Abstract
Systemic complications often occur in patients with advanced liver disease. In particular, the development of renal complications (acute kidney injury, hepatorenal syndrome), acute-on-chronic liver failure, cardiopulmonary diseases, or relative adrenal insufficiency can be serious in patients with advanced liver disease and may determine the patient’s quality of life and prognosis. Therefore, the early diagnosis of possible complications is the key to the prompt initiation of specific treatments that can improve quality of life and survival. For this purpose, networking with reference centers where multidisciplinary units are available is essential so that every patient is evaluated in clinical discussions involving specialists from different fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute kidney injury (AKI) is frequent in patients with advanced liver disease and impacts negatively on patients’ survival. Early differential diagnosis and adequate classification are critical. |
Hepatorenal syndrome (HRS) is a severe complication in patients with cirrhosis and ascites. Early intervention with vasoconstrictors in combination with albumin infusions are determining factors in the success of therapy and increased survival. |
Acute-on-chronic liver failure (ACLF) is a common and severe complication in patients with chronic liver disease, but the pathophysiology, clinical course, and prognosis differ from acute hepatic decompensation. Liver transplantation should be evaluated according to the possible risk-benefit, considering the degree of ACLF. |
Cardiopulmonary complications associated with portal hypertension are often underdiagnosed and include liver hydrothorax, hepatopulmonary syndrome, portopulmonary hypertension (PoPH), and cirrhotic cardiomyopathy. |
1 Introduction
Systemic complications often occur in patients with advanced liver disease. The liver has essential but diverse functions in metabolism, detoxification and excretion, the formation and inactivation of mediators, and non-specific defense mechanisms. Because of the high blood flow through the liver from the hepatic artery and portal vein, severe or advanced liver disease can cause changes in the systemic, portal, or cardiopulmonary circulation. Patients with advanced liver disease may develop several hemodynamic changes, leading to a hyperdynamic circulation, which is based on a progressive decrease in mean arterial pressure, a compensatory increase in cardiac output, and hypo-responsiveness to vasopressors. The current knowledge has linked these hemodynamic changes with the increase in portal pressure, bacterial translocation phenomena, and a progressive chronic systemic inflammatory state. These alterations manifest in extrahepatic symptoms that are often decisive for the disease course. In particular, renal and cardiopulmonary complications of advanced liver disease can be serious and may determine the patients’ quality of life and prognosis [1]. This review summarizes the diagnosis and management of the most important complications of advanced liver disease: renal impairment, liver failure, adrenal insufficiency, and cardiopulmonary disease.
2 Acute Kidney Injury
2.1 Definition and Classification
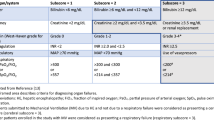
Acute kidney injury (AKI) is a common condition in patients with advanced liver disease and negatively impacts patient survival, therefore early differential diagnosis (hepatorenal syndrome [HRS], acute tubular necrosis, prerenal failure, or nephrotoxicity) and proper classification are critical. AKI in cirrhosis is defined as an increase in serum creatinine (sCR) levels of ≥ 0.3 mg/dL (≥ 26.5 mmol/L) in 48 h, or an sCr increase of ≥ 50% from baseline levels in the last 7 days [2].
Classification of the severity of AKI is essential. The International Club of Ascites-Acute Kidney Injury in cirrhosis (ICA-AKI) criteria provide a relevant and straightforward staging system for AKI in patients with liver cirrhosis based on the relative increases in sCr (Table 1) [2]. More recent studies indicate that stage 1 disease should be further subdivided into two subgroups with different outcomes, mainly sCr stage 1A (SCr < 1.5 mg/dL) and stage 1B (SCr ≥ 1.5 mg/dL) [3].
2.2 Prevalence
AKI is a frequent clinical problem that usually presents at and during hospitalization in cirrhotic patients; the estimated prevalence is 27–53% [3, 4]. Therefore, it is vital to determine the nature of kidney failure in patients with advanced liver disease. Some types of kidney failure in patients with liver disease, such as HRS or acute tubular necrosis, are more aggressive and have a worse prognosis and higher short-term mortality.
3 Hepatorenal Syndrome
3.1 Definition and Classification
HRS is a severe complication in patients with cirrhosis and ascites and is defined as the occurrence of renal failure in a patient with advanced liver disease in the absence of another known cause of renal failure. Two forms of HRS have been described depending on the acuity and progression of kidney injury. The first form is characterized by acute impairment of kidney function, HRS-AKI, while the second is characterized by more chronic kidney dysfunction, HRS-non-AKI [1]. HRS-AKI has well-established diagnostic criteria in patients with decompensated cirrhosis. According to the International Club of Ascites, HRS-AKI can be defined as an acute impairment of kidney function in a patient with ascites without response to expansion with 48 h albumin, absence of shock, not taking nephrotoxic drugs, and absence of structural kidney damage. Ruling out structural kidney damage is challenging as kidney biopsy is a risky procedure and is not always available. To overcome this limitation, kidney Doppler ultrasound can be employed. Recently, several biomarkers have emerged, with urinary neutrophil gelatinase (NGAL) being the most promising. Several studies have shown that urinary NGAL [5], a marker of tubular damage, can be useful for differential diagnosis. Nevertheless, the optimal cut-off for diagnosis has not been established, and diagnosis based on biomarkers needs further evaluation. Finally, HRS non-AKI is characterized by gradual renal failure and glomerular filtration < 60 mL/min for < 3 months [6].
3.2 Pathogenesis
Portal hypertension is a hallmark of this disease [7, 8]. As cirrhosis progresses, systemic inflammation becomes more intense, leading to systemic and splanchnic vasodilation, reducing the effective circulating volume (ECV) and decreasing renal perfusion [9]. A decrease in cardiac output further aggravates this decrease in ECV due to cirrhotic cardiomyopathy and an increase in vasodilation due to bacterial translocation and systemic inflammation [10].
3.3 Treatment
3.3.1 Vasoconstrictors
Vasodilation is a fundamental aspect of the pathophysiology of HRS, therefore treatment is based on the use of vasoconstricting drugs. The main options include terlipressin, norepinephrine, and the combination of midodrine/octreotide. In Europe, the vasoconstrictor of choice is terlipressin, combined with intravenous albumin (20–25%) 20–40 g/day [11]. Intravenous albumin plays various roles: it increases the ECV, improves renal perfusion, and has immunomodulatory properties to reduce systemic inflammation [12]. Terlipressin compensates for pathological vasodilation by increasing ECV so that the combined use of terlipressin with albumin results in higher response rates than with albumin alone (77% vs. 33%; p = 0.03) [13]. Terlipressin can be administered by intravenous boluses at an initial dose of 1 mg every 4–6 h. However, continuous intravenous administration at an initial dose of 2 mg/day may reduce the rate of its adverse effects [14].
3.3.2 Diagnostic Algorithm and Treatment
In most cirrhotic patients with the mildest form of AKI (AKI 1A), the condition resolves with close monitoring and withdrawal of nephrotoxic drugs. However, in patients with more aggressive forms of AKI (AKI 1B, 2, 3), the withdrawal of nephrotoxic drugs is insufficient, and albumin expansion at a test dose of 1 g/kg should be administered for 48 h. HRS must be considered if the patient does not recover from renal failure or progresses despite this volume expansion (Fig. 1) [15]. Of note, non-selective β-blockers have also been associated with HRS and should be discontinued [9, 16]. Renal replacement therapy should be considered in the management of HRS patients who do not respond to vasoconstrictors or in those with end-stage kidney disease. The indications for renal replacement therapy are the same in patients with cirrhosis as in the general population (severe electrolyte or acid-base imbalance, volume overload, and symptomatic azotemia) [17]. These indications can be useful bridging therapy in very ill patients prior to liver transplantation.
3.3.3 Use of Non-steroidal Anti-inflammatory Drugs in Patients with Cirrhosis
Pharmacological nephrotoxicity, mainly from non-steroidal anti-inflammatory drugs (NSAIDs), is a frequent complication in patients with advanced liver disease. Portal hypertension and vasodilation that occur in cirrhosis results in a decrease in ECV. This is compensated for by the intrarenal release of prostaglandins, which produce vasodilation of the afferent artery and better renal perfusion. However, anti-inflammatory drugs block this compensatory mechanism and can compromise renal perfusion. Although the impairment of renal perfusion associated with NSAIDs is usually mild and transient, in about one-third of cases it has a persistent or progressive course; in these patients, the prognosis is potentially serious and the mortality rate is significantly higher [18].
4 Acute-on-Chronic Liver Failure (ACLF)
Acute-on-chronic liver failure (ACLF) is a syndrome in cirrhosis characterized by acute decompensation (AD), organ failure(s), and high short-term mortality [19]. According to the European Association for the Study of the Liver, based on the CANONIC study, ACLF is defined as hepatic and extrahepatic failure triggered by precipitating factors (hepatic and/or extrahepatic) in patients with acutely decompensated cirrhosis. In addition, the short-term mortality associated with ACLF is high (> 15% at 28 days), which differentiates it from acutely decompensated cirrhosis, where mortality is much lower. Acute organ failure, according to the Liver Failure Consortium scoring parameters is liver failure (bilirubin > 12 mg/dL), kidney failure (sCr > 2 mg/dL), severe encephalopathy, coagulopathy (international normalized ratio > 2.5), circulatory failure that requires vasoactive drugs, and respiratory failure (ratio of arterial oxygen partial pressure [PaO2] to fractional inspired oxygen [FiO2] < 200) [20].
The clinical presentation of ACLF is very heterogeneous and is classified according to the number of affected organs. Grade 1: organ failure in only the kidney or another organ accompanied by kidney and/or cerebral damage; grade 2: failure in two organs; grade 3: failure in three organs; grade 4: failure in four organs. This classification is essential for determining prognosis because a higher grade of ACLF corresponds to a worse prognosis (28-day mortality rate >15%) [Table 2]. ACLF is not a static process but a dynamic process, and up to 50% of ACLF cases improve or resolve, 20% worsen, and 30% remain stable [21].
4.1 ACLF Pathophysiology
Hepatic or extrahepatic precipitating factors (such as infections, alcohol, etc.) stimulate the release of factors that mediate inflammation and there is a higher degree of systemic inflammation than in decompensated cirrhosis. This massive release of inflammatory factors produces systemic inflammation and cellular oxidative stress, which cause tissue damage due to hypoperfusion, immune-mediated tissue damage, and direct mitochondrial damage, eventually causing multi-organ failure [22,23,24].
4.2 ACLF Treatment
Treatment is based on four pillars: management/removal of triggers, support techniques, artificial functionality techniques, and transplantation. Unfortunately, there is no specific treatment for ACLF. When infections are the suspected trigger, patients require intensive screening, early broad-spectrum antibiotic therapy, and daily reassessment. The prevention of ACLF progression, including early and aggressive treatment of infections, is crucial and the treatment response is conditioned by the degree of ACLF (52%, 42%, and 8% grade 1, grade 2, and grade 3, respectively) [24]. Patients with alcoholic hepatitis should be advised to abstain from alcohol and may benefit from the use of corticosteroids in the absence of active infections and when ACLF is severe. Patients with variceal bleeding require hemodynamic control, early antibiotic prophylaxis [25], and the application of transjugular intrahepatic portosystemic shunts (TIPS) as needed. Notably, the insertion of a pre-emptive TIPS has demonstrated a clear survival benefit in patients with ACLF who fulfill specific criteria (Child–Pugh B > 7 with active bleeding at the time of endoscopy and Child–Pugh C < 14) [26].
It is important to detect hemodynamic failure with regard to organ support, which can lead to kidney failure and the need for vasoactive drugs. In addition, it is also important to consider possible neurological failure (hepatic encephalopathy) that should be monitored and treated early with specific treatment (osmotic laxatives [lactulose] and non-absorbable antibiotics [rifaximin]) [27].
Two fundamental studies—RELIEF [28] and PROMETHEUS [29]—assessed artificial liver support techniques in these patients but neither demonstrated a short-term survival benefit with this approach.
Liver transplantation is the definitive treatment for ALCF, curing both the ACLF and the underlying liver disease. However, some concerns deserve consideration, particularly the brief accessibility of ACLF patients to liver transplantation, the complex evaluation of candidates, the poor survival outcomes of transplantation, and futility. The prognosis of patients with grade 3 ACLF is ominous regardless of their model for end-stage liver disease (MELD) score [30]. Liver transplantation positively impacts severe forms of ACLF and increases survival. Nevertheless, the result is highly conditioned by the degree of ACLF and number or type of organ failure in the patient, negatively impacting respiratory and hemodynamic function [31]. In addition, patients with three or more organ failures or CLIF-ACLF score > 64 must be carefully evaluated for other factors associated with poor survival after liver transplantation, including mechanical ventilation at the time of transplantation, lactate level > 4 mmol/L, normal leukocyte count, older age of the recipient, and use of marginal organs [32]. Therefore, early intervention is a fundamental factor in identifying and treating patients who may be appropriate candidates for transplantation.
5 Cardiopulmonary Complications in Cirrhosis
Up to 70% of patients with cirrhosis report dyspnea or hypoxemia at some point during disease progression [33]. Although several cardiopulmonary pathologies can coexist in patients with cirrhosis, some are pathophysiologically linked to portal hypertension: hepatic hydrothorax, hepatopulmonary syndrome (HPS), portopulmonary hypertension (PoPH), and cirrhotic cardiomyopathy.
5.1 Hepatic Hydrothorax
Hepatic hydrothorax is a relatively frequent complication (occurring in 5–10% of patients with cirrhosis) and is associated with high morbidity and mortality. Pathophysiologically, hepatic hydrothorax develops when ascitic fluid passes into the pleural cavity through communications in the diaphragm, assisted by the patient’s malnourished state/wasting syndrome and the increase in abdominal pressure [34].
Treatment of hepatic hydrothorax is a challenge. Initial treatment is a low-sodium diet with a diuretic (spironolactone + furosemide) and thoracocentesis, but if the patient does not respond to repeated thoracentesis, TIPS and transplantation should be considered (Fig. 2). Unfortunately, TIPS placement is less effective in controlling hydropic decompensation in patients with hepatic hydrothorax than in patients with pure refractory ascites [35].
5.2 Hepatopulmonary Syndrome
HPS is a relatively frequent complication in cirrhotic patients. It is defined by analytical characteristics (PO2 < 80 mmHg and a high alveolar-capillary gradient [> 15 mmHg]), structural features (arteriolar vasodilation and/or presence of intrapulmonary shunts), and the presence of portal hypertension due to either cirrhosis or non-cirrhotic causes [36].
The pathophysiology of HPS is characterized by systemic and pulmonary vasodilation and a progressive increase in cardiac output. This results in inadequate gas exchange, which is even more pronounced in the presence of a vascular shunt and inflammatory infiltrate at the interstitial level [36]. The characteristic signs and symptoms of HPS are platypneas with orthodeoxia or a state of desaturation when the patient is sitting due to a marked imbalance in the ventilation/perfusion ratio, caused by blood accumulating at the base of the lungs due to vasodilation. Diagnostic confirmation of this pulmonary vasodilation requires contrast echocardiography with agitated saline solution or scintigraphy with technetium-99m [37, 38]. Computed tomography angiography (CTA) can also reveal true intrapulmonary shunts, which support the diagnosis and opens up the therapeutic option of shunt occlusion.
No drug has shown efficacy in controlling this syndrome. TIPS appear to induce transient but not definitive improvements [39]. Currently, the only definitive treatment is liver transplantation, which is effective even in patients with severe HPS [40].
5.3 Portopulmonary Hypertension
PoPH is a severe form of Group 1 pulmonary arterial hypertension requiring vasodilator treatment and liver transplantation in selected cases. PoPH is defined as the presence of a mean pulmonary arterial pressure (mPAP) of ≥ 25 mmHg at rest, pulmonary vascular resistance ≥ 3 Wood Units, and pulmonary capillary pressure < 15 mmHg [41, 42]. Hemodynamic assessment is used to classify the severity of the disease as mild (mPAP < 35 mmHg), moderate (35–45 mmHg), or severe (> 45 mmHg).
The pathophysiology of PoPH is currently unknown but it is assumed that the hyperdynamic hypercirculation in cirrhosis induces shear stress in the pulmonary vascular endothelium [43]. Moreover, vasoconstriction mediated by enteric molecules that reach systemic circulation by portosystemic shunts can also contribute.
Any patient with portal hypertension, regardless of the cause, can develop PoPH. Nevertheless, it is important to rule out PoPH in candidates for liver transplantation, candidates for TIPS placement, those who present signs and symptoms of the disease, or those with suggestive electrocardiographic abnormalities such as right bundle branch block [44]. Therefore, the screening method of choice is the transthoracic echocardiogram.
Pharmacological treatment is not simple. Different drugs used in pulmonary hypertension have been tested and, to date, only macitentan has shown efficacy in randomized phase III studies (Table 3) [45]. Thus, treatment must be individualized depending on the experience of each center. Pulmonary vasodilators should be used cautiously in patients with ascites and patients closely monitored for possible adverse effects. Transplantation does not always reverse PoPH and it is difficult to establish which patients may benefit from transplantation. Therefore, severely ill patients are generally excluded and those with mild/moderate PoPH who respond well to vasodilators are selected.
5.4 Cirrhotic Cardiomyopathy
Systemic inflammation in patients with cirrhosis induces a series of changes that can lead to cardiac alterations [9]. Cirrhotic cardiomyopathy encompasses a set of common structural, functional, and electrophysiological alterations that have not been well defined. Consequently, the prognostic implications of this condition are not well known. The main echocardiographic feature is diastolic dysfunction, which is an early sign of cardiomyopathy in the setting of normal systolic function [46], although the clinical relevance of diastolic dysfunction is not clear [47, 48]. In contrast, some patients can develop systolic impairment, which can be detected by sophisticated techniques such as dynamic stress tests, myocardial strain imaging, and cardiac magnetic resonance imaging [49,50,51]. In addition, systolic dysfunction (i.e., low cardiac output) is implicated in the pathophysiology of HRS [8]. Finally, prolonging the QTc interval is common in cirrhosis and is associated with a worse outcome [52] but the risk of sudden death is unclear. There are two clinical scenarios where a detailed cardiac function is mandatory: patients who are candidates for liver transplantation [53], and as part of the assessment for TIPS insertion [54].
6 Adrenal Insufficiency in Cirrhosis
Adrenal insufficiency mainly appears in critically ill cirrhotic patients and implies a worse prognosis. Cortisol is a pluripotent hormone involved in adaptation to stress and is secreted by the adrenal gland in response to adrenocorticotropic hormone (ACTH). Pituitary secretion of ACTH is stimulated by various cytokines and other inflammatory mediators during stress, leading to fluctuating cortisol levels during critical illness and sepsis. Relative adrenal insufficiency may appear in 50–77% of critically ill patients with cirrhosis [55, 56]. Indeed, although cortisol levels may be within the normal range, they are still insufficient to guarantee vascular tone and prevent vascular permeability in cirrhosis. Several hypotheses explain the high incidence of adrenal insufficiency in patients with cirrhosis, although the most accepted is related to the state of chronic systemic inflammation and hypoperfusion of target organs in the most advanced stages of the disease. The diagnosis of adrenal insufficiency in cirrhosis is complex and relies on measuring plasma levels before and after adrenal stimulation with synthetic corticotrophin. At present, the diagnostic criteria are not clear. Nevertheless, baseline cortisol values of < 15 μg/dL or an increase in cortisol after ACTH of < 9 μg/dL in patients with baseline cortisol < 35 μg/dL have been proposed. There are contradictory data regarding the beneficial effects of hydrocortisone administration but it appears to shorten the length of stay in the intensive care unit [57,58,59].
7 Conclusions
Decompensated liver cirrhosis is a complex clinical scenario that requires specialized management. Increasing knowledge about the underlying pathophysiological processes and new therapeutic and prophylactic approaches may increase the survival of patients with cirrhosis. Renal impairment is crucial in the natural history of decompensation and should be recognized early. ACLF is a life-threatening complication where the treatment of precipitants and liver transplantation play the central therapeutic role. Cardiopulmonary complications are rare decompensation events but specific treatments are available.
References
European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60.
Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64(4):531–7.
Huelin P, Piano S, Solà E, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol. 2017;15(3):438-445.e5.
Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4(1):23.
Fagundes C, Pepin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267–73.
Wong F, Nadim MK, Kellum JA, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–9.
Erly B, Carey WD, Kapoor B, McKinney JM, Tam M, Wang W. Hepatorenal syndrome: a review of pathophysiology and current treatment options. Semin Interv Radiol. 2015;32(4):445–54.
Krag A, Bendtsen F, Henriksen JH, Moller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105–10.
Téllez L, Ibáñez-Samaniego L, del Villar CP, et al. Non-selective beta-blockers impair global homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73:1404–14.
del Arbol LR, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–47.
Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687.
Arroyo V, Fernandez J. Pathophysiological basis of albumin use in cirrhosis. Ann Hepatol. 2011;10:S6-14.
Ortega R, Ginès P, Uriz J, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36(4 Pt 1):941–8.
Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion vs. intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983–92.
Solé C, Pose E, Solà E, Ginès P. Hepatorenal síndrome in the era of acute kidney injury. Liver Int. 2018;38(11):1891–901.
Téllez L, Albillos A. Non-selective beta-blockers in patients with ascites: the complex interplay among the liver, kidney and heart. Liver Int. 2022;42(4):749–61.
Sourianarayanane A, Raina R, Garg G, McCullough AJ, O’Shea RS. Management and outcome in hepatorenal syndrome: need for renal replacement therapy in non-transplanted patients. Int Urol Nephrol. 2014;46:793–800.
Elia C, Graupera I, Barreto R, et al. Severe acute kidney injury associated with non-steroidal anti-inflammatory drugs in cirrhosis: a case–control study. J Hepatol. 2015;63(3):593–600.
Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–45.
Moreau R, Jalan R, Gines P, CANONIC Study Investigators of the EASL–CLIF Consortium, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37 (1437.e1-9).
Gustot T, Fernandez J, Garcia E, CANONIC Study Investigators of the EASL-CLIF Consortium, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62(1):243–52.
Arroyo V, Jalan R. Acute-on-chronic liver failure: definition, diagnosis, and clinical characteristics. Semin Liver Dis. 2016;36:109–16.
Arroyo V, Moreau R, Jalan R, Gines P. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62:S131–43.
Arroyo V, Moreau R, Kamath PS, et al. Acute on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041.
Martínez J, Hernández-Gea V, Rodríguez-de-Santiago E, et al. Bacterial infections in patients with acute variceal bleeding in the era of antibiotic prophylaxis. J Hepatol. 2021;75(2):342–50.
Trebicka J, Gu W, Ibáñez-Samaniego L, et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73(5):1082–91.
Hernaez R, Sola E, Moreau R, Gines P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–53.
Bañares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57(3):1153–62.
Kribben A, Gerken G, Haag S, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142(4):782-789.e3.
Sundaram V, Jalan R, Wu T, et al. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation. Gastroenterology. 2019;156(5):1381-1391.e3.
Belli LS, Duvoux C, Artzner T, et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol. 2021;75(3):610–22.
Weiss E, Saner F, Asrani SK, et al. When is a critically ill cirrhotic patient too sick to transplant? Development of consensus criteria by a multidisciplinary panel of 35 international experts. Transplantation. 2021;105(3):561–8.
Filì D, Vizzini G, Biondo D, et al. Clinical burden of screening asymptomatic patients for coronary artery disease prior to liver transplantation. Am J Transplant. 2009;9(5):1151–7.
Jackson K, Davidson R, Aujayeb A. Hepatic hydrothorax in the management of cirrhosis. Gut. 2022;71(2):446–7.
Jindal A, Mukund A, Kumar G, Sarin SK. Efficacy and safety of transjugular intrahepatic portosystemic shunt in difficult-to-manage hydrothorax in cirrhosis. Liver Int. 2019;39(11):2164–73.
Krowka MJ, Fallon MB, Kawut SM, et al. International liver transplant society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016;100(7):1440–52.
Koch DG, Fallon MB. Hepatopulmonary syndrome. Curr Opin Gastroenterol. 2014;30(3):260–4.
Tonelli AR, Naal T, Dakkak W, Park MM, Dweik RA, Stoller JK. Assessing the kinetics of microbubble appearance in cirrhotic patients using transthoracic saline contrast-enhanced echocardiography. Echocardiography. 2017;34(10):1439–46.
Tsauo J, Zhao H, Zhang X, et al. Effect of transjugular intrahepatic portosystemic shunt creation on pulmonary gas exchange in patients with hepatopulmonary syndrome: a prospective study. J Vasc Interv Radiol. 2019;30(2):170–7.
Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology. 2014;146(5):1256-65.e1.
Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913.
Sharma M, Yong C, Majure D, et al. Safety of cardiac catheterization in patients with end-stage liver disease awaiting liver transplantation. Am J Cardiol. 2009;103(5):742–6.
Téllez L, Martínez J, Moreira V, Albillos A. Pulmonary hypertension and hepatic cirrhosis. Revista Clínica Española (English Edition). 2015;215:324–30.
Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53(1):1802148.
Krowka M, Cottreel E, Hoeper MM, et al. Macitentan improves risk categorization for liver transplant mortality in patients with portopulmonary hypertension: A PORTICO study post hoc analysis. Liver Transpl. 2020;26(7):935–40.
Moller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15.
Nazar A, Guevara M, Sitges M, et al. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013;58:51–7.
Ruiz-del-Arbol L, Achecar L, Serradilla R, et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732–41.
Kim MY, Baik SK, Won CS, et al. Dobutamine stress echocardiography for evaluating cirrhotic cardiomyopathy in liver cirrhosis. Korean J Hepatol. 2010;16:376–82.
Krag A, Bendtsen F, Dahl EK, Kjaer A, Petersen CL, Moller S. Cardiac function in patients with early cirrhosis during maximal beta-adrenergic drive: a dobutamine stress study. PLoS One. 2014;9:e109179.
Wiese S, Hove JD, Mo S, et al. Cardiac dysfunction in cirrhosis: a 2-yr longitudinal follow-up study using advanced cardiac imaging. Am J Physiol Gastrointest Liver Physiol. 2019;317(3):G253–63.
Zhao J, Qi X, Hou F, Ning Z, Zhang X, Deng H, et al. Prevalence, risk factors and in-hospital outcomes of QTc interval prolongation in liver cirrhosis. Am J Med Sci. 2016;352:285–95.
Yotti R, Ripoll C, Bermejo J, Bañares R. Cardiac function, a key component in evaluation for liver transplant. Liver Transpl. 2018;24(1):7–8.
Billey C, Billet S, Robic MA, Cognet T, Guillaume M, Vinel JP, et al. A prospective study identifying predictive factors of cardiac decompensation after transjugular intrahepatic portosystemic shunt: the toulouse algorithm. Hepatology. 2019;70(6):1928–41.
Tsai MH, Peng YS, Chen YC, et al. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–81.
Fernández J, Escorsell A, Zabalza M, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288–95.
Piano S, Favaretto E, Tonon M, et al. Including relative adrenal insufficiency in definition and classification of acute on chronic liver failure. Clin Gastroenterol Hepatol. 2020;18(5):1188–96.
Acevedo J, Fernández J, Prado V, et al. Relative adrenal insufficiency in decompensated cirrhosis: relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology. 2013;58(5):1757–65.
Vu T, Vallabh M, Laine G. Adrenal insufficiency and response to stress dose hydrocortisone in patients with cirrhosis and vasopressor dependency using cirrhosis-specific cortisol thresholds. Ann Pharmacother. 2020;54(8):742–9.
Sibon O, Bosch J, Cottreel E, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med. 2019;7(7):594–604.
Ghofrani HA, Galiè N, Grimminger F, et al., PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40.
Acknowledgements
The authors thank Ana Isabel Ortega for writing assistance on behalf of Springer Healthcare Ibérica, and Catherine Rees from Springer Healthcare for copyediting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure statement
This article has been published as part of a journal supplement wholly funded by Eisai.
Funding
Support for medical writing assistance was funded by Eisai Farmacéutica. S.A.
Conflict of interest
Luis Téllez and Antonio Guerrero declare they have no conflicts of interest.
Ethics approval
This article was based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the conception and design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and final approval of the version to be published.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Téllez, L., Guerrero, A. Management of Liver Decompensation in Advanced Liver Disease (Renal Impairment, Liver Failure, Adrenal Insufficiency, Cardiopulmonary Complications). Clin Drug Investig 42 (Suppl 1), 15–23 (2022). https://doi.org/10.1007/s40261-022-01149-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01149-3