Abstract
Background and Objectives
Aclidinium bromide was approved in the European Union for the treatment of chronic obstructive pulmonary disease (COPD) in adult patients in 2012 and in a fixed-dose combination with formoterol in 2014. We characterised new users of aclidinium, aclidinium/formoterol and other COPD medications and evaluated off-label prescribing of these medications in three European populations.
Methods
We described demographic characteristics, comorbidities, comedications, COPD severity and off-label prescribing of new users of aclidinium, aclidinium/formoterol and other COPD medications in patients with COPD aged ≥ 40 years in the Clinical Practice Research Datalink (CPRD, UK), Danish National Health Databases, and German Pharmacoepidemiological Research Database (GePaRD) between 2015 and 2017.
Results
We included 17,668 new users of aclidinium (CPRD, 4871; Denmark, 2836; GePaRD, 9961) and 14,808 new users of aclidinium/formoterol (CPRD, 2153; Denmark, 2586; GePaRD, 10,069). Study patients were of similar age, except in GePaRD, where users of long-acting beta2-agonists (LABA)/inhaled corticosteroids were younger. Patients had multiple comorbidities and used multiple comedications—most frequently hypertension (50–79%) and short-acting beta2-agonists (26–84%). Aclidinium users in CPRD and long-acting anticholinergics/LABA users in Denmark and GePaRD had the highest frequency of severe/very severe COPD. Off-label prescribing of aclidinium (5.0% [CPRD]–8.9% [Denmark]) and aclidinium/formoterol (2.6% [GePaRD]–3.2% [CPRD]) was low, and the main reason was asthma without a COPD diagnosis.
Conclusions
Aclidinium and aclidinium/formoterol were mostly prescribed according to label, with preference given to older patients with more severe COPD and to patients with a high prevalence of comorbidities and comedication use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aclidinium and aclidinium/formoterol users showed similar characteristics to those of other LAMA users. |
Among the study medication groups, aclidinium and aclidinium/formoterol users, and LAMA users in general, were older and had a high frequency of severe COPD, chronic comorbidities and use of COPD comedications. |
Off-label prescribing of aclidinium and aclidinium/formoterol is low. |
1 Introduction
In July 2012, aclidinium bromide 322 µg twice daily (under the brand name Eklira/Bretaris Genuair) was approved in the European Union (EU) for maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD) [1]. In September 2014, aclidinium bromide in fixed-dose combination with formoterol, 340 µg/12 µg twice daily (under the brand name Duaklir Genuair), was approved in the EU for the same indication [2].
It is common that in real world clinical practice, medication is given to a more diverse group of patients than those included in clinical trials; for example, patients with asthma, severe kidney or liver impairment, children, and pregnant women were not included in randomised controlled trials of aclidinium [3,4,5]. Furthermore, new drugs are often selectively prescribed to patients with severe types of the respective disease or to those not responding to available treatment [6].
A drug utilisation study (DUS) is an important component of a medication risk management plan (RMP) for the evaluation of the effectiveness of routine risk minimisation measures, such as the summary of product characteristics, a communication tool through which it is expected that physicians who prescribe a medication do so in line with the approved indication. Additionally, DUSs provide information on how the medications are used in patients for which information is missing in clinical trials. As part of the marketing authorisation in Europe, the RMP for aclidinium bromide included the conduct of a European multidatabase DUS to characterise new users of aclidinium, aclidinium/formoterol and other COPD medications, and to evaluate potential off-label prescribing of aclidinium, including use in children and patients with asthma.
A first DUS conducted in the United Kingdom (UK), Denmark, and Germany—DUS1 (September 2012–December 2015)—included new users of aclidinium bromide as monotherapy and in non-fixed-dose combination with formoterol (hereafter, aclidinium) [7, 8]. Here we report the results of the second drug utilisation study (DUS2) conducted in the UK, Denmark and Germany—(January 2015–December 2017)—after marketing authorisation was received for a fixed-dose aclidinium/formoterol product in 2014 in Europe.
2 Methods
2.1 Study Design
This non-interventional, multinational, European study describes new users of aclidinium, aclidinium/formoterol fixed-dose combination, and other selected COPD medications with the use of secondary data collection.
2.2 Setting
We conducted the study using data from the Clinical Practice Research Datalink, General Practitioner Online Database (CPRD GOLD) in the UK, the Danish National Health Databases, and the German Pharmacoepidemiological Research Database (GePaRD) in Germany between 2015 and 2018 (see data sources in Table S1, Online Supplementary Material (OSM)). The recording of medical information was based on primary care electronic medical records in CPRD, inpatient and outpatient hospital discharge diagnoses in Denmark, and insurance claims from outpatient care visits and hospitalisations in GePaRD. Use of medications was based on general practitioner prescriptions in CPRD, dispensations in community pharmacies in Denmark, and outpatient dispensations in GePaRD.
2.3 Cohort Selection
The study groups included new users of aclidinium, aclidinium/formoterol, tiotropium, other long-acting muscarinic antagonists (other LAMA) (glycopyrronium bromide, umeclidinium), LAMA/long-acting beta-2 agonists (LAMA/LABA) (glycopyrrolate/formoterol, glycopyrronium/indacaterol, umeclidinium/vilanterol and tiotropium/olodaterol), LABA/inhaled corticosteroids (LABA/ICS) (fixed-dose combinations: formoterol/budesonide, formoterol/beclometasone, formoterol/mometasone, formoterol/fluticasone, salmeterol/fluticasone propionate, and vilanterol/fluticasone), and LABA (formoterol, salmeterol, indacaterol, and olodaterol). Drugs were selected based on anatomical therapeutic chemical (ATC) codes in GePaRD and Denmark and using Multilex/British National Formulary codes mapped to ATC codes in CPRD. New users were defined as patients with > 1 year of continuous enrolment in the study data sources who were prescribed a study medication of interest during the study inclusion period and who did not receive a prescription/dispensing for the same medication or medication group within a 6-month washout period before the date of first prescription/dispensing (the index date) for that medication (OSM Fig. S1) in CPRD, UK and Denmark or who did not have exposure of the same medication or medication group during the 6 months before the date of first prescription for that medication in GePaRD (Germany). A patient may have had one or more prescriptions/dispensings for a different study drug at any time prior to the index date or may have had one or more prescriptions/dispensings for the same study drug before the 6-month washout prior to the index date. A patient could qualify as a new user for more than one study group. In each data source, new users of the study medications of interest were selected on the date of the first prescription occurring within the patient inclusion period that they fulfilled all the inclusion criteria. Inclusion of new users stopped 1 year before the end of the study period to allow for 1 year of potential follow-up (OSM Table S2).
2.4 Variables
Comorbidities diagnosed at any time before the index date (except in GePaRD, where the 12 months before index date was used because of data availability) and prior use of medications in the 12 months before the index date were ascertained by using diagnosis and medication codes specific to each data source (OSM Fig. S1). Smoking status was ascertained based on the latest available information recorded before or at the index date in CPRD and using proxy information (recorded diagnosis related to severe smoking or smoking cessation drugs) in Denmark and GePaRD. Alcohol abuse was ascertained based on the latest information available on alcohol consumption before or at the index date in CPRD and based on the presence of diagnoses for alcohol abuse or dispensings of medications indicated for treatment of alcohol abuse in Denmark and GePaRD. Chronic obstructive pulmonary disease was defined as a recorded diagnosis code for COPD, chronic bronchitis or emphysema for any time before the index date. Severity of COPD was evaluated according to a modified version of the algorithm developed by Verhamme et al. [9] and was classified as mild, moderate, severe or very severe (OSM Table S3). Other individual markers of COPD severity were also measured at the index date [10].
Patterns of use of each study medication were ascertained during the first year of follow-up. Assessment included duration of use and persistence. The duration of each single prescription/dispensation was defined based on the number of supply days in CPRD, the amount of defined daily doses in GePaRD, and the waiting-time approach in Denmark. Duration of the index episode was estimated through the length of consecutive prescriptions/dispensings concatenated (after stockpiling; i.e., for overlapping dispensings, the beginning of the consecutive dispensing was shifted to the day after the end of the previous dispensing to allow for stockpiling) by using an allowed gap that was no greater than the length of the previous prescription/dispensing in CPRD and GePaRD. In Denmark, the sequences of dispensings that belonged to the same treatment episodes were identified by the waiting-time approach. The waiting-time approach is modelled on the actual, observed distance between dispensings of the same medication [11]. Persistence of use of each study medication was measured by the percentage of individuals remaining on therapy (persistent) from the index date until the end of follow-up (i.e., the earliest of 1 year after the index date, death, loss to follow-up/disenrollment/emigration in Denmark/end of insurance coverage in GePaRD). The time window for ascertainment of the study variables is presented in OSM Fig. S1.
Prescribing was considered off-label in the following user groups: patients aged < 18 years, adult patients with a diagnosis of asthma and no recorded diagnosis of COPD, and patients with an unknown indication (i.e., absence of a recorded diagnosis of COPD or asthma) (OSM off-label use criteria).
The age- and sex-specific annual prevalence of use of each study medication was calculated as the number of patients receiving at least one prescription for the medication of interest in a specific year divided by the overall population in each data source in that year. The age and sex distribution of the European standard population on 1 January 2017 was used to standardise the prevalences.
Information on diagnoses and medications codes used in the study is available in the study protocol.
2.5 Statistical Analyses
Data describing the characteristics of the study population (lifestyle, medical history, comedications, severity of COPD and off-label use of aclidinium and aclidinium/formoterol) are presented as counts and percentages and as mean values and interquartile ranges (age, duration of index episode of use) as appropriate. The age- and sex-standardised annual prevalence of use of each study medication was estimated with 95% confidence intervals. All research partners conducted the analyses following a common protocol and analysis plan.
In the CPRD and GePaRD, all analyses were conducted using SAS software, version 9.4 (SAS Institute Inc.; Cary, NC, USA). In Denmark, all analyses were conducted using Stata/MP software, release 16.1 (StataCorp LP, College Station, TX, USA).
2.6 Approvals and Regulatory Review
The study was reviewed by the RTI International institutional review board (IRB), the Independent Scientific Advisory Committee of CPRD, the Danish Data Protection Agency, and the statutory health insurance providers and the German Federal (Social) Insurance Office in Germany. The IRB determined that since this study did not involve private, identifiable, human subjects’ data nor interaction with any human subjects, informed consent was not needed. None of the members of the research team had access to identifying patient information when analysing the data.
The study protocol (version 2.1, 29 July 2014) was endorsed by the European Medicines Agency, approved by the European Commission on 19 November 2014, and updated on 2 June 2015 (v2.2). The study and its protocol were registered in the EU PAS Registry on 15 May 2014 with the register number EUPAS6559.
3 Results
3.1 Cohort Participants
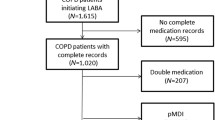
The study included 17,668 new users of aclidinium (4871 in CPRD, 2836 in Denmark and 9961 in GePaRD) and 14,808 new users of aclidinium/formoterol (2153 in CPRD, 2586 in Denmark and 10,069 in GePaRD) (Fig. 1).
Number of users of aclidinium, aclidinium/formoterol and other COPD medications before and after fulfilling inclusion criteria in each data source. The different order of columns for the National Health Databases in Denmark reflects a different order in the application of eligibility criteria driven by data access and protection rules in Denmark. COPD chronic obstructive pulmonary disease, CPRD Clinical Practice Research Datalink, GePaRD German Pharmacoepidemiological Research Database, ICS inhaled corticosteroids, LABA inhaled long-acting beta2-agonist, LAMA long-acting muscarinic antagonists, UK United Kingdom
3.2 Baseline Characteristics
Prevalence of aclidinium use per 100,000 population was 122 in the CPRD in 2017, 38 in Denmark in 2017 and 80 in GePaRD in 2015 (Table 1). For aclidinium/formoterol, the prevalence of use per 100,000 population was 54 in the CPRD in 2017, 30 in Denmark in 2017 and 60 in GePaRD in 2015. The prevalence of use of aclidinium and aclidinium/formoterol was lower than that for other treatment groups, except for other LAMA in GePaRD.
In the three data sources, new users of study medications with a diagnosis of COPD who were aged ≥ 40 years showed similar age, the median age ranged from 69 to 70 years in CPRD, from 70 to 72 years in Denmark, and from 63 to 70 years in GePaRD, where users of LABA/ICS were younger (median age 63 years). New users of LAMA medications had a higher percentage of men than new users of LABA/ICS or LABA. In Denmark, however, overall, there were more females than males in all study medications (Table 1). Current smoking was more frequent in the CPRD than smoking proxy estimators in Denmark and GePaRD for all study medications (OSM Table S4).
Frequent baseline comorbidities in new users with COPD who were aged ≥ 40 years are presented in Table 2. Hypertension was the most frequent comorbidity across the study medications in CPRD (50–53%), Denmark (76–79%) and GePaRD (60–72%). The second most frequent comorbidity was depressive disorders in CPRD (39–43%) and Denmark (50–54%) and ischaemic heart disease in GePaRD (19–32%). Other frequent comorbidities were diabetes, urinary tract infection, obesity, pneumonia and arrhythmias. For all the study medications, the pattern of comorbidities was similar across the three databases except for GePaRD, where comorbidities among users of LABA/ICS were observed less frequently than for the other study drugs. Other less frequent comorbidities are presented in the OSM Table S5.
Short-acting beta2-agonists (SABA) were the most frequent respiratory medications prescribed in the 12 months before the index date among COPD patients aged ≥ 40 years in CPRD (77–84%), in Denmark (48–60%) and in GePaRD (26–41%) (Table 3). In general, prior use of SABA and oral corticosteroids was more frequent in users of LAMA medications, while prior use of ICS was more frequent in users of LABA/ICS or LABA. Prior use of LABA/ICS was very frequent among new users of LAMA medications. Cardiovascular medications (63–76%) and antibiotics (54–75%) were among the most frequently prescribed medications across all study groups in the three countries (OSM Table S6).
Severe and very severe COPD was more frequent in Denmark (47–66%) than in CPRD (28–41%). In GePaRD, severe COPD ranged from 12 to 28%, and very severe COPD could not be evaluated. Users of LAMA had a higher frequency of severe and very severe COPD than users of LABA/ICS or LABA in Denmark (Fig. 2). The most frequent individual criterion that qualified patients into the severe or very severe categories were use of oral corticosteroids in CPRD (15–27%), prior COPD hospitalisation in Denmark (35–53%), and prior COPD exacerbation without hospitalisation in GePaRD (8–20%). Other proxies of COPD severity are presented in OSM Table S7.
Distribution of COPD severity among new users of study medications aged 40 years or older with COPD, by study medication and data source. COPD chronic obstructive pulmonary disease, CPRD Clinical Practice Research Datalink, GePaRD German Pharmacoepidemiological Research Database, ICS inhaled corticosteroids, LABA inhaled long-acting beta2-agonist, LAMA long-acting muscarinic antagonists, UK United Kingdom
The percentage of new users considered to have received aclidinium according to the label (i.e., adult patients who had a recorded diagnosis of COPD with or without a recorded diagnosis of asthma) was 89% of users in the CPRD, 87% of users in GePaRD, and 56% of users in Denmark. For aclidinium/formoterol, the percentage was slightly higher in the three countries: 90% in the CPRD, 93% in GePaRD and 58% in Denmark (Table 4). In Denmark, 35% of new users of aclidinium and 39% of new users of aclidinium/formoterol did not have a recorded diagnosis of COPD or asthma, and indication was considered unknown. Potential off-label prescribing of aclidinium occurred in 5.0% of new users in CPRD, 8.9% in Denmark and 5.4% in GePaRD and of aclidinium/formoterol occurred in 3.2% of new users in CPRD, 3.0% in Denmark and 2.6% in GePaRD. The most frequent reason for potential off-label prescribing was presence of a recorded diagnosis of asthma without any recorded code for COPD. The number of users of aclidinium and aclidinium/formoterol who were of paediatric age ranged from 0 to only a few.
3.3 Pattern of Use of Study Medications
The patterns of use of the study medications during the first 12 months of treatment are presented in OSM Table S8. For all the study medications, the duration of the index episode was longer in Denmark. The median duration of the index episode was 4.3 months for aclidinium and 4.7 months for aclidinium/formoterol in CPRD, 6.2 months for aclidinium and 8.7 months for aclidinium/formoterol in Denmark, and 3.0 months for aclidinium and 3.9 months for aclidinium/formoterol in GePaRD. Persistence of use during the first 12 months of treatment was higher among new users of LAMA medications than among new users of LABA/ICS or LABA in GePaRD. Persistence of use at 12 months from initiation of treatment was higher for aclidinium/formoterol than for aclidinium in the three countries.
4 Discussion
This study included patients with COPD initiating medications considered in the pharmacological treatment recommended for initial and maintenance treatment of patients with COPD. According to the current Global Initiative for Chronic Obstructive Lung Disease 2021 guidelines, initial pharmacological treatment of COPD depends on symptoms and history of exacerbations assessment. While patients in group A are recommended to receive any bronchodilator, patients in group B receive LABA or LAMA, patients in group C receive LAMA, and those in group D receive LAMA or LAMA+LABA or LABA+ICS. Following implementation of initial therapy and based on reassessment of dyspnoea, exacerbations and eosinophil counts, physicians may consider for maintenance therapy the potential addition of LAMA if the patient is taking only LABA or vice versa, the addition of ICS if the patients is already taking both, or further adding roflumilast or azithromycin.
Patients with COPD aged ≥ 40 years included in the study as new users of COPD medications frequently had several comorbidities and used multiple respiratory and non-respiratory medications. Hypertension, depressive disorders and ischaemic heart disease were the most frequent comorbidities, and SABA, LABA/ICS, cardiovascular medications, and antibiotics were the most frequent baseline comedications. LAMA medications, including aclidinium, aclidinium/formoterol, tiotropium, other LAMA and LAMA/LABA, were preferentially prescribed to patients with more severe COPD.
In this study, off-label use of aclidinium was low and off-label use of aclidinium/formoterol was lower than that for aclidinium. The main category of off-label use was having a diagnosis of asthma in the absence of a diagnosis of COPD, and paediatric use was negligible.
Approximately one-third of users of aclidinium and aclidinium/formoterol continued treatment at 1 year of follow-up in the CPRD. This proportion was higher in users of aclidinium/formoterol in Denmark, possibly due to a higher proportion of patients with severe or very severe COPD and differences in the calculation of duration of treatment episodes. It was lower in users of aclidinium in GePaRD.
In general, the clinical characteristics of users of the study medications were consistent with those reported in the literature for patients with COPD [12], although the prevalence of specific comorbidities was higher in our study. In studies conducted in the UK, the most frequent comorbidities in patients with COPD were cardiovascular disease (46%), hypertension (34%), diabetes (14–19%), depressive disorders (3–15%), osteoporosis (⁓ 11%) and myocardial infarction (8%) [13, 14]. These prevalences were even lower in a study conducted in a large cohort of patients with COPD in Denmark [15]. In our study, the maximum prevalences for these disorders in CPRD were 53% for hypertension, 43% for depressive disorders, 18% for diabetes, 20% for osteoporosis and 9% for myocardial infarction. The differences across studies may be explained by the different inclusion criteria used. Patients with COPD included in the UK studies were probably less severely affected because they included patients with a diagnosis of COPD regardless of treatment, while the COPD population in our study was restricted to patients initiating a medication for COPD.
Overall, the characteristics of new users and the pattern of use of aclidinium in the present study were similar to those observed in the previous study (DUS1, 2012–2015) conducted in the same data sources [7, 8]. Off-label prescribing of aclidinium and aclidinium/formoterol was low in the three countries. Prescription for asthma appears to be the main category of off-label prescribing. However, it cannot be ruled out that a patient may have had childhood asthma that later resolved or evolved to asthma-COPD syndrome in adulthood. The impact of off-label prescribing for asthma is very low. In Denmark, the indication was unknown in approximately one-third of users of aclidinium and aclidinium/formoterol. Chronic conditions such as asthma, COPD and other respiratory conditions are usually managed in primary care unless patients require specialist care at an outpatient hospital clinic, or they have aggravation of symptoms or complications that require inpatient care. Diagnostic information from primary care was not available in Denmark, and although medication use is captured, the potential indication of COPD may not have been captured. This may explain the higher percentage of users with no diagnosis of COPD or asthma.
Differences in the characteristics of the study data sources may explain the variability of results across them, in particular on the evaluation of comorbidities, the potential indication for use of study medications, and the severity of the indication based on recorded diagnoses. Some information desired for research in COPD was only available in CPRD (e.g., life habits such as smoking and alcohol consumption, spirometry values). Diagnostic information recorded in the CPRD is based on primary-care electronic medical records, and for half of the population, complemented with hospital discharge diagnoses from HES; in Denmark, it is based on hospital discharge inpatient and outpatient clinic diagnoses; and in GePaRD, Germany, it is based on insurance claims from outpatient care visits and hospitalisations. To minimise under-ascertainment of comorbidities in Denmark, we identified diabetes, hypertension, urinary tract infections and depression from prescriptions for specific medications in addition to diagnosis codes. A similar approach to identify diabetes and hypertension was used in GePaRD. Ascertainment of the use of medications was based on prescribed (CPRD) or dispensed (Denmark, GePaRD) medications but not on the actual use of medications by patients, and this could introduce misclassification of exposure. Adherence and persistence of medication use among patients with COPD are frequently low [16,17,18].
The algorithm for severity of COPD categorised most patients as having moderate or severe COPD. Severity of COPD was defined according to a clinical algorithm that has been validated in a primary-care database in the Netherlands. The algorithm was adjusted to the available data in the study data sources, which could result in some misclassification of COPD severity in Denmark and GePaRD, where some variables were not available or proxies were included. The lack of information in GePaRD on patients receiving oxygen therapy and those on a waiting list or scheduled for lung transplant prevented the assessment of very severe COPD. Also, the higher degree of COPD severity in Denmark than in CPRD or GePaRD is consistent with the nature of the data source, which is based on inpatient and outpatient hospital discharge diagnoses, and only those patients with COPD that were hospitalised or required a hospital specialist visit could be identified.
Another limitation of this study is that information on the listed indication of a prescription was not available in the study databases. The lack of information about indication could have introduced some degree of misclassification on the severity of COPD. Further, in Denmark, underascertainment of the potential indication of aclidinium (e.g., COPD, asthma) affected the evaluation of off-label prescription of aclidinium, as over 40% of users did not have a recorded diagnosis of COPD or asthma. This percentage was approximately 4–5% for aclidinium and approximately 3% for aclidinium/formoterol in CPRD and GePaRD, where both primary-care and hospital diagnoses are available. Also, substantial underrecording of COPD in the Danish National Patient Registry among hospitalised patients has been reported [19].
5 Conclusions
Overall, this study indicates that users of aclidinium and aclidinium/formoterol have a high prevalence of chronic comorbidity, high use of comedications and more severe COPD. Hypertension, depressive disorders and urinary tract infections were the most frequent comorbidities in users of the study medications with COPD. Severe COPD was frequent in users of LAMA medications.
This study shows that aclidinium and aclidinium/formoterol are mainly prescribed according to the labelling. Off-label use of aclidinium bromide and aclidinium/formoterol was low in the three countries, although in Denmark information on diagnoses was limited to the inpatient and outpatient hospital setting.
References
EMA. Summary of opinion (initial authorisation). Eklira Genuair: aclidinium bromide. European Medicines Agency, Committee for Medicinal Products for Human Use; 24 May 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002211/WC500127779.pdf. Accessed 3 Feb 2014.
EMA. Summary of opinion (initial authorisation). Duaklir Genuair: aclidinium bromide/ formoterol fumarate dihydrate. European Medicines Agency, Committee for Medicinal Products for Human Use; 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003745/WC500173689.pdf. Accessed 7 May 2018.
Rennard SI, Scanlon PD, Ferguson GT, Rekeda L, Maurer BT, Garcia Gil E, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12-week efficacy and safety of twice-daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clin Drug Investig. 2013;33(12):893–904. https://doi.org/10.1007/s40261-013-0138-1.
Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–6. https://doi.org/10.1183/09031936.00225511.
Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD. 2012;9(2):90–101. https://doi.org/10.3109/15412555.2012.661492.
Jara M, Lanes SF, Wentworth C 3rd, May C, Kesten S. Comparative safety of long-acting inhaled bronchodilators: a cohort study using the UK THIN primary care database. Drug Saf. 2007;30(12):1151–60. https://doi.org/10.2165/00002018-200730120-00007.
Rebordosa C, Catsellsague J, Kristiansen N, Pottegård A, Plana E, Aguado J, et al. Severity of COPD in new users of aclidinium bromide and other COPD medications: a population-based study in the United Kingdom and Denmark [abstract 70]. Pharmacoepidemiol Drug Saf. 2017;26(Suppl 2):45–6.
Rebordosa C, Varas-Lorenzo C, Castellsague J, Plana E, Bui C, Aguado J, et al. Characteristics of new users of aclidinium bromide in the United Kingdom [abstract 1166]. Pharmacoepidemiol Drug Saf. 2016;25(Suppl 3):676.
Verhamme KM, Afonso AS, van Noord C, Haag MD, Koudstaal PJ, Brusselle GG, et al. Tiotropium Handihaler and the risk of cardio- or cerebrovascular events and mortality in patients with COPD. Pulm Pharmacol Ther. 2012;25(1):19–26. https://doi.org/10.1016/j.pupt.2011.10.004.
Curkendall SM, Lanes S, de Luise C, Stang MR, Jones JK, She D, et al. Chronic obstructive pulmonary disease severity and cardiovascular outcomes. Eur J Epidemiol. 2006;21(11):803–13. https://doi.org/10.1007/s10654-006-9066-1.
Pottegård A, Hallas J. Assigning exposure duration to single prescriptions by use of the waiting time distribution. Pharmacoepidemiol Drug Saf. 2013;22(8):803–9. https://doi.org/10.1002/pds.3459.
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. https://doi.org/10.1183/13993003.00164-2019.
Raluy-Callado M, Lambrelli D, MacLachlan S, Khalid JM. Epidemiology, severity, and treatment of chronic obstructive pulmonary disease in the United Kingdom by GOLD 2013. Int J Chron Obstruct Pulmon Dis. 2015;10:925–37. https://doi.org/10.2147/copd.s82064.
Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993–1002. https://doi.org/10.1183/09031936.00065013.
Schmidt SA, Johansen MB, Olsen M, Xu X, Parker JM, Molfino NA, et al. The impact of exacerbation frequency on mortality following acute exacerbations of COPD: a registry-based cohort study. BMJ Open. 2014;4(12): e006720. https://doi.org/10.1136/bmjopen-2014-006720.
Covvey JR, Mullen AB, Ryan M, Steinke DT, Johnston BF, Wood FT, et al. A comparison of medication adherence/persistence for asthma and chronic obstructive pulmonary disease in the United Kingdom. Int J Clin Pract. 2014;68(10):1200–8. https://doi.org/10.1111/ijcp.12451.
Mueller S, Wilke T, Bechtel B, Punekar YS, Mitzner K, Virchow JC. Non-persistence and non-adherence to long-acting COPD medication therapy: a retrospective cohort study based on a large German claims dataset. Respir Med. 2017;122:1–11. https://doi.org/10.1016/j.rmed.2016.11.008.
Ingebrigtsen TS, Marott JL, Lange P, Hallas J, Nordestgaard BG, Vestbo J. Medically treated exacerbations in COPD by GOLD 1–4: a valid, robust, and seemingly low-biased definition. Respir Med. 2015;109(12):1562–8. https://doi.org/10.1016/j.rmed.2015.10.015.
Thomsen RW, Lange P, Hellquist B, Frausing E, Bartels PD, Krog BR, et al. Validity and underrecording of diagnosis of COPD in the Danish National Patient Registry. Respir Med. 2011;105(7):1063–8. https://doi.org/10.1016/j.rmed.2011.01.012.
Acknowledgements
The authors would like to thank the general practitioners contributing information to the Clinical Practice Research Datalink (CPRD) in the UK and the German statutory health insurance providers that provided data for the study in GePaRD, namely the DAK Gesundheit, and the Die Techniker (TK). The authors also thank Drs. Cristina Varas-Lorenzo and Jordi Castellsague from Research Triangle Institute Health Solutions (RTI-HS) for leading the study until their retirements in 2016 and 2018, respectively; Nadine Wentzell, MSc, and Anna Julia Witzleb from the Leibniz Institute for Prevention Research and Epidemiology–BIPS for their contributions; Ana Frances and Esther García-Gil, former AstraZeneca employees, for contributing to the study design; Peter McMahon from AstraZeneca, for reviewing the manuscript; John Forbes (RTI-HS) for manuscript editing; and Bethan Pickering (RTI-HS) for graphic design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The aclidinium PASS programme was funded by Almirall S.A. In June 2015, AstraZeneca became the marketing authorisation holder of Eklira® and continued funding the programme, including this study. The contracts provide the research team independent publication rights. The authors had complete autonomy in the process of establishing the protocol, carrying out the analyses, and interpreting the results. The sponsors had no role in the data collection or analysis; however, in line with the Guideline on Good Pharmacovigilance Practices (GVP): Module VIII—Post-authorisation Safety Studies of the European Medicines Agency, the sponsors had the opportunity to view the results and interpretations included in the manuscript and provide comments prior to submission of the manuscript for publication.
Conflict of interest
Elena Rivero-Ferrer, Cristina Rebordosa, Estel Plana, Jaume Aguado, Núria Saigí-Morgui, and Susana Perez-Gutthann are employees of RTI Health Solutions and work on projects funded by pharmaceutical companies. As an employee of RTI Health Solutions, Susana Perez-Gutthann also participates in scientific advisory boards (for studies and medications) that are funded by pharmaceutical companies. Nina Sahlertz Kristiansen, Morten Olsen, Anton Pottegård and Jesper Hallas are employees of the University of Southern Denmark, Clinical Pharmacology, Pharmacy and Environmental Medicine. They have participated in studies funded by pharmaceutical companies (Alcon, Almirall, Astellas, Astra-Zeneca, Boehringer-Ingelheim, Pfizer, Menarini, Servier, Takeda), with money paid to their employer. Tania Schink is an employee of the independent, nonprofit scientific organisation Leibniz Institute for Prevention Research and Epidemiology—BIPS. Unrelated to this study, BIPS occasionally conducts studies financed by the pharmaceutical industry. Almost exclusively, these are post-authorisation safety studies (PASS) requested by health authorities. The design and conduct of these studies as well as the interpretation and publication are not influenced by the pharmaceutical industry. Annalisa Rubino, Sami Z Daoud, and Alejhandra Lei are employees of AstraZeneca.
Availability of data and material
This study uses a national healthcare database, a general practitioner database, and a claims database. Only the aggregated data, in the form of study results in the main manuscript and in the supplementary material can be shared.
Code availability
Programming codes are archived at BIPS (GePaRD, Germany), Southern Denmark University (Danish registers), and RTI Health Solutions (UK CPRD and aggregated tables across sites).
Authors’ contributions
ERF, AJW, MO, EP, JA, NSM, SPG, TS, NSK, JH, AP and CR provided substantial contributions to study design and interpretation of the data, performed the research, and wrote or critically reviewed the work for important intellectual content. MO, NSK, EP and JA also analysed the data. AR, SZD and AL provided substantial contributions to study design and interpretation of the data and critically reviewed the work for important intellectual content. All authors approved the final version of the manuscript and are accountable for all aspects of this research.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, based exclusively on routinely collected data, formal consent is not required. For more details on site-specific approvals, refer to the Methods section in the article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Prior postings and presentations
The summary of the regulatory report of the study is available in the EU PAS register [https://www.encepp.eu/encepp/openAttachment/studyResult/39220]. Partial results of this study have been presented at international conferences: Rebordosa C, Rubino A, Witzleb AJ, Olesen M, Plana E, Aguado J, Saigi N, Daoud SZ, Lei A, Perez-Gutthann S, Schink T, Kristiansen NS, Pottegård A, Rivero-Ferrer E. Characteristics of new users of aclidinium, aclidinium/formoterol, and other COPD medications in three European countries. Poster presented at the 2020 Virtual ERS International Congress; October 2020. [abstract] Eur Respir J. 2020 Oct 28; 56(Suppl 64):2071. https://doi.org/10.1183/13993003.congress-2020.2072. Rivero-Ferrer E, Witzleb AJ, Olesen M, Plana E, Aguado J, Saigí-Morgui N, Rubino A, Daoud SZ, Lei A, Perez-Gutthann S, Schink T, Kristiansen NS, Pottegård A, Rebordosa C. Are aclidinium and aclidinium/formoterol used according to their approved indication in Europe? Results of a multicountry drug utilization postauthorization safety study. Poster presented at the 36th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Virtual, September 16-17, 2020. [abstract] Pharmacoepidemiol Drug Saf. 2020 Oct; 29(Suppl 3):175.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rivero-Ferrer, E., Olesen, M., Plana, E. et al. Characteristics of New Users of Aclidinium Bromide, Aclidinium/Formoterol, and Other COPD Medications in the United Kingdom, Denmark, and Germany. Clin Drug Investig 42, 319–331 (2022). https://doi.org/10.1007/s40261-022-01120-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01120-2