Abstract
Background and Objectives
Tegoprazan is one of the potassium-competitive acid blockers (P-CABs). It exhibits its anti-secretory effects by competitively and reversibly blocking the availability of K+ of the H+, K+-ATPase. This study was designed to investigate the safety and pharmacokinetics of tegoprazan in healthy Chinese subjects.
Methods
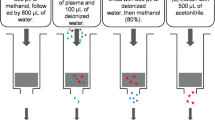
Thirty-eight healthy Chinese subjects were recruited in this randomized, single-center, double-blind, placebo-controlled study, with a single ascending dose of 50, 100, 200 mg and a multiple dose of 100 mg for 10 days. The plasma concentration of tegoprazan was determined by a validated liquid chromatography tandem mass spectrometry (LC–MS/MS) method. Pharmacokinetics were evaluated via non-compartmental and compartmental model analysis. Safety was assessed by physical examinations, vital signs, clinical laboratory tests, and electrocardiograms.
Results
No serious adverse event was observed in this study. After single-dose administration (50, 100 and 200 mg), tegoprazan was rapidly absorbed with a median maximum measure plasma concentration (Tmax) at 0.5 h and declined with a terminal (elimination) half-life (t1/2) of 3.87–4.57 h. The maximum measured plasma concentration (Cmax) for tegoprazan was 813.80, 1494.60 and 2829.00 ng/mL. Meanwhile, the corresponding area under the concentration–time curve (AUC) from time zero to infinity (AUC0−inf) was 2761.00, 5980.05 and 11,044.72 ng∙h/mL in 50, 100, 200 mg group, respectively. Dose-dependent increase was observed in the value of Cmax and AUC after administration of tegoprazan 50 to 200 mg. The two-compartment model well described the pharmacokinetic profile of tegoprazan. In the steady state, no accumulation was found after repeated administration at the 100-mg dose level. No experimental differences were found based on gender.
Conclusions
Tegoprazan was well tolerated in the dose range of 50–200 mg in single- and 100 mg in multiple-dose studies. Tegoprazan shows dose linearity with oral administration after a single dose of 50 to 200 mg and less drug accumulation after 10 days of continuous administration in 100 mg.




Similar content being viewed by others
References
Devault KR, Talley NJ. Insights into the future of gastric acid suppression. Nat Rev Gastroenterol Hepatol. 2009;6(9):524–32.
Mejia A, Kraft WK. Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol. 2014;2(3):295–314.
Shin JM, Vagin O, Munson K, et al. Molecular mechanisms in therapy of acid-related diseases. Cell Mol Life Sci. 2008;65(2):264–81.
Fock KM, Ang TL, Bee LC, et al. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet. 2008a;47(1):1–6.
CheyWd MRCL. Nighttime symptoms and sleep impairment among patients with gastro-esophageal reflux disease (GERD) receiving prescription (Rx) proton pump inhibitors (PPIs). Gastroenterology. 2008;134(4 Suppl. 1):A323–4.
Shin JM, Sachs G. Long-lasting inhibitors of the gastric H, K-ATPase. Expert Rev Clin Pharmacol. 2014;2(5):461–8.
Fock KM, Ang TL, Bee LC, et al. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet. 2008b;47(1):1–6.
Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl) -1H-pyrrol-3-yl]-N-methylmethanamineMonofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339(2):412–20.
Takahashi N, Take Y. tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther. 2018;364(2):275–86.
Parsons ME, Keeling DJ. Novel approaches to the pharmacological blockade of gastric acid secretion. Expert Opin Investig Drugs. 2005;14(4):411–21.
Berg A, Böttcher G, Andersson K, et al. Early stellate cell activation and veno-occlusive-disease (VOD)–like hepatotoxicity in dogs treated with AR-H047108, an imidazopyridine proton pump inhibitor. Toxicol Pathol. 2008;36(5):727–37.
Lee KJ, Son BK, Kim GH, et al. Randomised phase 3 trial: tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49(7):864–72.
Han S, Youn Choi H, Han Kim Y, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of escalating single and multiple oral doses of CJ-12420 (tegoprazan), a novel potassium-competitive acid blocker (P-CAB) in healthy male subjects. Clin Ther. 2017;39(8):e97–8.
Kim D, Lee KH, Kim SJ, et al. Effects of tegoprazan, a novel potassium-competitive acid blocker, on rat models of gastric acid-related disease. J Pharmacol Exp Ther. 2019;369:318–27.
Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636–48.
Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/non-Japanese Subjects. Clin Transl Gastroenterol. 2015;6(6):e94.
Acknowledgements
The authors thank all the volunteers who took part in the trial, as well as the staff that assisted with the trial at study site. The authors would also like to thank HK inno.N and Shandong Luoxin Pharmaceutical Group Stock Co., Ltd. for providing the study drug and reference standards.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Shandong Luoxin Pharmaceutical Group Stock Co., Ltd. China.
Conflict of interest
B. Zhang, J. Lu, and S. Chen are employees of Shandong Luoxin Pharmaceutical Group Stock Co., Ltd. China. J. He, G. Cao, J. Yu, X. Wu, J. Wang, J. Wu and J. Zhang are employed by the Huashan Hospital, Fudan University, Shanghai, China, which received funding from Shandong Luoxin Pharmaceutical Group Stock Co., Ltd. China to carry out this study. N. Cheng declares he has no conflict of interest.
Ethical approval
The study protocol and informed consent documents were approved by the Ethics Committee of Huashan Hospital, meanwhile the study was licensed in National Medical Products Administration (NMPA) in China with the registration number of 2017L04362, and the Identifier in ClinicalTrials.gov was NCT03458650. Study conduct was in accordance with the principles of International Conference on Harmonization, Declaration of Helsinki, NMPA, and Good Clinical Practice.
Consent to participation
Written informed consent was obtained from all individual participants included in the studies before commencing any study-related procedures.
Consent for publication
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
JH and GC contributed to subject recruitment, sample and data collection, biological analysis, and writing of the manuscript. JY and XW was contributed to sample collection and managed investigational drug. NC contributed to data analysis and manuscript review. JW contributed to collect samples as nurse. JW contributed to clinical observation as a doctor. JZ contributed to management of the clinical trial process.
Rights and permissions
About this article
Cite this article
He, J., Cao, G., Yu, J. et al. Safety, Tolerability and Pharmacokinetics of Single Ascending and Multiple Oral Doses of Tegoprazan in Healthy Chinese Subjects. Clin Drug Investig 41, 89–97 (2021). https://doi.org/10.1007/s40261-020-00986-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00986-4