Abstract
Background
Non-vitamin K antagonist oral anticoagulants (NOACs) are the preferred choice of anticoagulants to prevent stroke in most patients with atrial fibrillation (AF). NOAC's dosing algorithms are defined in the respective Summary of Product Characteristics (SmPC) but the European Heart Rhythm Association (EHRA) Practical Guide can also be used as it considers more complex clinical scenarios. Nevertheless, suboptimal dosing of NOACs compromises the efficacy and safety of this commonly prescribed therapy in the AF population. Clearer objectification of inappropriate dosing and its influencing factors is needed to optimise management of AF patients.
Objectives
The primary aim of this study was to investigate whether there is a difference in the perceived appropriateness of NOAC dosing with respect to the SmPC or the 2018 EHRA Practical Guide in AF patients criteria and influencing factors. The secondary aim was to explore if there were differences in appropriateness of NOAC dosing between primary care and specialist care, and when using different renal function formulas.
Methods
This retrospective study included AF patients treated with a NOAC in primary or in ambulatory specialist care in Antwerp (Belgium). Appropriateness of the NOAC dose was assessed according to the SmPC and 2018 EHRA recommendations. Univariate/multivariate analyses were performed to explore influencing factors for under- and overdosing of NOACs.
Results
Of the included 294 AF patients, 19.4% and 15.6% received an inappropriate dose according to the SmPC and the 2018 EHRA Practical Guide respectively (p = 0.003). Perceived frailty and higher weight were associated with underdosing relative to the SmPC, while a higher body mass index and the use of drugs/alcohol were associated with underdosing relative to the EHRA 2018 recommendations. Lower renal function and treatment with other NOACs than apixaban were associated with relative overdosing compared to both standards.
Conclusions
Inappropriate NOAC dosing is present in almost twenty percent of AF patients according to the SmPC and requires further education of health care professionals and frequent reassessment of NOAC dosing. However, a significant lower prevalence of underdosing was present when judged by the 2018 EHRA criteria, likely reflecting decision making in complex AF patients. Perceived frailty, weight, renal function and type of NOAC are the main determinants of deviated dosing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A significant proportion of non-vitamin K antagonist oral anticoagulants are inappropriately dosed, which compromises its efficacy in stroke prevention in patients with atrial fibrillation. |
Dosing of these oral anticoagulants can be based on different dosing recommendations. |
Insights into deviating dosing decisions can improve real-life stroke prevention, a cornerstone of atrial fibrillation management. |
1 Introduction
Non-vitamin K antagonist oral anticoagulants (NOACs) are now the standard of care for stroke prevention worldwide in patients with atrial fibrillation (AF) with high thrombo-embolic risk and in the absence of mechanical prosthetic heart valves or moderate/severe mitral stenosis, or severely depressed renal function [1, 2]. Currently, four NOACs are available in Europe, each with specific dose reduction criteria defined in their respective ‘Summary of Product Characteristics (SmPC) documents’. These criteria include age, renal function, weight and specific concomitant intake of medication. However, in daily practice, several less researched relevant aspects can influence the decision of clinicians to prescribe a different dose than recommended by the SmPC. This is why the European Heart Rhythm Association (EHRA) has developed sequentially updated practical guides for healthcare professionals concerning the use of NOACs in AF patients incorporating the SmPC criteria and important patient characteristics (e.g., frailty, concomitant use of antiplatelets) to provide support and scientific evidence concerning the dosing and use of NOACs [3,4,5,6]. Nevertheless, real-world studies have shown that a significant portion of AF patients treated with a NOAC receive inappropriate NOAC doses. Underdosing can lead to a higher risk of stroke, and overdosing can impair safety outcomes of these oral anticoagulants [7].
2 Aims
The primary aim of this retrospective study was to investigate whether there is a difference in the perceived appropriateness of NOAC dosing with respect to the SmPC or the 2018 EHRA Practical Guide in AF patients presenting at an outpatient visit at the cardiology department of the Antwerp University Hospital or at six primary care centres (all located in the Antwerp region).
The secondary aims were (i) to explore if there was a significant difference in appropriateness of NOAC dosing between primary care and specialist care, and (ii) when renal function was calculated according to different formulas.
3 Ethics Approval
The research protocol was approved by the Ethics Committees of the Antwerp University Hospital/University of Antwerp on the 12th of August 2019 and the study was conducted in compliance with the Declaration of Helsinki (local project reference 19/27/331).
4 Methods
4.1 Study Population and Enrolment Procedure
Patients were eligible for this study if they were (i) aged ≥ 18 years, (ii) diagnosed with AF or atrial flutter on an electrocardiogram and (iii) chronically treated with one of the four NOACs, namely apixaban, rivaroxaban, edoxaban or dabigatran. The indication of whether a patient should be treated with a NOAC was checked based on his/her CHA2DS2-VASc score. For the eligible patients of the Antwerp University Hospital, no explicit informed consent (IC) was needed as data were internally available and retrospectively retrieved by the study investigators; all patients of the hospital consented with inclusion in retrospective analyses. Enrolment of AF patients at the primary care centres was done consecutively by the general practitioner (GP), who explained the study to the patient and obtained the IC. Atrial fibrillation patients already enrolled in an interventional NOAC study or patients who were unable to sign the IC (i.e., language barrier) were excluded.
4.2 Data Collection
After approval of the research protocol, patients who had presented to any of the outpatient clinics after April 2018 (the date of publication of the EHRA 2018 Practical Guide) were retrospectively screened for inclusion. The inclusions were performed consecutively and equally spread over four cardiology subspeciality clinics (Interventional, Electrophysiology, Heart Failure and General Cardiology/Cardiac Imaging) to ensure a homogeneous AF cohort in follow-up by cardiologists. If a patient was found to have multiple visits, only the first clinic visit was assessed.
As mentioned previously, primary care patients were enrolled only after consent had been given. Recruitment was performed in AF patients presenting after approval of the research protocol (August 2019). Then, the medical file was reviewed retrospectively and the patients’ first GP visit after April 2018 was assessed for data extraction in order to have similar time periods evaluated in cardiology and GP patients.
Patients’ medical data were retrieved from the electronic patient record and included age, sex, actual body weight, height, body mass index (BMI), blood pressure, type of AF, prescribed NOAC and dose, concomitant medication and serum creatinine closest to the index consultation. Patients’ medical history was checked for components of the CHA2DS2-VASc and HAS-BLED scores. Moreover, a history of gastrointestinal bleeding, bleeding predisposition, recent surgery on a critical organ and available data to estimate frailty, based on the parameters used in the ENGAGE-AF TIMI 48 trial, were recorded as these factors also play a role in the 2018 EHRA Practical Guide [8].
Based on the collected data, calculation of renal function was estimated using the Cockcroft and Gault-(CG), the Modification of Diet in Renal Disease- (MDRD) and the Chronic Kidney Disease Epidemiology Collaboration- (CKD-EPI) equations [9,10,11].
Non-vitamin K antagonist oral anticoagulants dosing was evaluated by comparing the actual prescribed dose with the recommendations from the SmPC (Supplementary Table 1) and EHRA 2018 Practical Guide [12,13,14,15]. Classification was either appropriate or inappropriate in case of underdosing or overdosing. The EHRA 2018 Practical Guide incorporates additional clinical parameters that may justify dose adjustments and also includes an extensive list of interacting drugs that are not all included in the SmPCs (e.g., extended list of interactions with anticancer and antiepileptic drugs) [5]. This guide also uses a colour code with one important guide rule that recommends consideration of dose adjustment or the use of a different NOAC with fewer interactions (if available) in the presence of ≥ 2 ‘yellow’ criteria. Consequently, the EHRA 2018 guide is less stringent in case of a combination of ‘yellow’ criteria, which the SmPC dose adjustment criteria do not consider. For example, a 77-year-old patient with concomitant use of antiplatelets and a standard dose NOAC was classified as ‘appropriate’ for both classification systems (‘75+’ and ‘concomitant antiplatelet drugs’ are both yellow factors). The same patient on a reduced NOAC dose would be classified as ‘inappropriate’ according to the SmPC, but potentially ‘appropriate’ according to the EHRA 2018 Practical Guide.
Other principal colour codes include: ‘Orange’ = consider dose adjustment or different NOAC; ‘Red’ = contraindicated/not recommended; ‘Brown (dark)’ = contraindicated due to reduced NOAC plasma concentrations.
4.3 Sample Size
For the primary objective, a sample size of 152 AF patients in each arm was calculated using an alpha of 0.05 and a power of 0.80. This was based on a 10.4% difference in NOAC dosing appropriateness derived from a retrospective cohort study that investigated the correct prescription of NOACs in hospitalised patients comparing the SmPC prescription rules and the 2015 EHRA guide [16]. For the second objective of specialist care versus primary care, a sample size of 171 AF patients in each arm was calculated (alpha = 0.05 and power = 0.80), based on a substudy of the ORBIT AF-II registry, which reported data of incorrect NOAC dosing by different medical specialities (based on US approved package inserts) [17]. Combining these two sample size calculations, and anticipating 15% of incomplete patient files, an inclusion target of 197 AF patients, for both specialist and primary care was set forward (in total 394 patients). However, due to the COVID-19 pandemic, we did not reach the target inclusion rate in the GP cohort due to the severe impact on consenting procedures.
4.4 Statistics
Data were analysed using IBM SPSS version 27.0. Variables were described as numbers and percentages or as mean ± standard deviation, as appropriate. For continuous variables, differences between two (un)paired groups were compared using the paired-samples t test or independent-samples t test. The chi-squared test, the McNemar test and Fisher’s exact test were used for categorical variables, as appropriate. All comparisons were tested two-sided. P-values < 0.05 were considered statistically significant.
The relative risks (RRs) and odds ratios (ORs) were calculated and reported with their 95% confidence intervals (CIs) for significant categorical predictors for inappropriate dosing of NOACs (i.e., under- and overdosing). For continuous variables, univariate logistic regression models were used to calculate the ORs (with their 95% CI), and p-values were derived from the likelihood-ratio test. Candidate variables, categorical as well as continuous, with a p-value < 0.10 were considered for multivariate regression analysis and the optimal regression model was composed using a backward elimination strategy.
5 Results
5.1 Patient Characteristics
A total of 294 AF patients were included in this study, of which 200 (68.0%) patients were recruited at the cardiology outpatient clinic and only 94 patients (32.0%) at the GPs’ office (between September 2019 and February 2020) (Fig. 1).
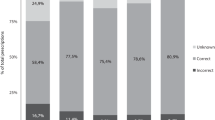
Table 1 presents the baseline characteristics of the included AF population. Mean serum creatinine was 1.09 ± 0.39 mg/dL for which the estimated renal functions calculated by the CG, MDRD and CKD-EPI formulae were 70.3 ± 28.8 mL/min, 71.2 ± 23.4 mL/min/1.73 m2 and 65.5 ± 20.3 mL/min/1.73 m2, respectively. Apixaban was the most commonly prescribed NOAC (41.5%) followed by rivaroxaban (34.4%), edoxaban (13.6%) and dabigatran (10.5%) with a reduced dose in 26.2%, 21.8%, 15.0% and 41.9% for each NOAC, respectively (p = 0.066; Table 2).
When comparing patients included in primary care versus specialist care, AF patients followed by GPs were older (78.6 ± 7.3 years). Consequently, they had a lower renal function calculated by the CG formula (65.1 ± 26.1 mL/min). These patients were also less known to have congestive heart failure (24.5% vs 37.5%) and took fewer antiplatelet drugs (3.2% vs 15.5%) (Table 1).
5.2 Appropriateness of NOAC Dosing
In general, according to the SmPC and EHRA 2018 guide, a rather high proportion of patients received an inappropriate dosage of NOAC, in 19.4% and 15.6% of patients (p = 0.003), respectively (Table 2). The significant difference was driven by a more lenient interpretation of potentially correctly underdosed NOACs by the EHRA 2018 (4.0%, p = 0.003). Translated in absolute numbers, of the 31 underdosed SmPC patients, 12 patients (38.7%) received a potentially correct NOAC dose according to the EHRA 2018 guide. These patients were more often classified as frail (RR = 5.46; 95% CI 1.85–16.06; p < 0.001) and more often used amiodarone (RR = 2.98; 95% CI 1.44–6.14; p = 0.022). Similar results were observed in overdosed patients when classified according to SmPC or EHRA 2018 (8.8%; n = 26).
Figure 2 shows dosing appropriateness per NOAC according to the SmPC guidelines and the 2018 EHRA Practical Guide.
5.3 Influencing Factors for Under- and Overdosing of NOACs
Inappropriate NOAC underdosing according to the SmPCs was univariately significantly related to the use of diuretics and to weight (or BMI) (all p < 0.05; Table 3), with borderline relations with perceived frailty and drug or alcohol use. In multivariate analysis, frailty and higher body weight were the only significant factors. Based on the EHRA 2018 Practical Guide only the use of drugs/alcohol and a higher BMI were correlated with an inappropriate reduced dose in both univariate and multivariate analysis (Table 3).
For overdosed NOAC patients (both according to the SmPC and EHRA 2018 guide as identified patients were identical), primary care, permanent AF, older patients, not taking apixaban, lower weight (or lower BMI) and lower renal function were factors significantly correlated with a higher risk for overdosing (univariate analysis). In multivariate analysis, patients not on apixaban and with lower renal function were associated with inappropriate overdosing of their NOAC (Table 4).
5.4 Primary Care Versus Specialist Care
Although the number of recruited patients in GP care was too low (caused by COVID-19 circumstances—see above), GP care versus cardiologist care was not retained in any multivariate analysis of factors related to underdosing or overdosing (Tables 3 and 4). Nevertheless, patients in GP care showed a higher rate of inappropriate dosing compared to cardiologists, which was non-significant based on the SmPCs (24.5% vs 17.0%; p = 0.131) but significant based on the EHRA 2018 guide (22.3% vs 12.5%; p = 0.03). This seems to be mainly the result of inappropriate overdosing (Table 4; p = 0.039 univariate p-value), which could be an indication that cardiologists consider more factors to reduce dose.
5.5 Influence of Different Renal Function Estimation Formulae
When comparing appropriateness of dosing based on renal function calculated by CG-, MDRD-, or CKD-EPI formulae, no significant differences were seen between these formulas, for either the SmPC- or for the EHRA 2018-based evaluation (Supplementary Table 2). On the other hand, the significant difference between the SmPC and EHRA 2018 Practical Guide as described with the CG formula in section 5.2 (p = 0.003) remained significant when reclassifying appropriateness using the MDRD and CKD-EPI formulae (with p-values of < 0.001 and 0.002, respectively) (Supplementary Table 3).
6 Discussion
This study in ambulatory AF patients found a high prevalence of inappropriate NOAC dosing (19.4%) according to the SmPC. When based on the EHRA 2018 Practical Guide, the proportion is significantly lower (15.6%) but still, 1 out of 7 AF patients, seem to be receiving an inappropriate dose of NOAC. The explanation,i.e. whether prescribers are incorrect, or whether prescribers have good reasons beyond the guidance to adapt the dose, remains a topic of study. We identified several factors associated with inappropriate NOAC dosing. Of note, reclassification of NOAC appropriateness based on the MDRD and CKD-EPI renal function estimation formulae (which are more readily available to clinicians than to the CG calculation) did not explain the difference in the classification of dosing.
6.1 Prevalence of NOAC Misdosing
As AF prevalence is expected to increase in the upcoming decades, optimal treatment of these patients is necessary to minimise AF complications and reduce the health burden, for both patients and for healthcare systems. A cornerstone of AF management is the prevention of stroke, for which NOAC treatment is the first-choice therapy [18,19,20,21]. Besides identifying and treating AF patients with a high risk of stroke, correct NOAC dosing is also of primordial importance to ensure efficacy and safety.
The range of ambulatory AF patients treated with an inappropriate dosage of NOACs in our study is in line with other large international investigations ranging between 12.8–31.1% [17, 22,23,24]. Two smaller Belgian studies by other centres in our country reported off-label dosing in 25.0% and 18.3% [16, 25]. Remarkably, in the aforementioned studies overdosing ranged between 3.4–7.8%; whereas in our study overdosing was slightly more prevalent in 8.8% of patients.
When applying the EHRA 2018 Practical Guide, an expected (but significant) decline of inappropriate dosing was found (− 3.8%) compared with the SmPC. This was driven by more lenient acceptance of reduced NOAC doses as potentially appropriate (from 10.5 to 6.8%). Moudallel et al reported NOAC underdosing in 17.4 versus 7.0% of patients according to the SmPC and EHRA 2015 Practical Guide, respectively, and is in line with our results regarding NOAC underdosing (6.1% were overdosed according to the SmPC but no data were reported concerning overdosing according to the EHRA 2015 guide) [16]. Two other European studies also evaluated NOAC dosing appropriateness according to the EHRA 2015 guide, but interpreted the presence of ≥ 2 ‘yellow’ interactions as an indication for a reduced dose. A retrospective subanalysis of the FANTASIIA Registry (a Spanish prospective, observational, multicentre study including adults with AF on anticoagulant evaluating the incidence of thrombo-embolic and bleeding events) found inappropriate doses in 32% of AF patients. More specifically, 15% were inappropriately overdosed and 17% were inappropriately underdosed (off-label dosing according to SmPC criteria was not reported) [26]. Capiau et al found an increase of inappropriate dosing from 18.3 to 23.4% according to the SmPC and EHRA 2015 guide, respectively, (for both systems 0.8% of NOACs were contra-indicated) [25]. Of the SmPC underdosed patients (9.8%), 21.9% were correctly dosed when classified by the 2015 EHRA guide, resulting in 7.6% underdosed patients. Therefore, the global increase of dose inappropriateness was related to the increase of overdosed patients according to the EHRA 2015 guide (from 7.8 to 15.0%). Of note, since this interpretation of “≥ 2 yellow factors” is suggested as a possibility in the EHRA Practical Guide, we considered a standard dose as a reduced dose ‘appropriate’ in such cases, which explains the overall lower prevalence of inappropriateness in our study.
Since the EHRA Practical Guide considers more factors with relevance for dosing, we anticipated that incorrect dosing would be less when judged by the Practical Guide than by the SmPC. It shows that in daily life, a large proportion of AF patients have a complex presentation. Nevertheless, even when evaluated by the EHRA Practical Guide, standard inappropriate dosing is prevalent. This could be explained in two ways. One is that physicians correctly take more clinical factors into consideration and hence, both the SmPC and EHRA PG still fall short of guide clinical practice. However, prior retrospective and observational data have clearly shown that dosing that deviates from recommendations is associated with increased risk of adverse events and even mortality [7, 27]. Therefore, the second explanation is that physicians still are falling short of making correct dosing decisions, which calls for more physician education to improve patient outcomes. This education could focus more specifically on some of the factors that our research has shown to be related with prescription errors. It also calls for better patient tailored (transmural) follow-up with frequent reassessment of NOAC dose to improve results.
6.2 Contributing Factors for Under- and Overdosing of NOACs
Prior studies have identified various univariate factors related to inappropriately reduced dosing, such as age, CHA2DS2-VASc score < 4, sex (female), ethnicity (non-Caucasian), acute coronary syndrome, vascular disease, prior stroke, diabetes and concomitant antiplatelet therapy [23, 24]. The FANTASIIA Registry found that the factors ‘younger age’ and ‘dabigatran use’ were also associated with inappropriately low NOAC dosing [26]. Our study, also retained the (univariate) association of drugs (i.e., antiplatelets or NSAID) and alcohol with underdosing. Of note, alcohol is a factor in the HAS-BLED score and not in the SmPC or EHRA Guide, and is a modifiable bleeding risk factor that should be addressed rather than lead to an adaption of the NOAC dose.
Based on the SmPC multivariate analysis, the factors ‘higher body weight’ and ‘frailty’ were associated with off-label underdosing.
For overweight or obese patients, this is a paradoxical finding: although higher weight is associated with both a higher volume of distribution and higher renal clearance, no specific (i.e., increased) NOAC dosing algorithm currently exists. At least the standard NOAC dose would be expected. This suggests that other factors that are not even part of the SmPC or EHRA Guide led physicians to paradoxically reduce the dose. One could postulate that some conditions for dose reduction are more prevalent in overweight patients (e.g., vascular disease for which antiplatelets are indicated), but our analysis could not identify such an explanation. This paradoxical finding certainly requires confirmation and further study.
Frailty is included as a ‘yellow’ parameters in the EHRA 2018 Practical Guide, and hence, in combination with other yellow factors, can justify an appropriately reduced dose according to this system.
Regarding factors related to NOAC overdosing, our study identified lower renal function and AF patients who were not treated with apixaban. This can be explained by the fact that prescription of a reduced dose of apixaban depends on the presence of a minimum of two out of three criteria (see Supplementary Table 1), which decrease the probability for an overdose. Renal function is a well-known risk factor as all NOACs are renally excreted and three of the four NOACs have absolute SmPC dosing reduction criteria depending on renal function [22].
Noteworthy, when reviewing the patients taking a NOAC concomitant with antiepileptic drugs (which can lower NOAC plasma concentrations), three patients (75.0%) were inappropriately dosed as classified by the two systems and one patient was appropriately dosed according to the SmPC but potentially underdosed according to the EHRA 2018 guide (apixaban 5 mg plus valproic acid, ‘dark brown’). This reflects the unawareness of the interaction of anti-epileptics with NOACs among clinicians, and the almost full absence of data on the clinical effect of plasma-lowering medication on the efficacy of NOACs. Further Phase 1 studies are needed in which NOAC plasma concentrations may be better defined under these combinations.
6.3 Primary Care Versus Specialist Care
Although one of the initial objectives of this study was to investigate the prescription patterns in primary care versus cardiologist care, well-founded conclusions cannot be made due to the underpowerment as the result of the cessation of inclusions by the COVID-19 pandemic. Moreover, data would need interpretation in the light of the different patient demographics, such as age and renal function (Table 1). These two parameters are critical factors in the dosing criteria of both SmPC and EHRA 2018 guides. Nevertheless, there is a higher rate of inappropriate dosing in GP care compared to cardiologist care, which seems mainly the result of inappropriate overdosing. Overall, inappropriate NOAC dosing in primary care in Portugal, Belgium and the UK has been reported by other investigators in a range between 18.3–30.3% [25, 28, 29]. So far, a proven difference with specialist care is lacking from the literature, although such findings might be important to tailor and focus educational initiatives.
6.4 Influence of Different Renal Function Formulae
Although the Cockcroft–Gault renal formula was used in all the landmark NOAC trials, and hence adopted in the SmPC guidelines and EHRA guide, laboratories cannot routinely report this value since they miss out information such as patient weight, and rather report estimated glomerular filtration rate (eGFR) based on the MDRD or CKD-EPI formulae. A post hoc analysis using these two eGFR formulae showed no significant impact on the classification of appropriateness according to the SmPC and EHRA 2018 Practical Guide, although a slightly higher proportion of patients received a non-significantly inappropriate NOAC dose when MDRD or CKD-EPI were used. Hence, recalculating renal function using the CG formula, especially in AF patients with borderline eGFR, could be helpful to improve prescription correctness among clinicians. Other studies in larger AF cohorts also investigated the influence of eGFR formulae on dosing appropriateness and recommended using the CG formula in patients with a GFR < 70 mL/min and/or elderly patients aged ≥ 75 years [30, 31].
6.5 Limitations
Several limitations have to be acknowledged. An important limitation was the underrepresentation of primary care patients, as already mentioned. Atrial fibrillation patients included at the cardiology outpatient clinic originated from one centre, which limits generalisability, although they were recruited from the different cardiology sub-specialty clinics. Some primary care patients could be in regular follow-up by other cardiologists than those of the Antwerp University Hospital. The size of our cohort did not allow for analyses of each NOAC separately. The same applies to the multivariate results, which need to be interpreted with caution. Furthermore, as this was a retrospective quantitative study, based on the factors for NOAC dose adaptation included in the SmPC and 2018 EHRA Practical Guide, other possible influencing factors could not be objectified. Additional prospective (qualitative) research in specialist and primary care can provide more insights into dosing decisions and improving AF care. Finally, the EHRA Practical Guide and its dose-adjustment chart has to be regarded as a guidance tool to support clinicians in rational decisions, although definitive evidence on outcomes is not yet available and further studies are needed.
7 Conclusion
Inappropriate NOAC dosing in AF patients in follow-up by cardiologists and primary care physicians still occurs regularly, i.e., in about one in five patients (19.4%), according to the SmPC. Based on the 2018 EHRA Practical Guide, this proportion is significantly lower (15.6%), likely because more complex patients can be accounted for, but it is still very high. This calls for further physician education, a structured and frequent reassessment of NOAC dosing in complex AF patients, and further investigation on what might be appropriate dosing in very specific patient situations.
References
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESCSD Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–32. https://doi.org/10.1016/j.jacc.2019.01.011.
Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P, European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–51. https://doi.org/10.1093/europace/eut083.
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–507. https://doi.org/10.1093/europace/euv309.
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H, ESCSD Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–93. https://doi.org/10.1093/eurheartj/ehy136.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Vanassche T, Potpara T, Camm AJ, Heidbuchel H, Lip GYH, Deneke T, Dagres N, Boriani G, Chao TF, Choi EK, Hills MT, Santos IS, Lane DA, Atar D, Joung B, Cole OM, Field M. European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021. https://doi.org/10.1093/europace/euab065.
Santos J, Antonio N, Rocha M, Fortuna A. Impact of direct oral anticoagulant off-label doses on clinical outcomes of atrial fibrillation patients: a systematic review. Br J Clin Pharmacol. 2020;86:533–47. https://doi.org/10.1111/bcp.14127.
Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y, Aylward P, White H, Zamorano JL, Antman EM, Ruff CT. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol. 2016;68:1169–78. https://doi.org/10.1016/j.jacc.2016.06.034.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. https://doi.org/10.1159/000180580.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Consortium. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. https://doi.org/10.7326/0003-4819-145-4-200608150-00004.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
European Medicines Agency. Rivaroxaban—Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf. Accessed 4 Aug 2022.
European Medicines Agency. Apixaban—Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf. Accessed 4 Aug 2022.
European Medicines Agency. Edoxaban—Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_en.pdf. Accessed 4 Aug 2022.
European Medicines Agency. Dabigatran—Summary of Product Characteristics https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf. Accessed 4 Aug 2022.
Moudallel S, Steurbaut S, Cornu P, Dupont A. Appropriateness of DOAC prescribing before and during hospital admission and analysis of determinants for inappropriate prescribing. Front Pharmacol. 2018;9:1220. https://doi.org/10.3389/fphar.2018.01220.
Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J, Singer DE, Peterson ED, Piccini JP, Investigators O-A and Patients. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68:2597–604. https://doi.org/10.1016/j.jacc.2016.09.966.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. https://doi.org/10.1056/NEJMoa1107039.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, EA-T Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. https://doi.org/10.1056/NEJMoa1310907.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. https://doi.org/10.1056/NEJMoa0905561.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, RA Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. https://doi.org/10.1056/NEJMoa1009638.
Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–90. https://doi.org/10.1016/j.jacc.2017.03.600.
Chan YH, Chao TF, Chen SW, Lee HF, Yeh YH, Huang YC, Chang SH, Kuo CT, Lip GYH, Chen SA. Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. 2020;17:2102–10. https://doi.org/10.1016/j.hrthm.2020.07.022.
Camm AJ, Cools F, Virdone S, Bassand JP, Fitzmaurice DA, Arthur Fox KA, Goldhaber SZ, Goto S, Haas S, Mantovani LG, Kayani G, Grierson Turpie AG, Antoon Verheugt FW, Kakkar AK, G-A Investigators. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. 2020;76:1425–36. https://doi.org/10.1016/j.jacc.2020.07.045.
Capiau A, De Backer T, Grymonprez M, Lahousse L, Van Tongelen I, Mehuys E, Boussery K. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation according to the drug labelling and the EHRA Practical Guide. Int J Cardiol. 2021;328:97–103. https://doi.org/10.1016/j.ijcard.2020.11.062.
Ruiz Ortiz M, Muniz J, Rana Miguez P, Roldan I, Marin F, Asuncion Esteve-Pastor M, Cequier A, Martinez-Selles M, Bertomeu V, Anguita M, Fs Investigators. Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors. A subanalysis of the FANTASIIA Registry. Europace. 2018;20:1577–83. https://doi.org/10.1093/europace/eux316.
Beyer-Westendorf J, Fay M, Amara W. The importance of appropriate dosing of nonvitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation. TH Open. 2021;5:e353–62. https://doi.org/10.1055/s-0041-1731777.
Cardoso CS, Sousa JA, Simoes P, Silva B, Albuquerque A, Esperanca A, Cibrao A, Correia A, Goncalves J, Mortagua J, Almeida JE, Oliveira L, Garcia J, Duarte M, Loureiro M, Costa ESM, Fraga M, Lopes P, Brandao R, Miguel S, Queiros T, Santo G, Silva F, Sargento-Freitas J. Misdosing of non-vitamin K antagonist oral anticoagulants in primary care. Clin Ther. 2020;42:1132-1136 e1131. https://doi.org/10.1016/j.clinthera.2020.04.008.
Garcia Rodriguez LA, Martin-Perez M, Vora P, Roberts L, Balabanova Y, Brobert G, Fatoba S, Suzart-Woischnik K, Schaefer B, Ruigomez A. Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK. BMJ Open. 2019;9: e031341. https://doi.org/10.1136/bmjopen-2019-031341.
Malavasi VL, Pettorelli D, Fantecchi E, Zoccali C, Laronga G, Trenti T, Lip GYH, Boriani G. Variations in clinical management of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation according to different equations for estimating renal function: post hoc analysis of a prospective cohort. Intern Emerg Med. 2018;13:1059–67. https://doi.org/10.1007/s11739-018-1857-3.
Andrade JG, Hawkins NM, Fordyce CB, Deyell MW, Er L, Djurdjev O, Macle L, Virani SA, Levin A. Variability in non-vitamin k antagonist oral anticoagulants dose adjustment in atrial fibrillation patients with renal dysfunction: the influence of renal function estimation formulae. Can J Cardiol. 2018;34:1010–8. https://doi.org/10.1016/j.cjca.2018.04.019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures in this study were in accordance with the 1964 Helsinki declaration (and its amendments). The research protocol was approved by the Ethics Committees of the Antwerp University Hospital/University of Antwerp on the 12th of August 2019 (local project reference 19/27/331).
Availability of Data and Material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent to Participate
All patients of the Antwerp University Hospital have consented with inclusion in retrospective analysis. All patients included at the primary care centres provided written informed consent.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Funding
This study is supported by the Antwerp University Hospital Cardiology Research Fund, and is part of Limburg Clinical Research Center, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital. The study team would like to thank the primary care physicians who participated in this study.
Conflict of Interest
Hein Heidbuchel and Lien Desteghe did not receive any personal honoraria; they received unconditional research support through Hasselt University or University of Antwerp from Bayer, Daiichi-Sankyo, Boehringer-Ingelheim, Bracco Imaging Europe, Medtronic, Boston-Scientific, Biotronik, and St. Jude Medical. None of the other authors has any personal conflicts of interest.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Arne Ballet, Cedric Hillegeer and Michiel Delesie. The first draft of the manuscript was written by Michiel Delesie and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delesie, M., Ballet, A., Hillegeer, C. et al. Appropriateness of Non-vitamin K Antagonist Oral Anticoagulants Dosing According to Different Prescription Guides Used in Belgian Ambulatory Care. Clin Drug Investig 42, 775–786 (2022). https://doi.org/10.1007/s40261-022-01190-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01190-2