27.6 ng/mL, respectively. Assay minimum required dilution was 1:50. The assays passed the validation criteria for all regulated validation parameters [18, 19].
For characterization of anti-eptinezumab antibodies in serum, a neutralizing ECL competitive ligand-binding two-tier assay (screening and confirmatory assay) were developed and validated [18, 19]. In the screening assay, test samples were pre-treated by an acid dissociation technique. After neutralization, samples were added to the eptinezumab-coated wells, which were pre-blocked. Labeled CGRP and SULFO-TAG–Strepavidin were added to the plate before the plate was washed to remove any non-specific bound complexes, and read buffer (2X) was added to each well. The plate was read on the MSD Quickplex 120. In the confirmatory assay, eptinezumab was added to serum samples and controls in excess prior to the acid dissociation step. The validated sensitivities of the screening and confirmatory assays were 213 ng/mL and 205 ng/mL, respectively. Assay minimum required dilution was 1:6.5. The assay passed the validation criteria for all regulated validation parameters [18, 19].
2.5 Statistical Methods
All participants who received eptinezumab and who had evaluable data (i.e., data for at least one of the pharmacokinetic parameters) were included in the pharmacokinetic analysis. On the basis of the individual concentration data and using standard non-compartmental methods, if data allowed, the following pharmacokinetic parameters for eptinezumab were determined:
-
Area under the curve from zero to time t (AUC0-t)
-
AUC from zero to infinity (AUC0-inf)
-
Extrapolated AUC of total AUC0-inf (AUCextr)
-
Systemic clearance (CL)
-
Maximum observed concentration (Cmax)
-
Time to Cmax (tmax)
-
Elimination half-life (t½)
-
Effective elimination half-life (t½(eff))
-
Steady-state volume of distribution (Vss)
-
Apparent volume of distribution (Vz)
Descriptive statistics were performed for pharmacokinetic results and safety and tolerability outcomes (AEs, including clinical safety laboratory test values, vital signs, weight, and ECG parameter values). Summary statistics [i.e., n, arithmetic mean, standard deviation (SD), median, lower and upper quartiles (except for demographics, vital signs, weight, and body temperature), and minimum and maximum values] are presented for continuous variables. In addition, geometric means and percent coefficient of variation (CV%) are presented for positive continuous pharmacokinetic parameters. Counts and, if relevant, percentages are presented for categorical variables. In the descriptive statistics for the pharmacokinetic parameters other than Cmax, all non-missing values were included. If one or more individual pharmacokinetic parameter values were missing, the N was flagged in the descriptive statistics, and a footnote was added explaining why the individual values were missing. The incidence of eptinezumab anti-drug antibodies (ADAs) was tabulated, and if possible, the magnitude (i.e., titer) of ADA responses was assessed and tabulated.
3 Results
3.1 Participants
Twenty participants were enrolled and randomized; 19 completed the trial, but all 20 were included in the pharmacokinetic and safety analyses (Fig. 1). The first participant visit occurred on 20 April 2021 (the date when the first consent form was signed), and the last participant visit occurred on 20 July 2021 (the date of the last protocol-specific contact with any participant). One participant receiving eptinezumab 100 mg withdrew prematurely due to pregnancy on Day 42. Participant demographics are summarized in Table 1. There were 10 women and 10 men, with a mean (SD) age of 28 (4.3) years and a mean (SD) BMI of 21.7 (1.60) kg/m2.
3.2 Pharmacokinetics
Plasma concentrations of eptinezumab following a single dose of 100 mg or 300 mg are illustrated in Fig. 2, and derived pharmacokinetic parameters are summarized in Table 2. Mean maximum plasma concentrations of eptinezumab occurred 1.0–1.5 h post start of infusion, and then slowly declined in a biphasic manner. Among participants who completed the study, plasma eptinezumab concentrations were quantifiable up to the last scheduled sampling point (Day 84), with plasma concentrations ranging from 1010‒2510 ng/mL and 4810‒11100 ng/mL for the 100 mg and 300 mg dose groups, respectively, at the final timepoint. Cmax and AUC generally increased in a dose-proportional manner with 3.2- and 3.6-fold increases in AUC and Cmax, respectively, with a 3-fold increase in dose. Dose-normalized values for Cmax were 0.311 and 0.332 μg/mL/mg, and for AUC0-inf were 156 and 185 h × μg/mL/mg in the 100 mg and 300 mg dose groups, respectively. In general, there was low between-subject variability, as assessed by the CV%, for the exposure parameters of AUC and Cmax in both the 100 mg and 300 mg dose groups. The geometric mean CV was 19.1% and 20.1% for AUC and Cmax, respectively, in the 100 mg dose group and 11.7% and 15.1% in the 300 mg dose group. The Vss values were also similar in the 100 mg and 300 mg dose groups (5.18 and 5.25, respectively), as were CL values [0.00643 L/h (0.15 L/day) and 0.00542 L/h (0.13 L/day)]. The terminal half-life was 22.5 days in the 100 mg group and 28.1 days in the 300 mg group.
3.3 Safety
AEs are summarized in Table 3. Overall, 13 participants experienced 27 AEs. A total of 9 out of 12 AEs in the 100 mg group and 14 out of 15 AEs in the 300 mg group were considered possibly or probably related to treatment. The most commonly reported AEs were increased blood triglycerides, increased blood glucose, and increased white blood cell count. One participant withdrew from the trial due to pregnancy. There were no serious AEs. There was no trend in clinical safety laboratory test values, vital signs, weight changes, or ECG findings. A total of seven participants had post-dose potentially clinically significant (PCS) clinical safety laboratory test values: six participants in the 100 mg dose group and one participant in the 300 mg dose group, with low high-density lipoprotein cholesterol being the most commonly reported PCS value. One participant had PCS high blood triglycerides, which was reported as an AE. There were no PCS vital signs, although AEs of increased orthostatic heart rate response were reported in two participants, and increased diastolic blood pressure was reported in one participant. No participants had PCS changes in weight. A single participant was positive for eptinezumab anti-drug antibodies (ADAs) at a single time point; however, the participant was negative for neutralizing antibodies, and there was no apparent effect on the pharmacokinetic values for this participant.
4 Discussion
The goal of this study was to evaluate the pharmacokinetic profile and safety of eptinezumab in a healthy Chinese population and to confirm the hypothesis that there are no meaningful pharmacokinetic differences between healthy Chinese participants and non-Asian individuals with migraine. To minimize bias, the healthy Chinese participants were randomly assigned to receive a single dose of either 100 mg or 300 mg of eptinezumab, and given the previously reported long terminal elimination half-life (27 days) [7], a single dose was considered sufficient to ensure systemic exposure to eptinezumab for the study duration. Results indicated that maximum plasma eptinezumab concentrations were achieved within 1–1.5 h after the start of the infusion, followed by a biphasic decline in concentrations. Notably, the Vss values in both dose groups were, as expected [7], close to the human plasma volume of 3 L [20]. Exposure to eptinezumab (assessed as AUC and Cmax) appeared to increase in an approximately dose-proportional manner. The derived pharmacokinetic results for exposure (i.e., AUC, Cmax), distribution (i.e., Vz), and elimination (i.e., CL) and the linearity of eptinezumab pharmacokinetics were consistent with the results of a population pharmacokinetic analysis of phase 2/3 clinical trials of eptinezumab conducted primarily in non-Asian patients with episodic migraine and chronic migraine [7]. Cmax (patients with migraine: ~37 µg/mL; healthy Chinese individuals: ~31 µg/mL), terminal half-life (patients with migraine: ~27 days; healthy Chinese individuals: 23.3 days), and clearance (patients with migraine: ~0.0062 L/h; healthy Chinese individuals: ~0.0064 L/h) were very similar at the eptinezumab 100 mg dose [7].
In addition, the population pharmacokinetic analysis found that patient and disease characteristics (e.g., body weight, renal function, disease state, and baseline mean migraine days) did not have clinically significant effects on the pharmacokinetic parameters of eptinezumab [7]. In this work, for instance, patients were healthy and between 19 and 38 years of age, with a BMI between 19.3 and 24.7 kg/m2; were excluded from the study if they had any clinically significant renal or other major disorder; and were not stratified by baseline mean monthly migraine days. Despite differences in patient and disease characteristics between this study’s cohort and that of the Baker et al. study [7], the latter of which included patients with episodic and chronic migraine, pharmacokinetic parameters were similar between all three populations. This is in line with the hypothesis that some adult patient characteristics (such as BMI) do not influence the pharmacokinetics of mAbs, but further investigations are still warranted, especially as measurements become more standardized [8, 12].
The lack of ethnic differences is to be expected on the basis of the intrinsic properties of mAbs and previous studies of other mAbs in Asian versus non-Asian populations [9,10,11,12]. Unlike small molecules, whose clearance can be influenced by genetic polymorphisms in hepatic cytochrome P450 enzymes, mAbs are eliminated via intracellular catabolism followed by either fluid-phase pinocytosis or receptor-mediated endocytosis [10]. In addition, since mAbs are administered via injection, there is little chance for dietary effects on absorption or food–drug interactions [10]. There was some variability, however, in the results of studies evaluating the pharmacokinetics of mAbs in Asian versus non-Asian individuals. One study evaluating erenumab found that AUC values were 70% higher in Chinese subjects compared with white subjects [21]. However, most analyses concluded that, while there are some differences in the pharmacokinetics of mAbs (including anti-CGRP mAbs) due to differences in body weight or in target expression levels between populations, these differences were generally clinically insignificant and did not warrant dosage adjustments based on ethnicity [9,10,11,12]. For example, one recent review of population pharmacokinetic modeling studies assessing pharmacokinetic variability of therapeutic mAbs found that most publications considered the influence of race to be clinically insignificant because of either the author’s clinical experience (n = 1) or the small effect on pharmacokinetic parameters (n = 12) [12].
This study also confirms that eptinezumab was well tolerated in Chinese individuals, with no new safety signals identified. There were no serious AEs, and the only treatment discontinuation was due to pregnancy. Thus, the safety results provide support for the good tolerability profile of eptinezumab in Chinese populations. Eptinezumab antibodies were present in a single participant in the 100 mg dose group at completion visit on Day 84, and no neutralizing antibodies were present in both the screening and confirmatory tests. There was no apparent effect on pharmacokinetic parameters in this patient. This is in line with previous studies that have reported that the incidence of ADAs is low and their presence (with or without neutralizing antibodies) has no effect on the efficacy or safety of eptinezumab [2, 3].
The primary limitation of this study is the small sample size; however, with 10 patients per eptinezumab dose, it was believed that sufficient information could be collected to evaluate the safety, tolerability, and pharmacokinetic parameters. However, the low interindividual variability of the current results and the similarity to the findings of other pharmacokinetic analyses of eptinezumab provides confidence in our hypothesis that the pharmacokinetic profile of eptinezumab is not different in Chinese individuals versus non-Asian individuals. This also confirms the experience with other anti-CGRP mAbs, which have, with the exception of erenumab mentioned above, found that patient characteristics (age, race, sex, body weight) do not have an effect on pharmacokinetic parameters and that, as a class, dosage adjustments for anti-CGRP mAbs are not required based on patient characteristics [22,23,24,25]. Finally, although there are no reasons to believe that there should be any differences in pharmacokinetics between healthy Chinese participants and Chinese participants with migraine, it is a limitation that this study only enrolled healthy Chinese participants.
5 Conclusion
In summary, the results indicate that eptinezumab is well tolerated in healthy Chinese individuals with pharmacokinetic parameters similar to those seen in previous studies with predominantly white populations with migraine. This supports the globally approved doses of 100 mg and 300 mg as being appropriate for Chinese patients with episodic migraine or chronic migraine.
References
Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, et al. Migraine: epidemiology and systems of care. Lancet. 2021;397(10283):1485–95.
Ashina M, Saper J, Cady R, Schaeffler B, Biondi DM, Hirman J, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–54.
Lipton RB, Goadsby PJ, Smith J, Schaeffler BA, Biondi DM, Hirman J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine. PROMISE-2. Neurology. 2020;94(13):e1365–77.
Martin V, Nagy AJ, Janelidze M, Giorgadze G, Hirman J, Cady R, et al. Impact of baseline characteristics on the efficacy and safety of eptinezumab in patients with migraine: subgroup analyses of PROMISE-1 and PROMISE-2. Clin Ther. 2022;44(3):389–402.
Goadsby PJ, Barbanti P, Lambru G, Ettrup A, Christoffersen CL, Josiassen MK, et al. Eptinezimab improved patient-reported outcomes and quality of life in patients with migraine and prior preventive treatment failures. Eur J Neurol. 2023;30:1089–98.
Winner PK, McAllister P, Chakhava G, Ailani J, Ettrup A, Krog Josiassen M, et al. Effects of intravenous eptinezumab vs placebo on headache pain and most bothersome symptom when initiated during a migraine attack: a randomized clinical trial. JAMA. 2021;325(23):2348–56.
Baker B, Schaeffler B, Beliveau M, Rubets I, Pederson S, Trinh M, et al. Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine. Pharmacol Res Perspect. 2020;8(2): e00567.
Baker B, Shen V, Cady R, Ettrup A, Larsen F. Eptinezumab administered intravenously, subcutaneously, or intramuscularly in healthy subjects and/ or patients with migraine: early development studies. Cephalalgia Rep. 2022;5:1–11.
Matsushima S, Huang Y, Suzuki H, Nishino J, Lloyd P. Ethnic sensitivity assessment—pharmacokinetic comparability between Japanese and non-Japanese healthy subjects on selected mAbs. Expert Opin Drug Metab Toxicol. 2015;11(2):179–91.
Zhou H, Tsukamoto Y, Davis H. Should clinical pharmacokinetic bridging studies between Caucasian and Asian populations be required for approval of monoclonal antibodies? J Clin Pharmacol. 2011;52(8):1273–6.
Chiba K, Yoshitsugu H, Kyosaka Y, Iida S, Yoneyama K, Tanigawa T, et al. A comprehensive review of the pharmacokinetics of approved therapeutic monoclonal antibodies in Japan: are Japanese phase I studies still needed? J Clin Pharmacol. 2014;54(5):483–94.
Bensalem A, Ternant D. Pharmacokinetic Variability of therapeutic antibodies in humans: a comprehensive review of population pharmacokinetic modeling publications. Clin Pharmacokinet. 2020;59(7):857–74.
World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. 2022. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 24 Mar 2023.
ICH. ICH Harmonised Guideline E6(R2): integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice. 2016. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Accessed 24 Mar 2023.
U.S. Department of Health and Human Services. Bioanalytical method validation: guidance for industry. 2018. p. 1–44. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed 24 Mar 2023.
Chinese Pharmacopoeia Commission. 9012—Guideline for Validation of Quantitative Analytical Methods for Biological Samples. Chinese Pharmacopoeia; 2020.
Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–7.
U.S. Department of Health and Human Services. Immunogenicity testing of therapeutic protein products—developing and validating assays for anti-drug antibody detection. 2019. https://www.fda.gov/media/119788/download. Accessed 24 Mar 2023.
NMPA. Technical Guidelines for Drug Immunogenicity Research. 2021.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Shen Q, Jin Y, Di X, Hu C, Liu R, Wang Y, et al. Pharmacokinetics and safety of erenumab after a single subcutaneous injection dose in healthy Chinese subjects. Clin Drug Investig. 2022;42(7):623–30.
VYEPTI [package insert]. Bothell, WA: Lundbeck Seattle BioPharmaceuticals, Inc.; 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761119s002lbl.pdf. Accessed 24 Mar 2023.
EMGALITY [package insert]. Indianapolis, IN: Eli Lilly and Company; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf. Accessed 24 Mar 2023.
AJOVY [package insert]. North Wales, PA: Teva Pharmaceuticals USA; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761089s002lbl.pdf. Accessed 24 Mar 2023.
AIMOVIG [package insert]. Thousand Oaks, CA: Amgen Inc.; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761077s009lbl.pdf. Accessed 24 Mar 2023.
Acknowledgments
The authors thank Bret Fulton, Ph.D., and Jessica A. Weaver, Ph.D., of The Medicine Group, LLC (New Hope, PA, USA) for providing medical writing support in drafting and revising the manuscript, which was funded by Lundbeck LLC (Deerfield, IL, USA) and in accordance with Good Publication Practice guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The clinical trial and publication support were funded by H. Lundbeck A/S, Copenhagen, Denmark.
Conflict of interest
XNL and HRX have no conflicts of interest to declare. EC, KBP, JR, AE, JBØ, and FL are full-time employees at Lundbeck.
Ethics approval
The trial was conducted in compliance with Good Clinical Practice and in accordance with the ethical principles described in the Declaration of Helsinki. All study procedures were approved by the Medical Ethics Committees of Fudan University, Zhongshan Hospital (approval number: 2020-110; approval date: 19 October 2020).
Consent to participate
Written informed consent was obtained from all individual participants included in the study prior to trial enrollment.
Consent for publication
Not applicable.
Data availability
In accordance with The European Federation of Pharmaceutical Industries and Associations (EFPIA) and the Pharmaceutical Research and Manufacturers of America (PhRMA) Principles for Responsible Clinical Trial Data Sharing guidelines, H. Lundbeck A/S is committed to responsible sharing of clinical trial data in a manner that is consistent with safeguarding the privacy of patients, respecting the integrity of national regulatory systems and protecting the intellectual property of the sponsor. The protection of intellectual property ensures continued research and innovation in the pharmaceutical industry. Deidentified data are available to those whose request has been reviewed and approved through an application submitted to https://www.lundbeck.com/global/our-science/clinical-data-sharing.
Code availability
Not applicable.
Authors’ contributions
All authors made substantial contributions to the conception of this manuscript, including design of the trial, analysis, and interpretation of data. All authors drafted the manuscript or revised it critically for important intellectual content.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, XN., Xu, HR., Cui, E. et al. Pharmacokinetics and Safety of Eptinezumab in Healthy Chinese Participants: A Randomized Clinical Trial. Clin Drug Investig 43, 873–881 (2023). https://doi.org/10.1007/s40261-023-01315-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01315-1