Abstract
Background and Objective
Nitazoxanide, a US Food and Drug Administration-approved antiparasitic agent, was reported to be effective in treating coronavirus disease 2019 (COVID-19). The lack of effective and precise treatments for COVID-19 infection earlier in the pandemic forced us to depend on symptomatic, empirical, and supportive therapy, which overburdened intensive care units and exhausted hospital resources. Therefore, the aim of this systematic review and meta-analysis was to assess the efficacy and safety of nitazoxanide for COVID-19 treatment.
Methods
A systematic review and meta-analysis synthesizing relevant randomized controlled trials from six databases (MedRxiv, WOS, SCOPUS, EMBASE, PubMed, and CENTRAL) until 17 May 2022 was conducted. Risk ratio (RR) for dichotomous outcomes was used and data with a 95% confidence interval (CI) are presented. The protocol was registered in PROSPERO with ID: CRD42022334658.
Results
Six randomized controlled trials with 1412 patients were included in the analysis. Nitazoxanide was effective in accelerating viral clearance compared with placebo (RR: 1.30 with 95% CI 1.08, 1.56, p = 0.006) and reducing oxygen requirements (RR: 0.48 with 95% CI 0.39, 0.59, p = 0.00001), but we found no difference between nitazoxanide and placebo in improving clinical resolution (RR: 1.01 with 95% CI 0.94, 1.08, p = 0.88), reducing the mortality rate (RR: 0.88 with 95% CI 0.4, 1.91, p = 0.74), and intensive care unit admission (RR: 0.69 with 95% CI 0.43, 1.13, p = 0.14). Moreover, nitazoxanide was as safe as placebo (RR: 0.9 with 95% CI 0.72, 1.12, p = 0.34).
Conclusions
Compared with placebo, nitazoxanide was effective in expediting viral clearance and decreasing oxygen requirements. However, there was no difference between nitazoxanide and placebo regarding clinical response, all-cause mortality, and intensive care unit admission. Therefore, more large-scale studies are still needed to ascertain the clinical applicability of nitazoxanide in COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nitazoxanide is potentially effective in accelerating coronavirus disease 2019 (COVID-19) viral clearance and reducing oxygen requirements compared with placebo. |
It showed no efficacy in improving clinical symptoms or reducing all-cause mortality and intensive care unit admission. |
1 Introduction
The coronavirus disease 2019 (COVID-19) pandemic is the worst global health crisis since the influenza pandemic of 1918. It crippled numerous healthcare systems around the world and tremendously downturned the global economy. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in a broad spectrum of clinical presentations. Most cases are asymptomatic or have mild-to-moderate symptoms; however, 5–14% develop a severe, potentially life-threatening disease [1–3]. The disease can progress to different clinical phenotypes ending with severe pneumonia, acute respiratory distress syndrome, cytokine storm, disseminated intravascular coagulopathy, multi-organ failure, shock, and eventually death [1, 4–7]. We can relate this progression to different reasons, including age, comorbidities, viral genotype, and viral load [8–12].
Earlier in the pandemic, the lack of effective and specific treatments for COVID-19 infection left us with symptomatic, empirical, and supportive therapies that overworked the intensive care units (ICUs) and depleted hospitals’ resources. Coupling vaccination programs, social distancing, and these therapies played a pivotal role in controlling the pandemic. Based on disease severity, treatment options included pre-existed antiviral therapies, anti-SARS-CoV-2 neutralizing antibody products, immunomodulatory agents, ventilation, and oxygen therapies [5]. With the focus on treating and developing new drugs for severe and complicated cases of COVID-19 infections, therapies for mild and moderate infections in outpatient settings are limited. Paxlovid and molnupiravir, two newly developed and US Food and Drug Administration-approved oral medications for COVID-19, have shown a significant reduction in hospitalization and death in mild to moderate infections [13, 14]. With barriers to worldwide access to these recently developed medications, the need to repurpose existing anti-microbial agents has been accepted as an alternative treatment option [15–17].
Nitazoxanide (NTZ) is a US Food and Drug Administration-approved antiparasitic drug with an excellent safety profile. It has been suggested as one of the alternative therapies for COVID-19 infection for different reasons. Hong et al. found that NTZ decreased the plasma level of interleukin (IL)-6 markedly when administered in mice [18]. Shou et al. also suggested that tizoxanide, the main active metabolite of NTZ, wielded anti-inflammatory effects in vivo [19]. This advocated for the possible beneficiary effects of NTZ in controlling cytokine storms where large amounts of IL-6 and other pro-inflammatory cytokines are released. Treatment with NTZ also showed wide antiviral activities with different mechanisms against various viral infections, including influenza, Middle East respiratory syndrome coronavirus, and other coronaviruses [20–25]. Jasenosky et al. also found that NTZ amplified the host’s innate immune response to viruses and inhibited Ebola virus replication [26].
To date, a total of 31 clinical trials have been registered on ClinicalTrials.gov to investigate the effect of NTZ on COVID-19 infections. Nitazoxanide was administered alone or combined with other drugs compared to a placebo. The results ranged from accelerated symptom resolution, a shorter time to hospital discharge, a decreasing viral load, and a well-tolerated safety profile to no difference between the placebo and NTZ groups [27–32]. Therefore, we performed this systematic review and meta-analysis to synthesize evidence from the published randomized controlled trials (RCTs) on the efficacy and safety of NTZ in patients with COVID-19 infection.
2 Methods
2.1 Protocol Registration
For this systematic review and meta-analysis, we rigorously adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [33] and the Cochrane Handbook for Systematic Reviews and Interventions [34]. The review protocol was registered in PROSPERO with ID: CRD42022334658.
2.2 Data Sources and Search Strategy
Until 17 May 2022, two reviewers (B.A. and M.A.) conducted a systematic search of the following electronic databases: MedRxiv, Web of Science, SCOPUS, EMBASE, PubMed (MEDLINE), and Cochrane Central Register of Controlled Trials (CENTRAL). There were no search filters applied. The search approach and results are outlined in Table S1 of the Electronic Supplementary Material (ESM).
2.3 Eligibility Criteria
We included RCTs with the following PICO criteria: population (P): patients with COVID-19 symptoms and confirmed by either chest computed tomography suggestive of viral pneumonia or a positive nasopharyngeal swab test for SARS-CoV-2 (reverse-transcriptase polymerase chain reaction [RT-PCR]); intervention (I): nitazoxanide regardless of dosage, route, and duration of administration; control I: placebo; outcomes (O): primary outcome: confirmed viral clearance by negative RT-PCR irrespective of the time of assessment. Our secondary outcomes are clinical resolution, all-cause mortality, oxygen supplementation, ICU admission or mechanical ventilation, and incidence of adverse events (diarrhea, nausea, vomiting, abdominal pain, pruritis, and headache). Animal studies, pilot studies, observational studies (cohort, case-control, cross-sectional, case series, and case reports), single-arm clinical trials, in vitro investigations (tissue and culture studies), book chapters, editorials, press articles, and conference abstracts were excluded.
2.4 Study Selection
After duplicates were deleted by Covidence online software [35], two reviewers (R.A. and F.L.) independently screened the titles and abstracts of the included records. The full texts of the relevant records were then screened for the preceding eligibility criteria. Any disagreements were resolved by inviting a third reviewer (M.A).
2.5 Data Extraction
Four reviewers (B.K., F.L., R.A., and A.A.) independently extracted the following data from the included trials using a pre-tested extraction sheet: study characteristics (first author name, year of publication, country, study design, total participants, the dose, route of administration, and duration of administration; time of viral eradication assessment of NTZ; and follow-up duration); baseline information (age, sex, viral load, race, basal metabolic index, and comorbidities); efficacy outcomes data (negative RT-PCR, all-cause mortality, oxygen supplementation, and ICU admission); and safety outcomes data (diarrhea, nausea, vomiting, abdominal pain, pruritis, and headache). Dissension was used to resolve conflicts.
2.6 Risk of Bias and Quality Assessment
Using The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials, four reviewers (M.A., F.L., R.A., and B.K.) independently assessed the included studies for risk of bias [36]. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias were considered. Conflicts were resolved by discussion. Two reviewers (M.A. and F.L.) used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group recommendation [37, 38] for the quality of evidence assessment. Inconsistency, imprecision, indirectness, publication bias, and bias risk were all considered. Our findings on the quality of evidence were justified, documented, and included in each outcome’s reporting. Any disputes were handled by the third reviewer (B.A.).
2.7 Statistical Analysis
The statistical analysis was carried out with RevMan version 5.3 software [39]. We pooled dichotomous outcomes using the risk ratio (RR) presented with the corresponding 95% confidence interval (CI). We used the I2 and Chi-square tests to examine heterogeneity; the Chi-square test determines if there is substantial heterogeneity, while the I2 determines the magnitude of heterogeneity. A substantial heterogeneity (for the Chi-square test) is defined as an alpha level below 0.1, according to the Cochrane Handbook for Systematic Reviews and Interventions (Chapter Nine) [34], while the I2 test is interpreted as follows: (0–40%: not significant; 30–60%: moderate heterogeneity; 50–90%: considerable heterogeneity). We utilized the fixed-effects model.
We conducted a sensitivity analysis in the case of considerable heterogeneity by deleting one study at a time and reconducting the analysis to see how each study affected the total effect size of the outcomes. We also conducted a subgroup analysis depending on the time of the viral clearance assessment. Because we only included fewer than ten studies in each outcome, we did not offer funnel plots to reveal publication bias, as advised by Egger et al. [40].
3 Results
3.1 Search Results and Study Selection
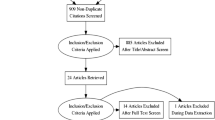
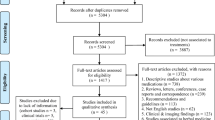
A total of 777 articles were collected by searching six databases: PubMed (110), Cochrane (45), Web of Science (106), Scopus (379), Embase (96), and MedRxiv (41), respectively. Two hundred and sixty-five duplicates were initially excluded. After title and abstract screening, 487 records were excluded leaving 25 articles for full-text screening. Sixteen articles were excluded after full-text screening (Table S2 of the ESM). Only six articles met our inclusion criteria. Figure 1 shows the selection process in a PRISMA flow diagram.
3.2 Characteristics of Included Studies
Our study included six RCTs: three conducted in Brazil [27, 29, 30], one in Egypt [28], one in Argentina [32], and another in the USA and Puerto Rico [31]. Our included studies had a total of 1412 participants who were randomized to receive either NTZ (n = 705) in the oral form or placebo (n = 707). Participants had a mean age of 48.1 years with a predominant white race (n = 714), then the black race (n = 108). Time of administration differed between one study and another, twice [27, 31], three [29, 30], or four [28, 32] times a day with a mean treatment duration of 8 days and a mean follow-up duration of 2 months. The method of COVID-19 assessment was RT-PCR in all our studies, with a mean viral eradication time assessment of 14 days. Further description of the summary and baseline characteristics of included trials can be found in Tables 1 and 2.
3.3 Risk of Bias and Quality of Evidence
We assessed the quality of the included studies according to the Cochrane risk of bias tool, as shown in Fig. 2. All studies had a low risk of bias regarding the “random sequence generation”. All studies had a low risk of bias regarding “allocation concealment” except Blum et al. [27] had an unclear risk of bias. All studies had a low risk of bias regarding “performance bias” except Medhat et al., an open-label study [28], with a high risk of bias. All studies had a low risk of bias regarding the “detection bias” except Medhat et al. [28] and Silva et al. [32], with a high risk of bias owing to a lack of outcome assessor blinding. Regarding the “attrition bias”, three studies had a low risk of bias [28, 30, 31]. However, Blum et al. [27], Rocco et al. [29], and Silva et al. [32] had a high risk of bias because of a significant loss of follow-up. All studies had a low risk of bias regarding “reporting bias” except Medhat et al. [28], which had a high risk of bias due to not reporting clinical response data. All studies had a high risk of bias regarding the “other bias” owing to the presence of a funding source, except the Silva et al. [32] study, which had a low risk of bias because of the absence of a funding source.
Using the GRADE system, all the included outcomes yielded very low-quality evidence. Details and explanations are clarified in Table 3.
3.4 Primary Outcome: Confirmed Viral Clearance by Negative RT-PCR
The pooled RR significantly favored NTZ over placebo (RR: 1.30 with 95% CI 1.08, 1.56, p = 0.006) [very-low quality evidence] (Fig. 3A, Table 3). Pooled studies were heterogenous (p = 0.03, I2 = 66%). To resolve heterogeneity, we conducted a sensitivity analysis. However, heterogeneity was not resolved by a sensitivity analysis. Furthermore, pooled RR showed no difference between NTZ and placebo after excluding Medhat et al. [28] and Rocco et al. [30] [(RR: 1.21 with 95% CI 1.00, 1.47, p = 0.06) and (RR: 1.16 with 95% CI 0.94, 1.44, p = 0.16), respectively] (Table S3 of the ESM). We conducted a subgroup analysis based on the time of assessment; pooled RR favored NTZ over placebo from 1 to 7 days (RR: 1.49 with 95% CI 1.07, 2.08, p = 0.02); however, we found no difference either from 8 to 14 days (RR: 1.16 with 95% CI 0.87, 1.54, p = 0.31) or from 15 to 21 days (RR: 1.26 with 95% CI 0.99, 1.61, p = 0.06) (Fig. 3B).
3.5 Secondary Outcomes
3.5.1 Clinical Resolution
The pooled RR showed no difference between NTZ and placebo (RR: 1.01 with 95% CI 0.94, 1.08, p = 0.88) [very-low quality evidence] (Fig. 4A, Table 3). Pooled studies were heterogenous (p = 0.11, I2 = 60%).
3.5.2 All-Cause Mortality
The pooled RR showed no difference between NTZ and placebo (RR: 0.88 with 95% CI 0.4, 1.91, p = 0.74) [very-low quality evidence] (Fig. 4B, Table 3). Pooled studies were homogenous (p = 0.36, I2 = 7%).
3.5.3 ICU Admission
The pooled RR showed no difference between NTZ and placebo (RR: 0.69 with 95% CI 0.43, 1.13, p = 0.14) [very-low quality evidence] (Fig. 4C, Table 3). Pooled studies were homogenous (p = 0.28, I2 = 21%).
3.5.4 Oxygen Requirement
The pooled RR significantly favored NTZ over placebo (RR: 0.48 with 95% CI 0.39, 0.59, p = 0.00001) [very-low quality evidence] (Fig. 4D, Table 3). Pooled studies were homogenous (p = 0.14, I2 = 39%). We conducted a subgroup analysis based on the day of assessment; pooled RR significantly favored NTZ over placebo on day 4 or 5 (RR: 0.4 with 95% CI 0.3, 0.52, p = 0.00001) and on day 7 (RR: 0.54 with 95% CI 0.36, 0.81, p = 0.003); however, we found no difference between NTZ and placebo on day 14 (RR: 0.67 with 95% CI 0.41, 1.08, p = 0.1) (Fig. 4D).
3.5.5 Safety (Incidence of Adverse Events)
The pooled RR showed no difference between NTZ and placebo in patients with at least one adverse event (RR: 0.9 with 95% CI 0.72, 1.12, p = 0.34) [very-low quality evidence] (Fig. 5A, Table 3). Pooled studies were homogenous (p = 0.45, I2 = 0%). Moreover, there was no difference between NTZ and placebo regarding the incidence of diarrhea, headache, nausea, abdominal pain, pruritis, and urticaria; however, NTZ was significantly associated with vomiting (Fig. 5B, Table S4 of the ESM).
4 Discussion
With the lack of a definitive antiviral therapy for SARS-CoV-2 infection, trials of repurposing existing medications started to trend. Nitazoxanide was considered a potential treatment for COVID-19 based on the existing evidence of its various antiviral and immunomodulatory properties either in vivo or in vitro [18–21, 23, 25, 26, 41, 42]. Our recent meta-analysis involving six RCTs demonstrated that NTZ is effective in increasing the viral clearance rate and decreasing oxygen requirements; however, we detected no difference between NTZ and placebo in reducing mortality, ICU admission, and improving clinical resolution. Additionally, NTZ was safe, well tolerated, and with similar rates of adverse events compared to placebo, except for vomiting.
Regarding viral clearance, NTZ was effective compared to placebo up to 7 days after initiating treatment but not effective afterward up to 14 and 21 days. On the one hand, Blum et al. [27] and Rossignol et al. [31] did not support NTZ. In Blum et al. [27], viral clearance was assessed on day 21 after treatment; hence, this difference can be attributed to the long duration of assessment after treatment, because in most patients, a longer duration would result in decreased viral load regardless of therapy [30]. This supports the findings of our subgroup analysis that NTZ was effective for only up to 7 days; however, only one to two studies were included in each subgroup, which can undermine our findings. In contrast, Medhat et al. [28] supported NTZ after 14 days, which can be attributed to some different methodological aspects, including using standard treatment along with NTZ, a longer treatment duration (14 days), and more frequent NTZ administration (four times a day). Moreover, Rossignol et al. [31] attributed this difference to the procedures used to collect, process, and quantify viral loads from nasopharyngeal swabs not being validated to predict viral load, inflammation, lung symptoms, or clinical outcomes at the patient or trial level. It is also still questionable if RT-PCR adequately detects infectious viruses because viral RNA can survive even in the absence of the replication-competent virus for a long duration [31]. On the other hand, Rocco et al. 2021 [30] and Medhat et al. [28] supported NTZ as they only included patients with mild disease with no mortality recorded and with only two patients admitted to the ICU in Rocco et al. [30].
Nitazoxanide may have an antiviral effect in more than one stage of the COVID-19 replication cycle; it suppresses viral RNA and DNA replication as well as direct viral protein production in a variety of viruses [20, 21, 43]. To clarify, NTZ has been reported to be effective against Middle-East respiratory syndrome severe acute respiratory syndrome-1 (SARS-CoV) [21, 44]. Given that the genomic similarity between COVID-19 and Middle-East respiratory syndrome is about 50% and between COVID-19 and SARS-Cov is about 79% [45], the effective therapeutic approaches against Middle-East respiratory syndrome and SARS-CoV, NTZ in our case, can be effective against COVID-19 [46].
Furthermore, it interferes with the host’s cellular metabolism by modulating interferon (IFN) surge [20, 21]. Nitazoxanide prevents COVID-19-induced IFN surge, subsequently preventing the development of a cytokine storm [47]. To clarify, the entry of the COVID-19 virus into the alveolar type II pneumocyte cells uses angiotensin-converting enzyme 2 receptors [48], which leads to cellular pyroptosis and a damage-associated molecular pattern release [49]. This is detected by alveolar macrophages, leading to the secretion of the pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), IL-6, IL-8, and macrophage inflammatory protein-1 alpha [50]. This cycle is inhibited and controlled by IFN-1, leading to diminished viral replication and decreased cellular damage [51]; however, IFN-1 is downregulated by COVID-19, leading to immunological escape and cytokine over-secretion leading to a cytokine storm [52]. This effect is inhibited by NTZ, preventing immunological escape and the subsequent cytokine storm [46]. Additionally, it interferes with host-regulated mechanisms responsible for viral replication of mammalian target of rapamycin complex 1 signaling [53]. Finally, NTZ has been reported to enhance autophagic cell death [46], which was reported to be beneficial in controlling COVID-19 [54]. The autophagy of necrotic cells, which is considered a pro-inflammatory trigger, can ameliorate the inflammatory process decreasing the amount of secreting cytokines [55].
Regarding inflammatory markers, acute inflammatory markers decreased significantly with NTZ in COVID-19. To clarify, C-reactive protein, which is associated with a worse prognosis in COVID-19 [56, 57], was decreased with NTZ [27]. Furthermore, IL-6 was reduced with NTZ; IL-6 is a key pro-inflammatory mediator involved in the development of the acute phase response, which results in a variety of local and systemic responses such as fever, leucocyte recruitment, activation, and hemodynamic effects [27]. It also predicts a higher risk of disease deterioration [58].
Furthermore, NTZ was also associated with TNF-α reduction [27]. Tumor necrosis factor-alpha is one of the main cytokines responsible for the immunological responses to COVID-19 [59, 60]. Hence, anti-TNF-α drugs can reduce COVID-19 respiratory insufficiency and mortality by lowering inflammatory-driven capillary leak [61]. Moreover, IL-8, a powerful pro-inflammatory cytokine that plays a key function in the inflammatory recruitment and activation of neutrophils, was decreased significantly with NTZ [27]. It is also possible that IL-8 has a role in the frequent neutrophilia seen in patients with COVID-19 [42]. Regarding cellular immunity, CD4+ HLA-DR+ T-cell lymphocytes were also significantly decreased with NTZ [27]. To conclude, NTZ can be beneficial in COVID-19 via its antiviral and immunomodulatory effects, decreasing IL-6, IL-8, TNF-α, and CD4 T cells [62].
Despite the previous effects, to effectively treat a viral infection resembling COVID-19, antiviral agents that act at different steps of the viral replication cycle must be used together [27, 29]. This would reduce the virus’s genetic variation and reduce the likelihood of the fast evolution of resistant strains [27]. Supporting this hypothesis, higher transmission rates and viral loads with COVID-19 can lead to new mutant strains, such as the Brazilian P.1 strain [63]. In this line, multiple trials have evaluated NTZ in combination with other drugs. To clarify, COVID-19 clearance from the nasopharynx was substantially faster with NTZ in conjunction with ribavirin, ivermectin, and zinc supplements compared with symptomatic treatment [53]. Another study assessed the efficacy of NTZ against COVID-19 in combination with azithromycin [53]. Furthermore, several in-vitro investigations revealed synergistic effects when NTZ is combined with other agents [64–66]. However, more research is still warranted in this regard.
Regarding the clinical resolution, we found no difference between NTZ and placebo; however, only two trials [29, 30] were included in our analysis. This can be attributed to symptoms in mild COVID-19 that can resolve spontaneously regardless of antiviral therapy [30], i.e., the median time from symptom onset to resolution was reported to be 8 (6.25–11.5) days [67]. Therefore, we can speculate that using clinical improvement as a marker of the efficacy of NTZ is inaccurate, especially when used in conjunction with symptomatic and supportive treatment [28]. However, Rocco et al. [29] reported a 1-day faster symptom resolution with NTZ compared with placebo, which can be attributed to the previous anti-inflammatory effects [29].
Accordingly, the magnitude of the effect of NTZ in managing COVID-19 is affected by the population being studied. To clarify, five out of six trials included only mild to moderate cases [27, 28, 30–32], with only Rocco et al. [29] including hospitalized patients with pneumonia. Additionally, despite the significant effect of viral load, the effects on substantial clinical data, such as mortality and ICU admission, were insignificant. Thus, our findings should be interpreted with caution, especially in the management of severe cases of pneumonia.
Regarding the all-cause mortality rates, we detected no difference between NTZ and placebo. In the RCT conducted by Rossignol et al. [31], two participants in the NTZ arm died. One because of severe COVID-19 infection and the other, who tested negative for SARS-CoV-2, because of secondary aspiration 19 days after completing the treatment. Both events were not tracked back to the study medication [31]. Rocco et al. [29] reported no difference by day 14 in the number of deaths between the NTZ group (six deaths) and the placebo group (five deaths). Blum et al. [27] reported a total of eight deaths; two in the NTZ group and six in the placebo group, all because of acute respiratory distress syndrome. The difference between the two groups was not statistically significant; however, they argued that this difference is clinically relevant, and a difference might be detected with a larger sample size [27]. Silva et al. [32] reported two deaths, one in each group. Both were aged older than 65 years and had other comorbidities [32]. The other two RCTs did not report any mortalities [28, 30] as they only included mild cases, as previously clarified.
Regarding the ICU admission, we detected no difference between NTZ and placebo. Rocco et al. [29] detected no difference between the NTZ and placebo group regarding ICU admission. However, he also found that participants who presented with oxygen saturation >90% on day 1 and were treated with NTZ had a lower odds of ICU admission compared with placebo [29]. Similarly, adding corticosteroids to NTZ decreased the odds of ICU admission compared with corticosteroids alone in the placebo group [29]. No ICU admission was reported in the rest of the included studies [28, 31, 32].
Oxygen requirements for treating the NTZ group were less than the placebo group. However, this effect showed a decreasing pattern with longer follow-ups, according to our subgroup analysis. Supporting our findings, Rocco et al. [29] found that NTZ reduced oxygen requirements of any type compared with placebo only from day 3 to day 7 [29]. He also found that the time on supplemental oxygen was reduced by a median of 2 days compared with placebo [29]. Blum et al. [27] reported a lower time to withdraw from oxygen supplementation in the NTZ group compared with the placebo group (3 vs 8 days, respectively). This effect is important because reducing the necessity of supplementary oxygen subsequently reduces the load on the healthcare system and perhaps enhances hospital capacity [29].
Regarding safety, there was no difference between the NTZ group and the placebo group in the incidence of at least one adverse event. Among the reported adverse events, only the incidence of vomiting was significantly associated with the NTZ compared with placebo. No severe adverse events associated with NTZ were reported in any of the included studies. The US Food and Drug Administration-approved dose of NTZ in treating parasite infection is 500 mg twice daily (BID). Different doses of NTZ have been suggested for their efficacy and safety against SARS-CoV-2 infection [68, 69]. Moreover, NTZ has a short half-life, thus its main action is achieved through its active metabolite, tizoxanide, which has a relatively long half-life. Maximum serum concentration (Cmax) and the time to reach Cmax determine the bioavailability (area under the curve) of NTZ and tizoxanide. Maximum serum concentration and time to reach the Cmax are affected by the formulation of the NTZ where the suspension form is 41% less bioavailable than tablets [70]. Furthermore, food affects the absorption and bioavailability of NTZ; when NTZ is taken with food its Cmax, T time to reach the Cmax, and area under the curve increase. The dosing interval also determines the area under the curve and concentration needed to inhibit 90% (IC90) of SARS-CoV-2 [70]. To clarify, with food, a plasma concentration of more than IC90 was expected most of the time and IC50 almost all the time by using NTZ 500-mg tablets every 6 h. However, with fasting, the same dose achieved only IC50. A less frequent dose of 500-mg tablets every 8 hours also achieved plasma concentrations more than IC50 with food [70]. Therefore, the variability noticed among the included studies regarding the formulation and associated food administration can affect our findings.
Rajoli et al. [69] reported a physiologically based pharmacokinetic model about the optimal doses of NTZ that provide plasma and lung concentrations above its reported in vitro 90% effective concentration against SARS-CoV-2 (4.64 μM or 1.43 μg/mL) [69]. Ninety percent effective concentration was achieved when given in the fasting state with the doses of 1200 mg four times a day (QID), 1600 mg three times daily (TID), or 2900 mg BID. While with food, the needed doses were 700 mg QID, 900 mg TID, or 1400 mg BID [69]. Nitazoxanide was also reported to be safe up to 4 g/day [68]; however, most of the RCTs investigating its efficacy and safety in COVID-19 infection have been using doses of less than 2 g daily [27–32]. Haffizulla et al. [25] investigated a higher dose of 600 mg BID to achieve antiviral activities against influenza without reporting safety issues [25]. In the same line, Blum et al. [27] and Rossignol et al. [31] investigated the same dose of 600 mg BID in cases of mild and moderate COVID-19 infection without safety issues as well [27, 31]. Rocco et al. used a higher dose of 500 mg TID in both mild and hospitalized cases of COVID-19 infection with no safety issues [29, 30]. Silva et al. [32] used a higher dose of 1 g TID in mild and moderate COVID-19 infection; however, they changed it sooner to 500 mg QID as the first two participants did not tolerate the first dose (3 g/day), but the latter dose (2 g/day) was well tolerated [32]. Medhat et al. [28] used a similar dose of 500 mg QID in mild and moderate COVID-19 cases without safety issues as well [28]. In the AGILE trial [71], a higher dose of NTZ (1500 mg BID) for 7 days was investigated in healthy volunteers to determine the optimal dose, safety, and efficacy of NTZ in preventing COVID-19 infection [71]. Only self-limited to moderate gastrointestinal disturbance, urine, and scleral discoloration were reported. No severe adverse events were reported [71]. Therefore, with the above evidence about the least effective doses of NTZ being 500 mg orally TID to achieve at least IC50 against SARS-CoV2, the optimal dosing regimen and formulation are still being investigated.
4.1 Strengths
To the best of our knowledge, this is the first systematic review and meta-analysis of the effectiveness of NTZ in the treatment of COVID-19 infection, constituting the most robust evidence in this regard. Moreover, we strictly followed the PRISMA statement [33] and the Cochrane Handbook for Systematic Reviews and Interventions [34] and prospectively registered and published our protocol. Moreover, the quality of evidence was assessed using the GRADE recommendations.
4.2 Limitations
Our review has a few limitations: first, we only included six RCTs with limited demographic distribution; three studies in south America [29, 30, 32], one in North Africa [28], and another in the USA [31]. Second, we could not control multiple confounding variables, including baseline viral load and comorbidities. Third, all of the included RCTs recruited patients with mild to moderate disease except Rocco et al. [29], who included patients with COVID-19 pneumonia requiring hospitalization. Fourth, the NTZ treatment regimen, including dosage, formulation, administration times, and duration of treatment varied among the included RCTs. Fifth, none of the included studies assessed the effect of NTZ against the different variants of COVID-19. Sixth, all of the included trials have a high risk of bias in different domains, as we previously clarified. Seventh, we could not conduct a dose-response meta-analysis based on the included data as we only included six RCTs with three different dosing regimens. Finally, we detected significant heterogeneity regarding the viral clearance, and the GRADE assessment yielded very low-quality evidence for all the included outcomes; thus, the generalizability of our findings is limited.
4.3 Implications for Future Research
Despite the globally available vaccination protocols, widespread vaccination will require a long period to adequately prevent further infection transmission. Therefore, a safe, well-tolerated, and easy-to-administer antiviral agent is required for mild to moderate COVID-19 treatment [31]. Nitazoxanide looks promising in this regard; however, further research is still required to ascertain the following: first, the most effective dosage regimen is still to be investigated with various regimens used in the previous trials. In this regard, we support Blum et al. [27] that given the lack of information about the most clinically applicable dosage, conducting a pharmacokinetic study is important. Second, more work is needed to evaluate the effect of NTZ in combination with other antiviral agents to prevent the evolution of NTZ-resistant strains on the wide implementation of a single NTZ treatment regimen. Additionally, the NTZ viral evasion in a high viral load is yet to be evaluated. Third, although multiple immunological effects of NTZ have been clarified, more work is still required to evaluate the effect of NTZ on monocytes and interferons (IFN-α and IFN-β), given their important role in COVID-19 pathogenesis [72–74]. Finally, more phase III, multi-center, large-scale clinical trials are still required to ascertain the effects of NTZ in COVID-19.
5 Conclusions
Despite the efficacy of NTZ in accelerating viral clearance compared with placebo, evidence regarding the efficacy of NTZ in improving clinical resolution, reducing all-cause mortality, reducing ICU admission, and oxygen requirements is uncertain. This warrants more large-scale clinical trials to yield more generalizable and clinically applicable findings.
References
Valencia DN. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12: e7386.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323:1239–42.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Rello J, Storti E, Belliato M, Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55(5):2001028.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708.
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'cytokine storm’ in COVID-19. J Infect. 2020;80:607–13.
Zhang L, Hou J, Ma F-Z, Li J, Xue S, Xu Z-G. The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis. Arch Virol. 2021;166:2071–87.
Liu W, Yang C, Liao Y-G, Wan F, Lin L, Huang X, et al. Risk factors for COVID-19 progression and mortality in hospitalized patients without pre-existing comorbidities. J Infect Public Health. 2022;15:13–20.
Lin L, Liu Y, Tang X, He D. The disease severity and clinical outcomes of the SARS-CoV-2 variants of concern. Front Public Health. 2021;9: 775224.
Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201:1435–8.
Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369: m1443.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20.
Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375: n2713.
McCullough PA, Alexander PE, Armstrong R, Arvinte C, Bain AF, Bartlett RP, et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev Cardiovasc Med. 2020;21:517–30.
Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA. Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med. 2020;21:611–4.
Kim PS, Read SW, Fauci AS. Therapy for early COVID-19: a critical need. J Am Med Assoc. 2020;324:2149–50.
Hong SK, Kim HJ, Song CS, Choi IS, Lee JB, Park SY. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int Immunopharmacol. 2012;13:23–7.
Shou J, Kong X, Wang X, Tang Y, Wang C, Wang M, et al. Tizoxanide inhibits inflammation in LPS-activated RAW264.7 macrophages via the suppression of NF-κB and MAPK activation. Inflammation. 2019;42:1336–49.
Rossignol J-F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103.
Rossignol J-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227–30.
Rossignol J-F, Bardin MC, Fulgencio J, Mogelnicki D, Bréchot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine. 2022;45: 101310.
Ashiru O, Howe JD, Butters TD. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores. Virology. 2014;462–463:135–48.
Belardo G, Cenciarelli O, La Frazia S, Rossignol JF, Santoro MG. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrob Agents Chemother. 2015;59:1061–9.
Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–18.
Jasenosky LD, Cadena C, Mire CE, Borisevich V, Haridas V, Ranjbar S, et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–90.
Blum VF, Cimerman S, Hunter JR, Tierno P, Lacerda A, Soeiro A, et al. Nitazoxanide superiority to placebo to treat moderate COVID-19: a pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine. 2021;37: 100981.
Medhat MA, El-Kassas M, Karam-Allah H, Al Shafie A, Abd-Elsalam S, Moustafa E, et al. Sofosbuvir/ledipasvir in combination or nitazoxanide alone are safe and efficient treatments for COVID-19 infection: a randomized controlled trial for repurposing antivirals. Arab J Gastroenterol. 2022;23(3):165–71.
Rocco PRM, Silva PL, Cruz FF, Tierno PFGMM, Rabello E, Junior JC, et al. Nitazoxanide in patients hospitalized with COVID-19 pneumonia: a multicentre, randomized, double-blind, placebo-controlled trial. Front Med. 2022;9:1–13.
Rocco PRM, Silva PL, Cruz FF, Melo MAC, Tierno PFGMM, Moura MA, et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021;58(1):2003725.
Rossignol JF, Bardin MC, Fulgencio J, Mogelnicki D, Bréchot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. eClinicalMedicine. 2022;45:1–10.
Silva AM, Espejo A, Pereyra ML, Lynch M, Thompson M. Efficacy of nitazoxanide in reducing the viral load in COVID-19. 2021; p. 1–17.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions. 2nd Ed. Chichester (UK): John Wiley & Sons; 2019.
Covidence systematic review software. Melbourne, Australia; http://www.covidence.org/. Accessed 19 Oct 2022.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–8.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
RevMan | Cochrane Training. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 3 Aug 2021.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284:29798–808.
Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9.
Hickson SE, Margineantu D, Hockenbery DM, Simon JA, Geballe AP. Inhibition of vaccinia virus replication by nitazoxanide. Virology. 2018;518:398–405.
Attallah NGM, El-Kadem AH, Negm WA, Elekhnawy E, El-Masry TA, Elmongy EI, et al. Promising antiviral activity of Agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study. Pharmaceuticals (Basel). 2021;14(12):1313.
Zhou H, Yang J, Zhou C, Chen B, Fang H, Chen S, Zhang X, Wang L, Zhang L. A review of SARS-CoV2: compared with SARS-CoV and MERS-CoV. Front Med (Lausanne). 2021;7(8): 628370.
Al-kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GES. Nitazoxanide and COVID-19: a review. Mol Biol Rep. 2022;49:11169–76. https://doi.org/10.1007/s11033-022-07822-2.
Dang W, Xu L, Ma B, Chen S, Yin Y, Chang KO, et al. Nitazoxanide inhibits human norovirus replication and synergizes with ribavirin by activation of cellular antiviral response. Antimicrob Agents Chemother. 2018;62(11):e00707-e718.
M Al-kuraishy H. Brain and peripheral neuronal injury in Covid-19: the panorama and dispute. Appl Med Res. 2021;8:1–3. https://doi.org/10.5455/amr.20211025.
Nguyen HT, Zhang S, Wang Q, Anang S, Wang J, Ding H, et al. Spike glycoprotein and host cell determinants of SARS-CoV-2 entry and cytopathic effects. J Virol. 2020;95(5):e02304-e2320.
Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. 2020;11:1518.
Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.
Darif D, Hammi I, Kihel A, El Idrissi SI, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153: 104799.
Elalfy H, Besheer T, El-Mesery A, El-Gilany AH, Soliman MAA, Alhawarey A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021;93:3176–83.
Bello-Perez M, Sola I, Novoa B, Klionsky DJ, Falco A. Canonical and noncanonical autophagy as potential targets for COVID-19. Cells. 2020;9(7):1619.
Pietrocola F, Bravo-San Pedro JM. Targeting autophagy to counteract obesity-associated oxidative stress. Antioxidants. 2021;10:1–14.
Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46:846–8.
Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19(1):18.
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–30.
Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–43.
Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258: 118167.
Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–9.
Kelleni MT. NSAIDs/nitazoxanide/azithromycin repurposed for COVID-19: potential mitigation of the cytokine storm interleukin-6 amplifier via immunomodulatory effects. Expert Rev Anti Infect Ther. 2022;20:17–21.
Naveca F, Nascimento V, Souza V, Corado A, Nascimento F, Silva G, et al. COVID-19 epidemic in the Brazilian state of Amazonas was driven by long-term persistence of endemic SARS-CoV-2 lineages and the recent emergence of the new variant of concern P.1. Res Sq. 2021;27:1–21. https://doi.org/10.21203/rs.3.rs-275494/v1. Accessed 26 Oct 2022.
Risner KH, Tieu KV, Wang Y, Bakovic A, Alem F, Bhalla N, et al. Maraviroc inhibits SARS-CoV-2 multiplication and s-protein mediated cell fusion in cell culture. bioRxiv [Preprint]. 2020. https://doi.org/10.1101/2020.08.12.246389.
Lian E, McAlister C, Ramirez G, Chernoff DN, Went G, Hoopes J, et al. Triple combination nitazoxanide, ribavirin, and hydroxychloroquine results in the multiplicative reduction of in vitro SARS-CoV-2 viral replication. bioRxiv. 2020;2020.11.25.399055. http://biorxiv.org/content/early/2020/11/26/2020.11.25.399055.abstract. Accessed 10 Oct 2022.
Bobrowski T, Chen L, Eastman RT, Itkin Z, Shinn P, Chen CZ, et al. Synergistic and antagonistic drug combinations against SARS-CoV-2. Mol Ther. 2021;29:873–85.
Chang D, Mo G, Yuan X, Tao Y, Peng X, Wang FS, et al. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am J Respir Crit Care Med. 2020;201:1150–2.
Lokhande AS, Devarajan PV. A review on possible mechanistic insights of nitazoxanide for repurposing in COVID-19. Eur J Pharmacol. 2021;891: 173748.
Rajoli RKR, Pertinez H, Arshad U, Box H, Tatham L, Curley P, et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br J Clin Pharmacol. 2021;87:2078–88.
Padmanabhan S, Padmanabhan K. The "devil is in the dosing": targeting the interferon pathway by repositioning Nitazoxanide against COVID-19. https://www.researchgate.net/profile/Srivatsan-Padmanabhan/publication/340902283_The_devil_is_in_the_dosing_-_targeting_the_interferon_pathway_by_repositioning_Nitazoxanide_against_COVID-19/links/602b2218299bf1cc26cb6617/The-devil-is-in-the-dosing-targeting-the-interferon-pathway-by-repositioning-Nitazoxanide-against-COVID-19.pdf. Accessed 10 Oct 2022.
Walker LE, FitzGerald R, Saunders G, Lyon R, Fisher M, Martin K, et al. An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2. Clin Pharmacol Ther. 2022;111:585–94.
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis. 2020;71:762–8.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.
Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2021;109:13–22.
Acknowledgments
We sincerely acknowledge Dr. Mostafa Eltobgy’s role in reviewing the screening process and confirming the included and excluded records.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests
Mohamed Abuelazm, Ahmed Ghanem, Ahmed K. Awad, Ramadan Abdelmoez Farahat, Fatma Labieb, Basant E. Katamesh, and Basel Abdelazeem have no conflicts of interest that are directly relevant to the content of this article.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data are available upon request from the corresponding author.
Code Availability
Not applicable.
Authors’ Contributions
MA conceived the idea. BA and MA designed the research workflow. BA and MA searched the databases. FL, RF, and BK screened the retrieved records, and MA resolved the conflicts. AK, FL, RF, and BK extracted relevant data, assessed the quality of evidence, and MA resolved the conflicts. MA and BA performed the analysis. MA and AG wrote the final manuscript. All authors have read and agreed to the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abuelazm, M., Ghanem, A., Awad, A.K. et al. The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig 42, 1031–1047 (2022). https://doi.org/10.1007/s40261-022-01213-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01213-y