Abstract
Background and Objectives
In clinical trials, the safety of drugs is summarized by the incidence of adverse events, while post-marketing reporting systems use disproportionate reporting of adverse drug reactions. Here, we propose a method to evaluate the novelty of a safety profile of a drug in a new class (in clinical trials), against that of those already on the market (using pharmacovigilance data).
Methods
Through Bayesian disproportionality analyses of the US Food and Drug Administration Adverse Event Reporting System (FAERS) data, we identified and ranked Preferred Terms for a pool of 30 antipsychotics. Adverse event rates in randomized, double-blind, placebo-controlled schizophrenia clinical trials were summarized by their class specificity. One study (N = 245) of the trace amine-associated receptor 1 (TAAR1) agonist ulotaront (SEP-363856) was compared with five studies of dopamine D2 receptor-based antipsychotics lurasidone (N = 1041), quetiapine (N = 119), olanzapine (N = 122), and placebo (N = 504).
Results
In clinical trials of antipsychotics, cumulative rates for adverse events at and above a threshold of disproportional reporting (Empirical Bayes Geometric Mean 50 > 3 in FAERS) were 52%, 42%, and 60% for lurasidone, quetiapine, and olanzapine, respectively, indicating that over half of the adverse events reported in clinical trials of an atypical antipsychotic are class-specific risks. In contrast, in the clinical trial of ulotaront, the cumulative rate was 23%, indicating a lower rate of antipsychotic class-specific risk.
Conclusions
These results demonstrate a novel approach to summarize adverse events in clinical trials, where the cumulative burden of class-specific risks describes the emerging safety profile of a new drug in clinical development, relative to reactions anticipated for drugs in an established pharmacological class.
ClinicalTrials.gov Identifiers
NCT0296938, NCT00088634, NCT00549718, NCT00615433, NCT00790192.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The safety profile of a drug in a new class can be explored by focusing on adverse drug reactions shared by marketed drugs with an established pharmacological class. |
Adverse events that occur in well-controlled clinical trials can be depicted according to the disproportional reporting of those class-specific Preferred Terms. |
Clinical trial data in schizophrenia for a new pharmacological class of compound ulotaront (TAAR1 agonist) were compared to the established pharmacological class of dopamine D2-based antipsychotics. |
1 Introduction
Since the 1950s, schizophrenia has been treated with the antipsychotic class of drugs defined by a common pharmacology: reducing the activity of dopamine at dopamine D2 receptors. Side effects common to the class, such as movement disorders, elevated prolactin, and metabolic disturbances, are also based on shared pharmacological effects at dopamine, serotonin, and other related receptors. The array of side effects seen with the use of antipsychotics in schizophrenia is also evident with their use in patient populations of bipolar disorder and depression [1,2,3,4,5,6,7,8].
Prescribing information of antipsychotic medications in the USA typically report the Preferred Terms (PTs) of adverse drug reactions (ADRs) that occur at a rate ≥ 2% and greater than placebo (2% tables). Common antipsychotic-class adverse reactions include the PTs of akathisia, parkinsonism, and dyskinesia, as reported in short-term clinical trials. However, rates of patients experiencing extrapyramidal symptoms, with first-generation or second-generation compounds, are typically higher in studies conducted outside of the trials for drug approval [9,10,11]. The high rates and broad range of antipsychotic side effects are a major cause of treatment dissatisfaction, discontinuation, and relapse, and together highlight the need for alternatives to D2-based antipsychotics [12,13,14].
Post-marketing pharmacovigilance data can be used, together with disproportionality analyses, to estimate the disproportional reporting of an ADR associated with any given drug, relative to all other ADRs and all other drugs. In the antipsychotic drug class, ADRs with elevated disproportional reporting, derived from post-marketing data, include the PTs reported in the 2% tables of US drug labels, but also include many additional PTs whose individual clinical trial incidence rates do not meet the threshold for inclusion in drug labeling. We hypothesized that clinical trial data may include a broader collection of PTs consistent with class-specific elevated disproportional reporting observed in pharmacovigilance data, but are not included in drug labels because of the low clinical trial incidence rates for each individual PT.
The undesirable effects of a drug in clinical development can be anticipated based on real-world experience with other drugs in the same class. Here, we sought to pilot an approach to characterize the adverse events (AEs) accumulated in clinical studies for the investigational drug (ulotaront) in a new pharmacological class, relative to specific side effects for compounds in an established pharmacological class anticipated from a disproportionality analysis of pharmacovigilance data.
Ulotaront is a trace amine-associated receptor 1 (TAAR1) agonist without pharmacological effect on dopamine D2 receptors in clinical development as a novel treatment for patients with schizophrenia [15,16,17,18,19]. We sought to examine the safety profile of ulotaront, placebo, and D2-based antipsychotics (lurasidone, quetiapine, olanzapine) relative to the adverse reactions associated with the established pharmacological class of D2-based antipsychotics in the US Food and Drug Administration Adverse Event Reporting System (FAERS).
2 Methods
The rates of drug-related AEs observed in clinical trials were calculated as a cumulative function of the PTs’ disproportional reporting with a pharmacological class of drugs with post-marketing pharmacovigilance data. Five randomized controlled trials were selected for inclusion based on the following criteria: available subject-level AE data and an adequate and well-controlled study design that was sufficient for submission of a New Drug Application for the US Food and Drug Administration.
2.1 Ulotaront AE Data
Patient-level AE data were obtained from two completed studies conducted by the authors (SCH, KSK): (1) a randomized, double-blind, placebo-controlled, 4-week study of ulotaront for the treatment of patients (N = 245) with an acute exacerbation of schizophrenia (NCT02969382) [16]; and (2) a 6-month, open-label extension study of ulotaront (NCT02970929) in patients (N = 157) who completed the initial double-blind trial.
2.2 Comparator AE Data
Patient-level AE data were obtained from two sources. First, pooled data from five double-blind, placebo-controlled, 6-week studies of lurasidone for the treatment of patients with an acute exacerbation of schizophrenia (N = 1786 patients). Study D105006 included AE data from 149 subjects receiving placebo or lurasidone at doses of 40 and 120 mg/day [20]. Study D1050196 (NCT00088634) included AE data from 180 subjects receiving placebo or lurasidone (80 mg/day) [21]. Study D105229 (NCT00549718) included AE data from 496 subjects receiving placebo or lurasidone (40, 80, 120 mg/day) [22]. Study D105231 (NCT00615433) included AE data from 475 subjects receiving placebo or lurasidone (40 or 120 mg/day) or olanzapine (15 mg/day) [6]. Study D105233 (NCT00790192) included AE data from 486 subjects receiving placebo or lurasidone (80 and 160 mg/day) or quetiapine extended release (600 mg/day) [23].
2.3 FAERS Query
A disproportionality analysis was conducted on the ADRs submitted to FAERS for a pool of 30 antipsychotics listed in Table 1. The FAERS data were accessed by Empirica™ Signal, Oracle’s pharmacovigilance software (version 8.1.1, release 2020Q2; Oracle Inc., Redwood City, CA, USA). The FAERS data included all pre-1997 Spontaneous Reporting System (SRS) data, Adverse Event Reporting System data through August 2012, and FAERS data from August 2012 to June 2020. Generic and trade names were mapped in Empirica data within Oracle’s curated and updated drug name mapping algorithms, including handling of all trade names with more than one generic name. Duplicate reports (referring to the same drug-event pair appearing more than once) were excluded from data mining via Oracle’s automated duplicate detection algorithm that identifies duplicated reports based on a sufficient overlap of matching records, equivalence of demographic fields, and a combination of manufacturer, drug, and event information, including drug and/or event start dates. Preferred Terms for a pool of 30 antipsychotics [Table in the Electronic Supplementary Material (ESM)] were ranked by a disproportionality analysis in FAERS, using the Empirical Bayes Geometric Mean (EBGM). In this analysis, the EBGM is calculated across a pool of 30 antipsychotics. As the pool of 30 antipsychotics spanned launch years from 1951 to 2016, the influences of confounding factors, important for any individual drug and/or individual drug-event pairings (e.g., Weber effect, polypharmacy, indication, competition, and notoriety biases), on the estimate of relative risk ratio (RRR), were reduced. The confidence limits (from EBGM05 to EBGM95) for each PT were also estimated (Table in the ESM). Preferred Terms in the ulotaront and lurasidone clinical trial databases were re-coded to the latest PTs of the Medical Dictionary for Regulatory Activities (version 23.1) used by FAERS in Empirica at the time of the data analysis.
2.4 Statistical Analysis
Adverse event data from the ulotaront and lurasidone clinical trial databases (randomized controlled trials) were sorted by PT according to their FAERS-EBGM ranking for the antipsychotic class. A disproportionality analysis was not utilized to detect safety signals for a single drug, but to make an overall class-specific estimate derived from the 30 atypical and typical antipsychotics. The aim was to utilize the post-marketing FAERS data to characterize the total burden of class-related AEs. The proportion of subjects in each clinical study having an AE was plotted as a cumulative function of each PT’s class-related disproportional reporting in a real-world reporting FAERS database. Cumulative AE curves, as a portion of all subjects, and as a portion of all subjects reporting an AE, were used to describe the AE profiles as a cumulative function of each PT’s class-related disproportional reporting in the real-world reporting FAERS database.
3 Results
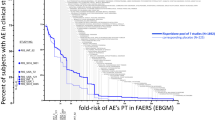
A total of 30 antipsychotics were pooled in a query of FAERS ADRs. The antipsychotics spanned chlorpromazine (launch date of 1951) to cariprazine (launch date of 2016). A total of 3.89 million ADRs were identified in FAERS (Table 1). The PTs of AEs observed in placebo-controlled clinical trial data were ranked by the disproportional reporting (EBGM) of that PT in FAERS for the class of antipsychotics in FAERS using a disproportionality analysis (Table in the ESM). In Fig. 1, the x-axis is the fold-increase in disproportional reporting of each PT for the 30-compound antipsychotic class within the FAERS database. The y-axis is the cumulative proportion of subjects having the AE in clinical trials at and above the disproportional reporting for each PT as ADRs in FAERS. Preferred Terms above three-fold increased disproportional reporting are labeled in Fig. 1. The PTs include movement-related disorders (akathisia, parkinsonism, extrapyramidal disorder), neuroendocrine and metabolic disturbances, as well as PTs related to the underlying disorder (schizophrenia, delusion, psychotic disorder).
Cumulative percent of subjects in clinical trials (y-axes) having indicated adverse events (AEs), as a function of the disproportional reporting (x-axes) for that Preferred Term (PT) in post-marketing pharmacovigilance data [US Food and Drug Administration Adverse Event Reporting System (FAERS)]. Individual PTs are separated by a comma. Individual clinical studies and their treatment arms are shown in the inset graphs by round symbols. The left inset indicates the percent of subjects having AEs of three-fold or greater class-specific disproportional reporting. The bottom inset indicates the value of Empirical Bayes Geometric Mean (EBGM) by which half the subjects have accumulated an AE
The cumulative curves in Fig. 1 are the profile of AEs in clinical studies in patients with an acute exacerbation of schizophrenia. The curves represent the AE burden accumulated (rising from right to left) across PTs in the class of antipsychotics. The AE rate in a clinical trial is accumulated (left to right) as a function of the disproportional reporting (EBGM) for the PT in the antipsychotic class. For example, akathisia is a common AE seen with atypical antipsychotics. In FAERS, the ADR of akathisia has a EBGM value of 15-fold increased disproportional reporting, relative to all other drugs and all other ADRs. In the pooled clinical trial data, the AE of akathisia was observed in 187 subjects, and accounts for a relatively large step in the cumulative curve shown in Fig. 1. Somnolence has a three-fold increased disproportional reporting for the class of antipsychotics and its corresponding step in the cumulative curves is visible for all drug treatment groups in Fig. 1.
The curves in Fig. 1 account for the portion of subjects having class-specific AEs, as a function of its class-specific disproportional reporting in post-marketing pharmacovigilance data. As indicated in Fig. 1, in the pool of clinical studies with lurasidone, approximately 52% of subjects have AEs with PTs having class-specific disproportional reporting (EBGM values) of three-fold or greater, compared with 37% of the placebo subjects. The corresponding rates for olanzapine and quetiapine were 60% and 42%, respectively. The corresponding rates in the clinical study with ulotaront and placebo were 23% and 22%, respectively.
In the clinical trial data for the atypical antipsychotic lurasidone, class-related AEs are accumulated at a greater rate than with placebo. The class-related AEs accumulated with ulotaront were similar to the AEs accumulated with placebo. The AE profile observed in the ulotaront short-term clinical study in schizophrenia is distinct from the profiles observed in lurasidone clinical studies, including the active comparator compounds quetiapine and olanzapine.
4 Discussion
Here, we demonstrate a novel method for summarizing and comparing safety in drug development based on class-related disproportional reporting in pharmacovigilance data. The cumulative class-effect curve characterizes side-effect profiles for compounds in clinical studies, based on the class-specific PTs reported for an established pharmacological class in pharmacovigilance data. Thus, clinical trial safety data may be summarized by a broader collection of PTs indicative of a class-specific disproportional reporting, even at low clinical trial incidence rates for each individual PT.
Typically, safety is summarized for the registration and approval of new drug treatments using a highly specific ontology of AE terminology (e.g., Medical Dictionary for Regulatory Activities). In this work, the pool of six clinical trials recorded AE data from over 2031 unique subjects and resulted in a total of 523 unique PTs (with associated EBGM values from FAERS). The unique PTs reported in clinical trial data were sorted according to the class-specific disproportional reporting observed in post-marketing data collected across the entire class of dopamine D2 binding drugs. A disproportionality analysis is typically applied to detect safety signals for a single drug, but here we adapted the disproportionality analysis to make an overall estimate of class specificity as derived from 30 atypical and typical antipsychotics. In contrast to standard pharmacovigilance approaches to signal detection, the methods here use post-marketing data to account for the total burden of the class-related AEs reported in the clinical trial data itself.
According to the Food and Drug Administration definition, an established pharmacological class is a text phrase to associate drugs in a common pharmacologic class with an approved indication of an active moiety that the Food and Drug Administration has determined to be scientifically valid and clinically meaningful [24]. Drugs indicated for the treatment of schizophrenia are classified by two closely related established pharmacological classes: typical and atypical antipsychotics, based on their shared molecular pharmacology of dopamine D2 with or without serotonin 5-HT2A antagonism. To date, comparisons of safety profiles within antipsychotics have focused on quantitative differences in the rates of the AEs among PTs common to the class [25,26,27]. Quantitative differences in the side effects can be dependent on dose, titration schedules, study duration, and differences in study populations. With the methods employed here, the cumulative curves represent a more objective approach to describe the qualitative differences in the AE profiles between drugs, as might arise from meaningful differences in pharmacological class.
Ulotaront, a TAAR1 agonist, does not mediate its effects via blockade of D2 or 5-HT2A receptors common to the current antipsychotic class [15, 16]. The profile of AEs accumulated in the clinical trial conducted to date with ulotaront appear to be distinct from those expected from the established classes of antipsychotics. Across all the PTs identified via FAERS data indicating high levels of class-specific disproportional reporting, the ulotaront AEs accumulated no greater with ulotaront than with placebo. The AEs reported for ulotaront and greater than placebo were somnolence and gastrointestinal symptoms [16], and each identified as having very low (less than two-fold) antipsychotic class-specific disproportional reporting.
In this work, we defined class-specific PTs at three-fold or greater disproportional reporting in FAERS. The antipsychotic class-specific disproportional reporting spans categories of movement disorders, metabolic and neuroendocrine disorders, but also psychiatric symptoms related to the underlying conditions and populations for the conditions treated. As highlighted in an analysis by Khouri et al. [28], the relative risks of more objective PTs (such as movement disorders, metabolic and neuroendocrine in our query) are likely to be more correlated between meta-analyses of clinical trial data and disproportionality analyses of spontaneous reporting databases compared with the PTs associated with the underlying condition (e.g., psychiatric symptoms of schizophrenia).
To decrease the chance of introducing subjectivity by the manual curation of PTs, we decided to retain all the PTs in the class-specific query. Inclusion of PTs that may appear to be more directly related to the psychiatric symptoms of the underlying conditions, rather than from the use of the medication itself, avoided the introduction of bias that arises in a manual curation of PTs for drug-related queries. Manual curation would be necessary to remove the influence of such indication bias; however, in this application to randomized controlled clinical trials, indication bias is controlled by the reporting of drug-placebo differences. Indeed, the incidence of PT schizophrenia is increased on the placebo treatment vs drug treatment.
In the investigation of drug safety issues in pharmacovigilance and clinical development, standardized Medical Dictionary for Regulatory Activities queries are validated pre-determined sets of PTs grouped together to support safety analysis and reporting. The method piloted here with schizophrenia drug trials, where the AE burden is accounted for as it is accumulated across PTs as a function of their class-specific disproportional reporting, is a prototype for a novel approach to summarize and compare datasets of clinical trial data, and can be used to supplement the standard methods of summarizing safety during drug development. For any one drug, the nature of post-marketing case reporting of ADRs in pharmacovigilance data limits conclusions about risk. Post-marketing estimates of risk rely on event counts while, in contrast, clinical trial data capture the total number of subject exposures. To better represent class-related disproportional reporting and to normalize the uncertainties of single-drug event counts, in this approach, we used a pool of 30 drugs to span much larger (3.89 million) reports than any single drug. Selecting PTs from pharmacovigilance data is a more objective method to quantify the accumulated burden of class-specific side effects in drug-development trials and provides a measurable benchmark for future schizophrenia treatments developed in a novel pharmacological class. The cumulative AE curves of this work will be sensitive to a lack of efficacy, as specific disease-related symptoms are not manually excluded in the query for class effects. For example, placebo treatment accumulated class-specific AEs, indicative of a lack of efficacy with disorder-related PTs such as psychosis, schizophrenia, and hallucinations.
The identification of PTs for a class-effect query utilizing post-marketing data sources such as FAERS is influenced by the limitations of pharmacosurveillance itself. The PTs selected rely on spontaneous reporting, and are limited by under-reporting, reporter biases, variability in reporting standards, inclusion of non-healthcare professional confirmed (consumer) reports, and incomplete data. The inclusion of the selected PTs is further influenced by the inability to filter out those events that are due to underlying disease, confounding co-morbidities, other risk factors, or other concomitantly administered drugs.
A limitation of this work is the sample size of ulotaront clinical study data relative to the available lurasidone clinical trial data used as an example of the antipsychotic class. In addition, as ulotaront is the first TAAR1 agonist with clinical trial data in patient populations, no comparison data for its class exist in real-world use. Because the class-related ADRs are pharmacologically driven, comparisons between antipsychotics are influenced by dose. In contrast, the approach described in this work seeks to normalize the effect of dose by focusing on the qualitative profile of the AEs seen in clinical trials, across all doses. Cumulative ADR profiles of other established antipsychotics, not just the one used here, should be reported. Future studies on novel compounds should be performed to depict the emerging clinical trial data in this way. A full list of the PTs whose EBGM05 > 3 for the pool of 30 antipsychotics is provided in the Table in the ESM for use with other clinical trial data sets. Although this work used 30 antipsychotics for the selection of class-specific PTs, it is feasible to apply the analysis to different classes of antipsychotics, or even to different drug classes entirely, as a valuable method to establish class-wide effects and to create more informative comparisons with treatments in novel classes.
5 Conclusions
In controlled clinical trials, the TAAR1 agonist ulotaront exhibited a distinct safety profile when compared to D2-based antipsychotics (lurasidone, quetiapine, olanzapine), where over half of the AEs experienced with atypical antipsychotics in clinical trials are class specific. Application of a Bayesian disproportionality analysis of post-marketing reports collected from established drugs can meaningfully describe class specificity of emerging safety profiles of new treatments in clinical trials.
References
Citrome L, Yatham LN, Patel MD, Barabassy A, Hankinson A, Earley WR. Cariprazine and akathisia, restlessness, and extrapyramidal symptoms in patients with bipolar depression. J Affect Disord. 2021;31(288):191–8.
Correll CU, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–80.
Earley W, Durgam S, Lu K, Laszlovszky I, Debelle M, Kane JM. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: a pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int Clin Psychopharmacol. 2017;32(6):319–28.
Earley WR, Guo H, Nemeth G, Harsanyi J, Thase ME. Cariprazine augmentation to antidepressant therapy in major depressive disorder: results of a randomized, double-blind, placebo-controlled trial. Psychopharmacol Bull. 2018;48(4):62–80.
Loebel A, Cucchiaro J, Silva R, Kroger H, Hsu J, Sarma K, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160–8.
Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957–67.
Suppes T, Silva R, Cucchiaro J, Mao Y, Targum S, Streicher C, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2016;173(4):400–7.
Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232–40.
Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127–48, viii.
Miller DD, Caroff SN, Davis SM, Rosenheck RA, McEvoy JP, Saltz BL, et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry. 2008;193(4):279–88.
Juncal-Ruiz M, Ramirez-Bonilla M, Gomez-Arnau J, Ortiz-Garcia de la Foz V, Suarez-Pinilla P, Martinez-Garcia O, et al. Incidence and risk factors of acute akathisia in 493 individuals with first episode non-affective psychosis: a 6-week randomised study of antipsychotic treatment. Psychopharmacology. 2017;234(17):2563–70.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23.
Girgis RR, Zoghbi AW, Javitt DC, Lieberman JA. The past and future of novel, non-dopamine-2 receptor therapeutics for schizophrenia: a critical and comprehensive review. J Psychiatr Res. 2019;108:57–83.
Read J, Sacia A. Using open questions to understand 650 people’s experiences with antipsychotic drugs. Schizophr Bull. 2020;46(4):896–904.
Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Pharmacol Exp Ther. 2019;371(1):1–14.
Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, et al. A non-D2-receptor-binding drug for the treatment of schizophrenia. N Engl J Med. 2020;382(16):1497–506.
Hopkins SC, Dedic N, Koblan KS. Effect of TAAR1/5-HT1A agonist SEP-363856 on REM sleep in humans. Transl Psychiatry. 2021;11(1):228.
Galluppi GR, Fisher JM, Hopkins SC. Population pharmacokinetic analysis of ulotaront in subjects with schizophrenia. Pharmacomet Syst Pharmacol. 2021;10:1245–54.
Galluppi GR, Polhamus DG, Fisher JM, Hopkins SC, Koblan KS. Population pharmacokinetic analysis of ulotaront in subjects with schizophrenia. CPT Pharmacomet Syst Pharmacol. 2021;10:1245–54.
Ogasa M, Kimura T, Nakamura M, Guarino J. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology. 2013;225(3):519–30.
Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829–36.
Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47(5):670–7.
Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145(1–3):101–9.
US FDA. Labeling for human prescription drug and biological products: determining established pharmacologic class for use in the highlights of prescribing information. Silver Spring: US FDA; 2009.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51.
Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62.
Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Kissling W, et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull. 2012;38(1):167–77.
Khouri C, Petit C, Tod M, Lepelley M, Revol B, Roustit M, et al. Adverse drug reaction risks obtained from meta-analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J Clin Epidemiol. 2021;134:14–21.
Acknowledgements
Sunovion discovered ulotaront in collaboration with PsychoGenics based in part on a mechanism-independent approach using the in vivo phenotypic SmartCube® platform and associated artificial intelligence algorithms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Sunovion Pharmaceuticals Inc.
Conflict of interest
All authors are employees of Sunovion Pharmaceuticals Inc.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Available from the corresponding author upon request.
Code availability
Available from the corresponding author upon request.
Authors’ contributions
SCH: conceptualization, writing, and methodology; AO: methodology, formal analysis, and data curation; MAW: validation, resources, and writing; and KSK: conceptualization and writing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hopkins, S.C., Ogirala, A., Worden, M. et al. Depicting Safety Profile of TAAR1 Agonist Ulotaront Relative to Reactions Anticipated for a Dopamine D2-Based Pharmacological Class in FAERS. Clin Drug Investig 41, 1067–1073 (2021). https://doi.org/10.1007/s40261-021-01094-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01094-7