Abstract
Background and Objective
Gastric cancer has been associated with notable geographic heterogeneity in previous multi-regional studies. In particular, patients from Japan have better outcomes compared with patients from other regions. Here, we assess patient-focused outcomes for the subgroup of Japanese patients in the global RAINBOW study.
Methods
Quality of life (QoL) was assessed using the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire-Core 30 (QLQ-C30) at baseline and 6-week intervals. Investigators assessed performance status before each 4-week cycle. Time-to-deterioration in each QLQ-C30 scale was defined as randomization to first worsening of ≥ 10 points (on a 100-point scale). Time-to-deterioration in performance status was defined as first worsening to ≥ 2. Hazard ratios were estimated using Cox proportional hazards models.
Results
The Japan subgroup contained 140 patients (ramucirumab plus paclitaxel, n = 68; placebo plus paclitaxel, n = 72); baseline QoL data were available for all patients. At baseline, QLQ-C30 scores were similar between study arms. Of the 15 QLQ-C30 scales, nine had a hazard ratio < 1, indicating similar or numerically longer time-to-deterioration in QoL for ramucirumab plus paclitaxel; all 95% confidence intervals included 1. Best mean change from baseline numerically favored ramucirumab plus paclitaxel in most QoL scales. The hazard ratios for time-to-deterioration of performance status to ≥ 2 were 0.64 in the Japan subgroup and 0.88 in the non-Asian subgroup. The Japan subgroup had better QoL at baseline compared with the non-Asian subgroup.
Conclusions
Treatment with ramucirumab plus paclitaxel maintained QoL and performance status over time compared with placebo plus paclitaxel in the Japan subgroup of the RAINBOW trial. These data suggest that the heterogeneity in gastric cancer between geographic regions includes multiple measures of QoL.
Trial registration number
NCT01170663 (first submitted 21 July, 2010).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The phase III RAINBOW trial showed ramucirumab plus paclitaxel versus placebo plus paclitaxel significantly improved survival in the second-line treatment of patients with gastric cancer in the overall intention to-treat population, as well as the subgroup in Japan. |
The RAINBOW trial also demonstrated quality of life was better maintained in the ramucirumab plus paclitaxel arm of the intention-to treat population. |
This study investigated the impact of ramucirumab plus paclitaxel on patient-focused outcomes in the Japan subgroup of the RAINBOW trial and showed quality of life and performance status were better maintained over time compared with placebo plus paclitaxel. Furthermore, the Japan subgroup had better quality of life at baseline compared with the non-Asian subgroup. |
1 Introduction
Globally, gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third leading cause of cancer-related deaths [1], and is often diagnosed at an advanced stage [2]. In Japan, it is the most common type of cancer diagnosed in men. Contrary to worldwide trends, early detection is often possible in Japan because of widely performed screening [2], yet the population is still high risk for GC [3]. Moreover, the aggressive nature of the disease often results in poor outcomes and worsening quality of life (QoL).
Recently, QoL has emerged as an increasingly important outcome in GC to complement traditional oncologic outcomes such as overall survival (OS), and tumor-related outcomes such as progression-free survival (PFS) and objective response rate (ORR) [4, 5]. Quality of life is a subjective multidimensional concept, which encompasses physical, psychological, and social issues [6]. In addition to having prognostic value in advanced GC [7,8,9], QoL has been associated with tumor-related outcomes and may provide a more complete assessment of treatment effectiveness for patients and clinicians.
Quality of life is typically assessed through questionnaires completed by the patient. One of the most reliable and widely used QoL questionnaires is the European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life Questionnaire-Core 30 (QLQ-C30) [10]. The EORTC QLQ-C30 measures 15 scales: one global health status/QoL scale, five functional scales (physical, role, emotional, cognitive, and social functioning), three multi-item symptom scales (fatigue, nausea/vomiting, and pain), and six single items for specific symptom scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties) [10]. Dimensions of QoL most impacted by advanced GC include global QoL, fatigue, pain, and appetite loss [11]. Baseline QLQ-C30 data have predicted survival in patients with advanced GC in both first- and second-line settings [7, 9].
In patients with advanced GC, improvement in OS remains the main goal. However, with the limited OS improvements demonstrated to date, patient-reported outcomes including QoL are increasingly recognized as critical endpoints in cancer treatment [11,12,13]. Unfortunately, only a few studies have demonstrated OS improvement and, as a result, QoL data in conjunction with clinical outcomes remain limited and the understanding and interpretation of these instruments are still poor.
RAINBOW, a phase III randomized trial, evaluated ramucirumab plus paclitaxel as compared to placebo plus paclitaxel in patients with metastatic or non-resectable GC or gastroesophageal junction adenocarcinoma who had progressed on first-line chemotherapy [14]. In this global trial, ramucirumab plus paclitaxel resulted in significantly improved OS (median 9.6 vs 7.4 months, hazard ratio [HR]: 0.807; p = 0.017), PFS (median 4.4 vs 2.9 months, HR: 0.635; p < 0.0001), and ORR (28% vs 16%; p = 0.0001) compared to placebo plus paclitaxel, along with a manageable safety profile [14]. In addition, the safety and efficacy of this treatment was also reported in the Japanese population of the RAINBOW trial (median OS 11.4 vs 11.5 months, HR: 0.88; p = 0.5113; median PFS 5.6 vs 2.8 months, HR: 0.50; p = 0.0002, and ORR 41% vs 19%, p = 0.0035) [15]. Results of the RAINBOW trial also demonstrated that QoL was maintained in the intention-to-treat population [13].
There is remarkable geographic heterogeneity in the incidence and mortality of GC. In particular, outcomes for patients from Japan are better than patients from Western regions [16,17,18]. The reasons for this heterogeneity are unclear, although factors such as nationwide screening and high post-progression treatment rates in Japan may contribute to this phenomenon [19, 20]. In this article, we describe the impact of second-line ramucirumab plus paclitaxel treatment on QoL in the Japan subgroup of the RAINBOW trial. The treatment arms were compared with respect to patient-reported functioning and symptoms (disease and treatment related), as measured by the EORTC QLQ-C30. In addition, deterioration in investigator-assessed performance status (PS) was evaluated. To our knowledge, this analysis is the first to assess QoL in a Japanese subgroup of a multi-regional study of GC and provides new insights into the geographic heterogeneity of GC. Furthermore, the results of this study may help refine the design of future GC studies, minimize gaps in healthcare between Japan and other regions, and ultimately benefit patients with GC.
2 Patients and Methods
2.1 Study Design
The design of the global, randomized, double-blind, phase III RAINBOW trial, as well as the study flowcharts for the intention-to-treat population and Japan subgroup, have been published previously [14, 15]. Briefly, eligible patients had locally recurrent/advanced, measurable or non-measurable, metastatic or nonresectable GC or gastroesophageal junction adenocarcinoma that had progressed despite first-line platinum- and fluoropyrimidine-based treatment, and an Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1. Patients were randomly assigned, in a 1:1 ratio, to receive either ramucirumab 8 mg/kg or placebo intravenously on days 1 and 15 plus paclitaxel 80 mg/m2 intravenously on days 1, 8, and 15 of a 28-day cycle. Randomization was stratified by geographic region (region 1: Europe, Israel, Australia, and the USA; region 2: Argentina, Brazil, Chile, and Mexico; and region 3: Japan, South Korea, Hong Kong, Singapore, and Taiwan), time to progression after the first dose of first-line therapy (< 6 months vs ≥ 6 months), and disease measurability (measurable vs non-measurable). The primary endpoint was OS, and secondary endpoints included PFS, ORR, QoL, and safety. The study was approved by the ethical and local institutional review boards for the participating sites, and was conducted in accordance with the CONSORT 2010 Statement, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Patients enrolled from Japan were considered for the purpose of this analysis and were called the Japan subgroup. To draw comparisons at baseline between the Japanese and global populations, patients in regions other than East Asia (i.e., patients enrolled from Argentina, Australia, Brazil, Chile, Europe, Israel, Mexico, and USA) were grouped in the non-Asian subgroup. Patients completed the QLQ-C30 (version 3.0) at baseline, every 6 weeks while receiving study therapy, and at the end of treatment. Radiological tumor assessments were performed at baseline and every 6 weeks (Response Evaluation Criteria in Solid Tumors, version 1.1). Performance status was evaluated at baseline and before every 28-day cycle.
2.2 Statistical Considerations
For the QLQ-C30, compliance at each assessment time point was defined as the number of patients who completed the questionnaire divided by the expected number of patients at that time point; the expected number of patients at any post-baseline visit was equal to the number of patients who were alive and without disease progression. The QoL data were scored according to EORTC QLQ-C30 guidelines, with all scales reported from 0 to 100. Higher scores represented better QoL for the functioning and global QoL scales. For the symptom scales, higher scores meant a greater symptom burden. A change of at least 10 points (an increase of ≥ 10 points for the functional scales and the global QoL scale or a decrease of ≥ 10 points for the symptom scales) was considered clinically meaningful [21].
For each patient and each QoL scale, change from baseline was calculated for each post-baseline assessment. For each treatment arm, the mean of the best post-baseline change and the mean difference between arms were estimated. This summary was limited to those patients with at least one post-baseline assessment. The QLQ-C30 response analysis characterized each post-baseline assessment as improved or deteriorated if the change was ≥ 10 points, and stable if the change was < 10 points for each of the scales. Of interest was the proportion of patients with improved or stable scores.
The QLQ-C30 time-to-deterioration (TTD) was defined as the time from randomization to the first deterioration of ≥ 10 points from baseline for each scale. Censoring occurred at the date of the last QLQ-C30 assessment if no deterioration was observed. The QLQ-C30 TTD was compared between the treatment arms using an unstratified log-rank test. The TTD HRs and 95% confidence intervals were estimated using the Cox proportional hazards model with assigned treatment and baseline score as covariates.
The ECOG PS TTD was defined as the time from randomization to the first time an ECOG PS score of ≥ 2 was observed. If no deterioration was observed, censoring occurred at the date of the last ECOG PS assessment. The ECOG PS TTD was compared between the treatment arms using an unstratified log-rank test and was presented using the Kaplan–Meier method. The HR was estimated using the Cox proportional hazards model.
3 Results
3.1 Patient Demographics and Baseline QLQ-C30 Data (Japan vs Non-Asian)
Of the 665 patients randomized globally, 140 patients from the sites in Japan were included in this analysis. Of these, 68 were in the ramucirumab plus paclitaxel arm and 72 in the placebo plus paclitaxel arm, and the mean age was 64 and 63 years, respectively. The primary tumor site was gastric for the majority of patients (96% and 90% in the ramucirumab plus paclitaxel and placebo plus paclitaxel arms, respectively). Table 1 presents the baseline demographics and disease characteristics.
In the Japan subgroup, a higher number of patients had an ECOG PS of 0 at baseline (54% and 60% in the ramucirumab plus paclitaxel and placebo plus paclitaxel arms, respectively), whereas amongst the non-Asian subgroup patients, approximately one-third had an ECOG PS of 0 in both treatment arms. In the Japanese population, baseline QLQ-C30 data were available for all patients. Among symptoms, the highest level of impairment was reported in fatigue, followed by appetite loss, constipation, insomnia, pain, and dyspnea based on the mean scores at baseline. Overall, the QLQ-C30 scores were similar between the two treatment arms. The QLQ-C30 scores observed for the Japan subgroup at baseline showed a better trend for almost all parameters than the non-Asian subgroup. A summary of baseline QLQ-C30 scores by treatment arm and region (Japan vs non-Asian) is shown in Fig. 1.
Quality-of-Life Questionnaire-Core 30 (QLQ-C30) scores by treatment arm at baseline a Japan and b Non-Asian. Non-Asian comprised patients in regions other than East Asia (i.e., patients enrolled from Argentina, Australia, Brazil, Chile, Europe, Israel, Mexico, and USA). *The baseline data were not available for all patients and ‘N’ varied (ramucirumab and paclitaxel arm: 214–215; placebo plus paclitaxel arm: 215–217). Error bars represent standard deviation; scores range from 0 to 100 (y-axis runs to 120 because of error bars); for functional scales and global quality of life (QoL), higher score = better QoL; for symptom and financial difficulties scales, lower score = better QoL
3.2 EORTC QLQ-C30 Compliance
As stated above, all patients completed the QLQ-C30 at baseline. The number expected to be completed at each scheduled assessment decreased over time due to the decrease in the number of patients who remained on study therapy, with a more rapid decrease in the placebo plus paclitaxel arm. Based on expected assessments, a percentage compliance of > 70% was observed at the early assessment times in both ramucirumab plus paclitaxel and placebo plus paclitaxel arms (Table 1 of the Electronic Supplementary Material).
3.3 EORTC QLQ-C30 Mean of Best Change from Baseline
All of the patients in the ramucirumab plus paclitaxel arm and 97% of the patients in the placebo plus paclitaxel arm were included in the best change analyses. In the ramucirumab plus paclitaxel arm, improvement from baseline was observed in all scales (functional scales range: 2.3–7.9, symptom scales range: − 0.5 to − 12.0 points) (Fig. 2). In the placebo plus paclitaxel arm, worsening of symptoms was observed in nausea/vomiting (0.6 points) and pain (0.4 points), and the mean changes from baseline had ranges of 0.4–5.4 and 0.4 to − 7.3 in functional scales and symptoms, respectively. A clinically meaningful improvement from baseline was observed in appetite loss (mean change from baseline: − 12.0 points) and constipation (mean change from baseline: − 11.7 points) in the ramucirumab plus paclitaxel arm. In the placebo plus paclitaxel arm, no clinically meaningful improvements were observed. Overall, the highest treatment-arm difference was observed for pain with a mean improvement from baseline of 8 points in the ramucirumab plus paclitaxel arm and a worsening of 0.4 points in the placebo plus paclitaxel arm, resulting in a total difference of 8.4 points in the mean change from baseline between arms. Diarrhea was the only symptom scale that did not favor ramucirumab plus paclitaxel (treatment difference: 1.1).
3.4 EORTC QLQ-C30 Response Analysis Over Time
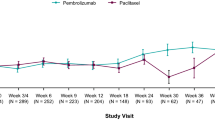
At week 6, similar percentages of patients reported improved or stable scores between treatment arms; however, from week 12 onwards, higher percentages were observed for the ramucirumab plus paclitaxel arm in all of the scales. Figure 3 summarizes the QLQ-C30 response over time for select scales of global QoL, physical functioning, and role functioning scores, as well as appetite loss, pain, and fatigue symptom scores. Similar patterns were observed for the other QoL scales (data not shown). At week 6 in the ramucirumab arm, the highest number of improved or stable scores relative to baseline were observed for physical functioning and emotional functioning (72% each) among functional scales, and nausea/vomiting (76%) among symptom scales. Across all time points, each scale had a greater number of patients with ‘no data’ in the placebo plus paclitaxel arm than in the ramucirumab plus paclitaxel arm.
Summary of European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire-Core 30 (EORTC QLQ-C30) response analysis over time for select scales a global quality of life; b physical functioning; c role functioning; d fatigue; e pain; and f appetite loss. All patients included to derive percentages (ramucirumab plus paclitaxel, n = 68; placebo plus paclitaxel, n = 72); ‘No data’ was primarily due to discontinuation of therapy related to tumor progression. The QLQ-C30 response analysis characterized each post-baseline assessment as improved or deteriorated if the change was ≥ 10 points, and stable if the change was <10 points for each of the scales
3.5 EORTC QLQ-C30 Time to Deterioration
Of the 15 QLQ-C30 scales, nine had a HR < 1 (range 0.65–0.99), indicating similar or numerically longer TTD in QoL in patients treated with ramucirumab plus paclitaxel as compared to those treated with placebo plus paclitaxel. These included physical functioning, emotional functioning, nausea/vomiting, and pain. Hazard ratios that favored placebo plus paclitaxel included appetite loss and diarrhea. A summary of HRs for TTD is presented in Fig. 4.
3.6 Eastern Cooperative Oncology Group Performance Status Time to Deterioration
The analysis of TTD in ECOG PS (Fig. 5) showed that the HRs for treatment with ramucirumab plus paclitaxel for TTD of PS to ≥ 2 were 0.64 in the Japan subgroup and 0.88 in the non-Asian subgroup. As a result of censoring, medians could not be estimated in the Japan subgroup.
4 Discussion
The phase III RAINBOW trial demonstrated that the addition of ramucirumab to paclitaxel significantly increases OS compared with placebo plus paclitaxel, and can be regarded as a standard second-line treatment for patients with advanced GC [14]. It was also found that the safety and efficacy of this treatment observed in the Japanese population was consistent with that observed in the overall population [15]. Furthermore, a pooled analysis of patient-reported outcomes and QoL in two phase III ramucirumab trials (RAINBOW and REGARD) established that QoL could be an additional tool to assess tumor status for patients with non-measurable disease [11]. Additionally, baseline QoL has been shown to be a prognostic factor for GC that is associated with improved clinical outcomes [22]. It is pertinent from these findings that, although improved survival is key, preserving QoL is a critically important consideration in this setting. There have been very few positive phase III studies in patients with GC and understanding the QoL of these patients is important when considering extended survival benefit.
The present analyses aimed to provide a source of patient-reported outcome data to better understand treatment and disease burden in patients with advanced GC in Japan receiving second-line systemic therapy. The Japan subgroup had better QoL at baseline compared with the non-Asian subgroup. The more favorable baseline QoL scores in the Japan subgroup compared with the non-Asian subgroup is consistent with the higher percentage of patients with baseline ECOG PS of 0. The ECOG PS is a function of cancer symptom burden [23,24,25] and an association between QoL and PS in patients with GC has already been reported [11]. Other reasons for the better QoL at baseline in the Japan subgroup may be related to differences in disease etiology, such as a lower number of distal tumors, and younger age and earlier stage at diagnosis, or differences in medical practice between the regions [26,27,28,29]. These factors are worth exploring in future studies.
The HRs for TTD to PS ≥ 2 with ramucirumab plus paclitaxel vs placebo plus paclitaxel were 0.64 in the Japan subgroup and 0.88 in the non-Asian subgroup. Neither treatment arm in the Japan subgroup reached the median for TTD of PS to ≥ 2, which is in contrast to the non-Asian subgroup. These data suggest that the better baseline QoL scores and PS maintenance during treatment observed for Japanese patients partially explain why Japanese patients with GC tend to have longer survival compared with non-Asian patients [15, 30]. These findings provide new insights into the geographic heterogeneity of advanced GC, and may help refine the design of future GC studies and improve outcomes for patients with GC.
In the Japan subgroup, QoL was similar between the ramucirumab plus paclitaxel and placebo plus paclitaxel arms at baseline. Consistent with the global population, fatigue and appetite loss had the poorest baseline scores amongst symptom scales in the Japan subgroup. Both of these symptoms are closely related to the advanced GC disease state and hence are expected to present with poorer scores [13]. At week 6, approximately 80% of patients in each arm provided QLQ-C30 data, with more than half of these patients reporting improved or stable scores, with stable scores more frequent. After week 6, a lower proportion of patients in the ramucirumab plus paclitaxel arm were classified as ‘no data,’ consistent with the longer PFS observed. The ‘no data’ group was considered relevant to account for over time as the data are not missing at random and assumed to be most similar to those with deteriorated QoL scores.
Over time, numerically more patients in the ramucirumab plus paclitaxel arm reported improved or stable scores. Results of the response analysis therefore supported the TTD in QoL results. It is worth noting that since an improvement is not possible when a patient is relatively asymptomatic or has good QoL at baseline, a treatment with benefits in QoL is more likely to demonstrate maintenance of QoL/symptoms functioning. These Japan subgroup findings were supported by the similar analysis in the global population in which a consistently numerically higher percentage of patients in the ramucirumab plus paclitaxel arm experienced stable or improved QoL parameters at each assessment, compared with patients in the placebo plus paclitaxel arm [13].
The TTD in QoL scales in the Japan subgroup was consistent with the results from the global population [13], but with more variability in point estimates for HRs. As observed in the global population, the highest non-favorable HR was reported for diarrhea, which was consistent with the higher rate of any-grade diarrhea reported by investigators in both the global and Japanese populations [13, 15]. As diarrhea is a known adverse event reported with the ramucirumab plus paclitaxel combination, the observed HR was not unexpected.
Similarly, the results from TTD in ECOG PS were consistent with those reported previously in the global RAINBOW population [13]. Although primarily utilized for its prognostic value, physicians commonly assess ECOG PS to monitor a patient’s physical functioning. The ECOG PS has been considered a surrogate when patient-reported functioning has not been assessed, with values ≥ 2 associated with impaired mobility and self-care. In addition, continuation of treatment may not be warranted if PS deteriorates as chemotherapy is usually not indicated for patients with PS of > 2 [31].
In the Japan subgroup of the RAINBOW trial, the addition of ramucirumab to second-line paclitaxel therapy extended median PFS in patients with advanced GC [15]. Additionally, the ORR (ramucirumab plus paclitaxel: 41% vs placebo plus paclitaxel: 19%) and disease control rate (ramucirumab plus paclitaxel: 94% vs placebo plus paclitaxel: 75%) were also improved. The current QoL analysis indicates that greater tumor shrinkage and longer PFS with the addition of ramucirumab to paclitaxel also leads to better QoL outcomes. This can be considered as meaningful when taking into account the median duration of treatment for patients in the ramucirumab plus paclitaxel arm compared to patients in the placebo plus paclitaxel arm (23 weeks vs 12 weeks) [15]. A longer duration of ramucirumab plus paclitaxel treatment resulted in efficacy results without deteriorating patients’ QoL in the Japanese population.
There were limitations associated with this study. Although compliance with completing the QoL questionnaire was high at baseline, this compliance decreased over time, more so in the placebo plus paclitaxel arm. This limitation is common in cancer QoL studies and may be attributed to multiple factors, including deterioration in clinical status [32,33,34,35]. In future studies, compliance may be improved with staff commitment and education, as well as taking advantage of existing tracking systems [36]. A further limitation was the small number of patients in this subgroup analysis, which may have led to the variability in results that prevented detailed description. Thus, overall conclusions are based on consistent trends in results across the presented analyses, with particular consideration of the goal of maintaining QoL.
5 Conclusions
Treatment with ramucirumab plus paclitaxel maintained QoL over time as compared with placebo plus paclitaxel in the Japan subgroup of the RAINBOW trial. The Japan subgroup also showed better QoL at baseline compared with the non-Asian subgroup. These data suggest that the heterogeneity in GC between geographic regions includes multiple measures of QoL. The results could aid decisions pertaining to clinical care and treatment options in the advanced GC setting.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: gastric cancer version 2. 2019. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed 4 Jun 2019.
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48.
Al-Batran SE, Ajani JA. Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer. 2019;116:2511–8.
Conroy T, Marchal F, Blazeby JM. Quality of life in patients with oesophageal and gastric cancer: an overview. Oncology. 2006;70:391–402.
Padmaja G, Vanlalhruaii C, Rana S, Tiamongla, Kopparty S. Quality of life of patients with cancer: a determinant of the quality of life of their family caregivers. J Cancer Educ. 2017;32:655–61.
Fuchs CS, Muro K, Tomasek J, Van Cutsem E, Cho JY, Oh S, et al. Prognostic factor analysis of overall survival in gastric cancer from two phase 3 studies of second-line ramucirumab (REGARD and RAINBOW) using pooled patient data. J Gastric Cancer. 2017;17:132–44.
Park SH, Cho MS, Kim YS, Hong J, Nam E, Park J, et al. Self-reported health-related quality of life predicts survival for patients with advanced gastric cancer treated with first-line chemotherapy. Qual Life Res. 2008;17:207–14.
Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer: pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–403.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Chau I, Fuchs CS, Ohtsu A, Barzi A, Liepa AM, Cui ZL, et al. Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: exploratory analysis of RAINBOW and REGARD phase 3 trials. Eur J Cancer. 2019;107:115–23.
McCall MD, Graham PJ, Bathe OF. Quality of life: a critical outcome for all surgical treatments of gastric cancer. World J Gastroenterol. 2016;22:1101–13.
Al-Batran SE, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. Quality-of-life and performance status results from the phase 3 RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol. 2016;27:673–9.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35.
Shitara K, Muro K, Shimada Y, Hironaka S, Sugimoto N, Komatsu Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer. 2016;19:927–38.
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47:2306–14.
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, et al.; COUGAR-02 Investigators. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86.
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–44.
Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006;24:2188–96.
Kim R, Tan A, Choi M, El-Rayes BF. Geographic differences in approach to advanced gastric cancer: is there a standard approach? Crit Rev Oncol Hematol. 2013;88:416–26.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44.
Abdel-Rahman O. Prognostic impact of baseline quality of life status among patients with advanced gastric cancer; results from two randomized studies. Expert Rev Pharmacoecon Outcomes Res. 2019;19:711–5.
Shitara K, Muro K, Matsuo K, Ura T, Takahari D, Yokota T, et al. Chemotherapy for patients with advanced gastric cancer with performance status 2. Gastrointest Cancer Res. 2009;3:220–4.
Shitara K, Takahari D. A case of advanced gastric cancer with poor performance status which improved by chemotherapy. Case Rep Oncol. 2010;3:262–7.
Wang J, Qu J, Li Z, Che X, Zhang J, Liu J, et al. A prognostic model in metastatic or recurrent gastric cancer patients with good performance status who received first-line chemotherapy. Transl Oncol. 2016;9:256–61.
Takahari D, Boku N, Mizusawa J, Takashima A, Yamada Y, Yoshino T, et al. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist. 2014;19:358–66.
Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965–71.
Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–6.
Davis PA, Sano T. The difference in gastric cancer between Japan, USA and Europe: what are the facts? What are the suggestions? Crit Rev Oncol Hematol. 2001;40:77–94.
Sawaki A, Yamada Y, Yamaguchi K, Nishina T, Doi T, Satoh T, et al. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer. 2018;21:429–38.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Blazeby JM, Nicklin J, Brookes ST, Winstone K, Alderson D. Feasibility of quality of life assessment in patients with upper gastrointestinal tract cancer. Br J Cancer. 2003;89:497–501.
Tabernero J, Alsina M, Shitara K, Doi T, Dvorkin M, Mansoor W, et al. Health-related quality of life associated with trifluridine/tipiracil in heavily pretreated metastatic gastric cancer: results from TAGS. Gastric Cancer. 2020;23:689–98.
Jiao L, Dong C, Liu J, Chen Z, Zhang L, Xu J, et al. Effects of Chinese medicine as adjunct medication for adjuvant chemotherapy treatments of non-small cell lung cancer patients. Sci Rep. 2017;7:46524.
Kawahara M, Tada H, Tokoro A, Teramukai S, Origasa H, Kubota K, et al. Quality-of-life evaluation for advanced non-small-cell lung cancer: a comparison between vinorelbine plus gemcitabine followed by docetaxel versus paclitaxel plus carboplatin regimens in a randomized trial: Japan Multinational Trial Organization LC00-03 (BRI LC03-01). BMC Cancer. 2011;11:356.
Atherton PJ, Burger KN, Pederson LD, Kaggal S, Sloan JA. Patient-reported outcomes questionnaire compliance in Cancer Cooperative Group Trials (Alliance N0992). Clin Trials. 2016;13:612–20.
Acknowledgements
We thank the patients, caregivers, and investigators who participated in this study. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this manuscript to be published. Medical writing support, funded by Eli Lilly and Company, was provided by Anchal Sood and Andrew Sakko, and editorial support was provided by Antonia Baldo, Kayla Kirby, and Dana Schamberger of Syneos Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Eli Lilly and Company. The study sponsor provided the study drug and collaborated with the investigators to design the study; collect, analyze, and interpret the data; and write this report. The corresponding author had access to study data, and all authors approved submission for publication.
Conflicts of interest/Competing interests
Kensei Yamaguchi received grants and personal fees from Eli Lilly and Company. Shuichi Hironaka received personal fees from Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Yakult Honsha Co. Ltd., Daiichi Sankyo Co. Ltd., Eli Lilly and Company, Chugai Pharmaceutical Co. Ltd., Nihonkayaku, and Tsumura and Co. Yoshito Komatsu reports fees from Ono Pharmaceutical Co. Ltd., Merck Sharp & Dohme, Eli Lilly and Company, AstraZeneca, Daiichi Sankyo Co. Ltd., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Linical Co. Ltd., Tennessee Coalition for Open Government, Nassau Community College, Kyowa Hakko Kirin Co. Ltd., Takeda Pharmaceutical Co. Ltd., Sanofi, Yakult Honsha Co. Ltd., Bristol-Myers Squibb, Boehringer Ingelheim, Bayer, Pfizer, and Novartis. Takao Tamura reports grants and personal fees from Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Ono Pharmaceutical Co. Ltd., Merck Serono Co. Ltd, Bristol-Myers Squibb, and Takeda Pharmaceutical Co. Ltd. Yongzhe Piao, Gosuke Homma, Min-Hua Jen, and Astra M. Liepa are employees of Eli Lilly and Company and received salaries. Min-Hua Jen and Astra M. Liepa are also shareholders of Eli Lilly and Company. Yasuhiro Shimada, Naotoshi Sugimoto, Tomohiro Nishina, Yasushi Omuro, and Kei Muro have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Ethics approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Consent to participate
Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Authors’ contributions
YP, GH, MHJ, and AML conceived and designed the study. KY, YS, SH, NS, YK, TN, YO, TT, and KM collected the data. M-HJ analyzed the data. All authors contributed to the interpretation of the data and the writing of the manuscript.
Consent for publication
Not applicable.
Code availability
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yamaguchi, K., Shimada, Y., Hironaka, S. et al. Quality of Life Associated with Ramucirumab Treatment in Patients with Advanced Gastric Cancer in Japan: Exploratory Analysis from the Phase III RAINBOW Trial. Clin Drug Investig 41, 53–64 (2021). https://doi.org/10.1007/s40261-020-00979-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00979-3