Abstract
Background and Objective
Golimumab is a fully human anti-tumor necrosis factor monoclonal antibody approved for the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS). This study estimated rates of prespecified outcomes in patients with RA, PsA or AS initiating golimumab versus matched patients initiating non-biologic systemic (NBS) medications.
Methods
Patients enrolled in a US health plan with rheumatic disease who initiated a study medication were accrued between April 2009 and November 2014. Golimumab initiators were matched by propensity score to NBS initiators in a 1:4 ratio. Outcomes were identified through September 2015. As-treated, as-matched, and nested case–control (NCC) analyses were conducted in the matched cohorts. Sensitivity analyses evaluated the impact of residual confounding and nondifferential misclassification of exposure and outcomes.
Results
Risks of outcomes were similar between golimumab and NBS initiators. In the as-treated analysis, the rate ratio (RR) for depression was elevated during current golimumab use versus golimumab non-use in the NBS cohort [RR 1.45, 95% confidence interval (CI) 1.31–1.61]. This finding was not replicated in as-matched (RR 1.08, 95% CI 0.97–1.19) or NCC (odds ratio 1.01, 95% CI 0.78–1.31) analyses, which focused on incident cases. Sensitivity analyses suggest that depression was sensitive to misclassification, and the RR changed from greater than to less than one across a plausible range of specificity.
Conclusions
This study suggests that there is no association between exposure to golimumab and an increased risk of prespecified outcomes. Increased depression risk in the as-treated analysis was not replicated in other analyses and may be associated with residual imbalance in baseline history or severity of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study suggests that there is no association between exposure to golimumab and an increased risk of prespecified outcomes. |
The results of this study are consistent with golimumab’s overall safety profile and generally comparable with observations from other studies in patients with treated rheumatic disease. |
1 Introduction
Golimumab (Janssen Biotech, Inc.) is a fully-human tumor necrosis factor (TNF)-alpha inhibitor indicated for the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and ulcerative colitis in the USA, with additional indications for non-radiographic axial spondyloarthritis and polyarticular juvenile idiopathic arthritis in the European Union. Golimumab was first approved in the USA in 2009 for once-monthly subcutaneous administration in RA, PsA, and AS and in 2013 for intravenous administration in RA.
RA is characterized by chronic, progressive inflammation of the joints that may lead to deformity, disability, and in some cases, premature death [1, 2]. The overall prevalence of RA ranges between 0.3 and 1% among adults worldwide and is more common with increased age and among women [3, 4]. AS and PsA are related chronic inflammatory arthritides that are distinct from and less prevalent than RA. The goals of rheumatic disease management are to decrease pain, to prevent or control joint damage, and to prevent loss of function [4]. Current treatments include nonsteroidal anti-inflammatory drugs, corticosteroids, and disease-modifying anti-rheumatic drugs (DMARDs) [5,6,7]. Tumor necrosis factor inhibitors (TNFi) were the first biologic DMARDs (bDMARDs) approved for the treatment of these conditions and have revolutionized pharmacologic treatment by preventing disease progression [8,9,10].
Because of inherent immunological abnormalities associated with rheumatic disease and the immunosuppressive effects of therapeutic agents, patients may be at an increased risk of infection, hematologic malignancies, and other autoimmune diseases [11,12,13]. The incidence of infections requiring hospitalization or parenteral antibiotics in RA patients prior to the introduction of bDMARDs is estimated to be in the range of 0.02–0.12 per person-year, with infection-related deaths 6–9 times higher in RA patients compared to the general population [13, 14]. Risk factors of infection in RA patients include age, extra-articular manifestations, leukopenia, comorbidities, use of corticosteroids, skin breakdown, and joint surgery [14, 15].
Case reports and post-marketing data have documented the occurrence of serious and opportunistic infections in patients treated with bDMARDs [13, 16,17,18,19,20,21,22,23], which should not be used in patients with active infections [14]. However, not all studies have found an increased risk associated with their use [13]. As per current prescribing guidelines, use of bDMARDs is contraindicated in patients with severe active infections, and patients receiving these agents should be monitored for signs and symptoms of infection [14]. In addition to infections, malignancy, autoimmune conditions, hepatotoxicity, hepatitis B reactivation, and heart failure have been observed with use of TNFi [14].
Clinical trials of patients with RA, PsA, and AS have shown that golimumab is effective and generally well tolerated [24,25,26,27]. Due to the rarity of some outcomes and potential differences between patients enrolled in clinical trials and those under standard care, comprehensive assessments of risks associated with golimumab, a newer TNFi, among large populations reflecting routine practice are warranted. In this study, we aimed to provide a comprehensive overview of the safety of golimumab using claims data from a large US health insurer along with validation of outcomes using linked medical records and National Death Index (NDI) records. This study question is important as there is a lack of agent-specific safety data. We sought to estimate the risks of prespecified outcomes in a cohort of patients with RA, PsA, or AS initiating golimumab compared to a cohort of similar patients initiating non-biologic systemic (NBS) treatments, which is considered the standard of care and preferred comparator for biologic treatment [1, 2, 9].
2 Patients and Methods
2.1 Data Source
This prospective, observational, cohort study was conducted in the Optum Research Database (ORD), a proprietary research database containing claims and enrollment data dating back to 1993 for approximately 14 million members of a large US health plan with both medical and pharmacy benefit coverage and an average of 2.5 years of enrollment. The medical and pharmacy claims for these individuals form a longitudinal record of reimbursed medical services, irrespective of treatment site, along with detailed information on drug dispensing. The database is routinely updated and maintained. Access to a subset of patients’ medical charts (i.e. approximately 35% of all patients within the ORD) allows for confirmation of outcomes identified through claims. The underlying insured population is geographically diverse across the USA, representing approximately 4% of its population.
This study was approved by the New England Institutional Review Board and Privacy Board.
2.2 Patient Population
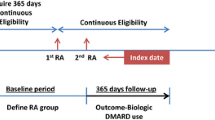
Patients who initiated golimumab or NBS treatments (methotrexate, azathioprine, cyclosporine, penicillamine, hydroxychloroquine, sulfasalazine, apremilast, tofacitinib, leflunomide, or gold compounds) were accrued from 24 April 2009 (date of first availability of golimumab in the USA) until 30 November 2014. The date of cohort entry (i.e. index date) is the date of the first claim of the cohort-defining drug with no previous claim of that specific drug in the prior 6 months (baseline period). Eligible patients included those of any age with complete medical coverage and pharmacy benefits who were continuously enrolled for at least 6 months prior (baseline period) to the index date.
The operational definition of an initiator included patients who were naïve to antirheumatic treatments, those who switched to the cohort-defining therapy from another antirheumatic treatment, and those who started the cohort-defining therapy as add-on treatment to existing therapy. For example, golimumab initiators included patients who began therapy with golimumab as the first prescription treatment for rheumatic disease observed in the claims database, and those who switched from or added to another prescription rheumatic disease treatment regimen. The cohort for NBS treatment initiators may include, for example, methotrexate initiators without any other antirheumatic treatment in baseline, and methotrexate initiators without prior methotrexate use but with prior use of another antirheumatic (including other NBS and biologic) treatment during baseline.
Initiators were required to have at least one claim for RA, PsA, or AS [International Classification of Diseases (ICD), 9th Revision diagnosis codes 714.xx, 696.0x, and 720.0x, respectively] in the 6 months before or up to 3 months following the index date.
2.3 Propensity Score Modeling and Matching
For the prediction model discriminating golimumab initiators from patients in the NBS cohort, using claims and membership data, a set of potential a priori confounders in addition to the 100 most frequently occurring diagnoses, procedures, and drug dispensing were included in the logistic regression model for the propensity score calculation. Age, sex, region, number of days enrolled before index date, calendar time, cost (total and associated with rheumatic disease), and healthcare utilization variables were forced into the model. Additional predictors were retained based on a stepwise regression technique with an entry criterion p-value of ≤ 0.10 and an exit criterion p-value of ≥ 0.30.
The golimumab cohort was matched by propensity score to NBS initiators using a standard greedy matching algorithm [28,29,30]. In order to achieve adequate power in the context of a medication with a large pool of comparators, the final cohort included up to 4 matched comparators for each golimumab patient, with the comparison cohort member selected at random from all potential comparators whose propensity scores were within the variable caliper applied by the matching algorithm that allows a maximum difference of 0.10 to that of the golimumab patients.
2.4 Outcome Identification and Follow-Up
Prespecified outcomes of interest were identified by a claim for an outpatient physician visit or inpatient hospitalization associated with the specific disease, procedure, or drug code as listed on claims for medical services. These outcomes include:
-
serious infection (i.e. requiring hospitalization or parenteral antibiotic therapy)
-
tuberculosis (TB)/non-TB mycobacterial infection
-
malignancy
-
lymphoma
-
systemic hypersensitivity
-
congestive heart failure (CHF)
-
hepatotoxicity
-
new onset hypertension (identified among those without a baseline claim for hypertension)
-
autoimmune disease
-
hematologic reaction
-
depression
-
mortality.
See Electronic Supplementary Materials (ESM) Appendix 1 for codes that were used to identify these outcomes.
Outcome identification began the day after cohort entry through the earliest of: (1) health plan disenrollment; (2) death; or (3) 30 September 2015. For systemic hypersensitivity and mortality, follow-up began on the index date to identify those outcomes that occurred immediately after exposure. Patients were censored for subsequent occurrences of the same outcome but were eligible for others. Person-time was calculated from the day after index date (or on the index date for systemic hypersensitivity and mortality) through end of follow-up.
2.5 Medical Record Review and Refinement of Outcome Definitions
Medical record review was undertaken to confirm potential outcomes identified in the claims data between 24 April 2009 and 31 May 2014. Medical records were adjudicated by a panel of 3 clinicians to determine whether each potential case met the clinical definition of the outcome. Using the results from adjudication, a positive predictive value (PPV) was calculated for each outcome as the number of confirmed cases divided by the number of charts adjudicated, excluding those cases with insufficient information to determine case status.
Outcomes with high PPVs (> 75%) were included in analyses using the original claims definition. In order to improve the PPV and increase the likelihood of identifying true cases, the claims definitions for outcomes with low-to-moderate PPVs (≤ 75%) were revised by requiring the codes (diagnosis, procedure, drug) used to identify each outcome to appear in the principal (primary) position on an inpatient or outpatient claim. PPVs were recalculated for the revised definition of each outcome as the number of confirmed cases with codes in the primary position on the healthcare claim divided by the total number of confirmed cases and non-cases with codes in the primary position and were included in analyses using the revised definition.
2.6 National Death Index
The NDI is considered a gold standard mortality assessment in studies based on health insurance claims data, and a NDI Plus search (including fact, date, and causes of death) was conducted on all golimumab initiators and matched comparators who disenrolled from the health plan during the study period with no evidence of re-enrollment.
After restricting to patients considered to have a high probability of being a true match based on an algorithm developed by the NDI, deaths occurring within 60 days of health plan disenrollment were selected as cases. Since exposure information is only available during enrolled time and deaths occurring after disenrollment may have been exposed to other medications, a 60-day window was chosen as a trade-off between completeness of mortality identification and uncertainty regarding medication exposure. Cause-specific mortality outcomes were identified from the NDI data using ICD-10 coded causes of death.
The mortality outcome used in statistical analyses included those deaths identified in the claims data through end of follow-up with an NDI search used to validate vital status for these patients.
2.7 Statistical Analyses
All analyses used SAS (Statistical Analysis Software™) version 9.2 (SAS Institute).
For non-cancer outcomes, the primary analysis was the as-treated analysis, where incidence rates (IR) per 1000 person-years and incidence rate ratios (IRR) were estimated during periods of current drug use and non-use among the matched golimumab and matched NBS cohorts. Drug exposure was characterized based on claims records during follow-up, including date of dispensing and days supplied or recommended number of days between injections/infusions. A 60-day “grace period” was added to account for medication non-adherence and the uncertainty surrounding the duration of biologic effect for these drugs.
-
Current use: dispensing/injection/infusion date + days supplied or recommended number of days between injection/infusion + 60 days. With each new dispensing/injection/infusion, a patient continues or reinitiates on-therapy current status
-
Non-use: days during which patients are not using the study drug(s) of interest, i.e. neither current nor prior use of the drug(s) in question during the follow-up. Person-time attributed to non-use of golimumab came from the matched NBS cohort before any golimumab was dispensed during follow-up; and vice versa, person-time attributed to non-use of NBS came from the matched golimumab cohort before any NBS was dispensed during follow-up.
Multivariable Poisson regression was used to estimate the IRR of non-cancer outcomes during periods of current drug use compared with non-use. Each model was stratified by matching ratio (number of NBS matches for each golimumab initiator) and adjusted for age and sex.
For cancer outcomes, the primary analysis was the as-matched analysis where IRs per 1000 person-years were calculated based on the cohort-initiating drug. In the as-matched analysis, Cox proportional hazards regression was used to compare golimumab to NBS initiators in the matched cohorts. Models for all outcomes were stratified by matching ratio and adjusted for propensity score. To avoid overfitting or lack of model convergence, the propensity score was used for adjustment since the number of covariates that could be included in the Cox proportional hazards models was limited.
To address the different potential mechanisms of cancer occurrence, the incidence of malignancy and lymphoma was estimated using a cumulative dose measure (considered a secondary analysis for cancer outcomes) in the matched cohorts. With each claim for the dispensing of a study drug during follow-up, cumulative dose was updated, and patients were categorized into mutually exclusive categories of dose (i.e. 1–5, 6–10, and ≥ 11 dispensings). If a patient had a cancer that occurred during follow-up, it was counted within the dose category it occurred, and the patient was no longer at risk for that outcome. Similar to the as-treated analysis, the Poisson regression models were stratified by matching ratio and adjusted for age and sex.
As an additional assessment of medication exposure in relation to occurrence of outcomes, a nested case–control (NCC) analysis was performed among propensity score-matched cohorts. Cases were defined as the first outcome identified during follow-up with no baseline claims for the outcome. Each risk set included all individuals from the propensity score-matched quintuplet of the larger drug cohorts who were still at risk of the event (controls). After excluding those controls with baseline claims for a specific outcome, for each case, up to 2 controls enrolled in the health plan were randomly selected among the cohort members in the risk sets defined by the case (matching preserved), and a case/control date was assigned as the outcome date of the matched case.
Exposure to study medications was assessed among cases and controls during the 6 months prior to the case/control date, and age, sex, region, and calendar time were assessed on this date. Cases and controls were compared with respect to study drug exposure (golimumab and NBS), with multivariable-adjusted odds ratios (OR) generated from conditional logistic regression models (conditioned on the matched set) and adjusted for age, sex, region, and calendar time.
2.8 Sensitivity Analyses
Three sensitivity analyses were conducted. First, as-treated and as-matched analyses were repeated using a narrowed definition of depression where initiators with a baseline claim of a diagnosis of depression or dispensing of an antidepressant were excluded from each matched cohort. During follow-up, the first occurrence of a diagnosis for depression or antidepressant claim was identified. If both a diagnosis and dispensing occurred within 14 days of one another, then the date of the later diagnosis or dispensing was assigned as the outcome date.
Second, residual confounding in the propensity score model by covariates not included in the propensity score model or directly ascertainable in claims data (e.g. disease severity, duration) was assessed. A standard epidemiological spreadsheet was used to assess the robustness of the results in the presence of an unmeasured confounder [31]. The results of the primary analyses and ranges of association between an unmeasured confounder and disease outcome were entered into the spreadsheet and used to calculate the difference from the observed result if the unmeasured confounder was accounted for. In addition to using the adjusted IRR from the primary analyses, the value of the observed lower 95% CI was also plotted to determine the range in which the 95% CI would cross the null. The prevalence of the unmeasured confounder was fixed at 40%, which provided close to maximum potential confounding in many situations while the prevalence of the exposure was calculated as the number of person-years during current use divided by the number of person-years during non-use. The resulting figures include the range of unmeasured confounder characteristics assuming varying levels of strength of the confounder-disease association and strength of association with current golimumab use that would result in a change in the point estimates. Detailed assumptions for conducting this analysis are described in ESM Appendix 2.
For the last sensitivity analysis, another standard epidemiological spreadsheet was used to adjust primary analysis point estimates by exploring different pairs of sensitivity and specificity values in order to assess different degrees of nondifferential misclassification [32]. To assess the potential effect of nondifferential exposure misclassification, sensitivity and specificity were set to 99, 95, and 90%, respectively, to assess the effect on primary as-treated analysis point estimates in the golimumab cohort matched to NBS. Similarly, to determine the potential effect of nondifferential outcome misclassification, sensitivity was assumed to be 99% while specificity was defined as a transformation of the PPV based on contingency table analysis. The original PPV was used for malignancy, lymphoma, and autoimmune disease while the revised PPV was used for the remaining outcomes. A PPV of 50% was used to estimate misclassification bias for mortality. The specificity was varied by ± 5% to assess the effect on primary analysis point estimates across a plausible range of alternate specificity values. Detailed assumptions for conducting this analysis are described in ESM Appendix 3.
3 Results
3.1 Cohort Characteristics
From 24 April 2009 through 30 November 2014, 1515 golimumab (97.0% dispensed subcutaneous formulation) and 48,975 NBS initiators were identified, and 1337 golimumab initiators (88.3% of those eligible) were matched to 4227 NBS initiators (3.2:1 matching ratio).
Prior to matching, differences were present between the golimumab and NBS initiators with respect to baseline characteristics (Table 1). Afterwards, balance improved on most of these variables (Table 2), with absolute standardized differences generally less than 0.1. A higher proportion of matched golimumab initiators than NBS initiators had ≥ 4 different treatments for rheumatic disease (33.7% vs 28.5%, standardized difference = 0.11). Both disease-specific and total healthcare utilization costs were also higher in the golimumab group (standardized differences = 0.11).
3.2 Outcome Chart Abstraction and Adjudication Results
Medical records were obtained and abstracted for 777 of 917 subjects with potential study outcomes (84.7%). Of these, 384 were assessed to have definite evidence supporting the diagnosis of the outcome while 364 did not contain supporting evidence. Determination of case status was not possible for 29 charts given the information available in the medical record.
The PPVs for malignancy, lymphoma, and autoimmune disease were > 75% (Table 3) so the original claims definitions for these outcomes were used for analyses. Claims definitions were refined for the remaining outcomes with moderate (30–75%) or low (< 30%) PPVs to require that the codes used to identify them occur in the primary position. Revisions resulted in increases in the PPV for outcomes other than serious infection, systemic hypersensitivity, and new onset hypertension, whose PPVs remained largely unchanged (Table 3).
3.3 NDI Results
Table 4 lists the number of NDI-identified death matches occurring within 60 days of health plan disenrollment for the matched golimumab and NBS cohort and the unmatched golimumab cohort by ICD-10 category. Person-time was calculated from the index date to the death date provided by the NDI or from the index date to the earliest of end of enrollment or data cut-off date (30 September 2015) plus 60 days, and crude IRs were calculated in order to compare the cohorts.
Four deaths were identified in the golimumab cohort matched to NBS for a crude IR of 1.22 per 1000 person-years (4 deaths/3270 person-years), and no deaths were identified in the unmatched golimumab cohort (383 person-years).
3.4 Outcome Analyses
In as-treated analyses, most findings were consistent with no increase in risk of the outcomes in the golimumab cohort (Table 5). Compared to golimumab non-use in the matched NBS cohort, the risk for depression was increased during current golimumab use (RR 1.45, 95% CI 1.31–1.61). There was no association between cumulative dose of golimumab and elevated risk of malignancy and lymphoma (Table 6).
As-matched analyses in Table 7 showed a decreased risk for malignancy and mortality in golimumab versus NBS initiators (RR 0.66, 95% CI 0.50–0.87 and RR 0.41, 95% CI 0.17–0.98, respectively). No elevated rate ratios were seen for any of the outcomes in the as-matched analyses, including depression (RR 1.08, 95% CI 0.97–1.19).
In NCC analyses (Table 8), no associations were observed between golimumab exposure and most outcomes. Golimumab use was associated with a decreased risk of systemic hypersensitivity and mortality (OR 0.57, 95% CI 0.37–0.90 and OR 0.23, 95% CI 0.08–0.67, respectively). No association between golimumab exposure and depression was seen (OR 1.01, 95% CI 0.78–1.31).
3.5 Sensitivity Analyses
Results using the restricted definition of incident cases (as opposed to prevalent cases) of depression had corresponding 95% CIs that suggested chance differences (Tables 9 and 10). The results of this sensitivity as-treated analysis reversed those seen in the primary as-treated analysis; the risk of incident depression included the null during current golimumab use compared to golimumab non-use (RR 0.71, 95% CI 0.30–1.69) (Table 9).
The assessment of the effect of residual confounding (i.e. as a result of an unmeasured or unavailable confounding variable omitted from the propensity score model) indicates that strong risk factors for the outcome that are also not balanced among the matched golimumab and NBS cohorts would be required in order to change the results of the analyses substantially. Most known independent risk factors were already included in the propensity score model; therefore, any unmeasured confounder of sufficient strength to alter findings would also have to be independent of the confounders that were included in the propensity score. See ESM Appendix 2 for outcome-specific results and figures.
In the assessment of nondifferential exposure or outcome misclassification, estimates in the golimumab cohort were only slightly altered, suggesting no change in inferences related to the point estimates. For depression, the observed as-treated result of an increased risk in current golimumab use versus non-use changed substantially when corrected for potential misclassification, suggesting that this result was sensitive to misclassification. See ESM Appendix 3 for outcome-specific results.
4 Discussion
This post-approval observational study assessed the risk of several safety outcomes in a population of golimumab and NBS users over a 6-year period. The as-treated analysis showed no elevation in risk for most of the outcomes associated with use of golimumab compared to NBS, with the exception of depression during current golimumab use. In the cumulative drug exposure analysis for malignancy and lymphoma, no evidence of a dose–response effect for golimumab was observed. In the as-matched analysis that does not account for changes in exposure during follow-up, there were no elevated rates in the golimumab cohort versus the NBS cohort; similarly, no elevated rates were seen in the NCC analysis of golimumab.
A study strength is the use of automated medical claims that reflect routine care within a large, US insurance database, providing generalizability to patients aged < 65 with commercial health insurance. The comprehensive nature of the database means that any billable medical service would be recorded, including rare events. However, there are limitations inherent to using claims databases. Patients were identified based on claims for medication dispensings, but it is not known whether the medication was taken as prescribed. Actual use of the drug is inferred from the dispensings, which may result in exposure misclassification. Sensitivity analyses were conducted to examine the impact of a range of assumed misclassification of exposure on the effect estimates and indicated that nondifferential misclassification of exposure likely biased the effect estimates toward the null or did not change the inference of the point estimates. Also, diagnosis codes used for outcome identification do not necessarily indicate true disease. Accordingly, some degree of outcome misclassification may be present [33]. Medical record confirmation was used to revise the outcome claims definitions applied to the full population, which improved the specificity of outcome definitions.
The PPVs associated with each study outcome reflect the accuracy of the claims data and allow an interpretation of the RR with the knowledge that cases of outcomes with high to moderate PPVs are likely to be real cases of disease. For such outcomes, the results presented would be close to those that might be observed from a study that included confirmation of all study outcomes [34,35,36,37]. Among those outcomes with lower PPVs, the observed RRs in this study might diverge from that of RRs based on confirmed cases, and this divergence would in most cases tend to bias the effect estimate towards the null, potentially obscuring either a positive or negative treatment effect. The original and revised PPVs for depression in this study (42.5 and 71.4%, respectively) were consistent with a range of PPVs identified in a review that used various database algorithms (31.5–98.8%) [38], and with a study that focused on incident depression (48.6%) [33]. The results of the sensitivity analyses indicate that outcome misclassification of the extent indicated by the PPVs would not change effect estimates substantially, with the exception of depression. Additional limitations include limited follow-up time and lack of information on disease duration and disease activity.
Propensity score matching created cohorts that were similar with respect to available baseline data on underlying risk factors related to outcomes, claims proxies for disease activity and severity, healthcare utilization, and other factors that may be considered by clinicians to inform treatment selection. The labelled indications for golimumab do not specify a treatment sequence (such as requiring prior failure of NBS medications), although preferential prescribing (channeling) of sicker patients to biologic agents is plausible and reflected by the inability to find eligible matches for some golimumab patients (n = 178). However, this study accounted for an extensive list of important confounders, and comparison with NBS provides useful evidence for the benefit-risk balance in real-world practice not only for golimumab but also for other biologics when the majority of patients are treated by NBS. A limitation to this approach is the potential for confounding by unmeasured factors which may not be balanced by matching on the variables included in the propensity score model. Of note, a study drawing on medical chart data demonstrated that propensity score matching can lead to balance in potential confounders not directly measurable in claims (e.g. smoking status, BMI) but correlated with covariates included in the propensity score model [30]. A sensitivity analysis was conducted to address residual confounding associated with propensity score modeling and matching, but there are limitations associated with this analysis: (1) it was constrained to one binary confounder, which may not be informative if several confounders were unmeasured and the joint effect was unknown, and (2) it did not provide an assessment of the magnitude of existing residual confounding.
Given the purpose of the study to assess the safety profile of golimumab in the real-world and the value obtained by having a larger patient population and more potential outcomes, combining the different rheumatologic indications was warranted. Specifically, regarding the inclusion of ICD-9 diagnosis codes for juvenile idiopathic arthritis (714.3x), before matching, 5 patients in the golimumab cohort (5/1515 = 0.3%) and 557 patients in the NBS cohort (557/48,975 = 1.1%) had a claim for 714.3x. Among these 562 pre-matched initiators, zero golimumab and 3 NBS patients also had a claim for rheumatoid arthritis (ICD-9 714.0) during the baseline period, indicating little overlap in coding of these 2 conditions. After matching, the numbers with a claim for ICD-9 714.3 × dropped to 4 golimumab (4/1337 = 0.3%) and 16 non-biologic initiators (16/4227 = 0.4%), with zero golimumab and 2 non-biologics also identified with a claim for ICD-9 714.0 during baseline. The small numbers and good balance obtained on this variable means that these patients are unlikely to have any influence on study results.
Several types of analyses were undertaken in order to understand the association between golimumab use and the prespecified study outcomes. Based on the exposure definition used in this study, determining the temporal relationship between golimumab exposure and these outcomes, particularly chronic outcomes (i.e. malignancy and lymphoma) is difficult. Protopathic bias, a form of reverse causation in which treatment is initiated in response to symptoms of undiagnosed disease, cannot be ruled out. Protopathic symptoms of study outcomes may be mistaken for rheumatic disease activity leading to initiation of golimumab or NBS. The resulting association between the study drug and outcome could, in fact, be due to reverse causation, in which the underlying, undiagnosed disease leads to use of the study drug [39].
The current study design did not specifically account for the possibility of golimumab to be used in a manner that might produce protopathic bias. In order to address potential protopathic bias in the analysis of malignancy and lymphoma, a sensitivity analysis was conducted where person-time in the as-matched analysis that is particularly susceptible to protopathic bias (the first 6 months of exposure time) was removed, and the effect estimates recalculated [40]. Results from this sensitivity analysis were consistent with the primary findings, suggesting no strong protopathic bias effect (data not shown).
An elevated rate of depression during current golimumab use was observed in the as-treated analysis, but not in the as-matched or NCC analyses. Sensitivity analyses indicated depression is sensitive to misclassification, so this result should be interpreted cautiously. Furthermore, as patients with a baseline medical history of depression were excluded from the NCC and sensitivity analyses, the increased risk seen in the as-treated analysis may be related to an imbalance in baseline history of depression. Due to the design of the study, which focused on baseline balance of patient characteristics, the effect of changes in severity or treatment for depression after initiation of golimumab were not analyzed.
Assessment of depression in insurance claims databases presents challenges (e.g. patients underreporting symptoms due to stigma of mental illness, failure of physicians to recognize or treat depression). The definition of depression used here was comprehensive (i.e. any inpatient or outpatient claim with a diagnosis code in the primary position or a dispensing for an antidepressant) and captured more patients at the expense of generating false positive diagnoses. When a more stringent definition of depression that required a hospitalized diagnosis in the primary position was used, 3 cases were identified in the matched golimumab cohort among 1969 person-years (crude IR = 1.52 per 1000 person-years) and 19 in the NBS cohort among 6254 person-years (crude IR = 3.04 per 1000 person-years).
In chronic inflammatory diseases, comorbid depression is common. Among RA patients, prevalence of depression ranges from 14–48% [41,42,43] and is associated with higher levels of pain and disability, lower health-related quality of life, and increased mortality [44]. Evidence suggests a role of pro-inflammatory cytokines including interleukin (IL)-6, TNF-alpha, and IL-1 in promoting depression, and indeed, patients with severe RA have a higher risk of depression [45,46,47,48,49]. Specifically, TNF-alpha has been shown to be associated with depression and reducing its levels may actually reverse symptoms. In a randomized clinical trial investigating the effect of etanercept on fatigue and depression in patients with plaque psoriasis, improvements in depression were noted [50]. Smaller studies in patients with RA and inflammatory bowel disease confirm that therapy with TNFi could have a beneficial role in the treatment of depressive symptoms in patients with inflammatory conditions [51,52,53].
5 Conclusion
In summary, this study involving active surveillance of prespecified outcomes identified no specific safety concerns in patients with rheumatic disease treated with golimumab versus a NBS medication. The results are consistent with golimumab’s overall safety profile and generally comparable with observations from other studies in patients with treated rheumatic disease.
References
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis. 2002 Update. Arthritis Rheum. 2002;2002(46):328–46.
Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1–25.
World Health Organization. Chronic rheumatic conditions. Geneva: World Health Organization; 2017. http://www.who.int/chp/topics/rheumatic/en/. Accessed 24 Oct 2019.
Goldman L, Ausiello D, editors. Cecil medicine, 23rd edn. Philadelphia: Saunders Elsevier; 2007. http://www.mdconsult.com. Accessed 24 Oct 2019.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–89.
Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–48.
Mease P. Current treatment for psoriatic arthritis and other spondyloarthritides. Rheum Dis Clin N Am. 2006;S1:11–20.
Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77.
Urdaneta M, Jethwa H, Sultan R, Abraham S. A review on golimumab in the treatment of psoriatic arthritis. Immunotherapy. 2017;9:871–89.
Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93.
Chakravarty EF, Genovese MC. Associations between rheumatoid arthritis and malignancy. Rheum Dis Clin N Am. 2004;30:271–84.
Fleischmann R, Yocum D. Does safety make a difference in selecting the right TNF antagonist? Arthritis Res Therapy. 2004;6(Suppl 2):S12–8.
Cush JJ. Safety overview of new disease-modifying antirheumatic drugs. Rheum Dis Clin N Am. 2004;30:237–55.
Doran MF, Crowson CS, Pond GR, et al. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–300.
Schiff MH, DiVittorio G, Tesser J, et al. The safety of anakinra in high-risk patients with active rheumatoid arthritis. Arthritis Rheum. 2004;50:1752–60.
Weisman MA. What are risks of biologic therapy in rheumatoid arthritis? An update on safety. J Rheumatol Suppl. 2002;65:33–8.
Fleischmann R, Eqbal I, Nandeshwar P, Quiceno A. Safety and efficacy of disease-modifying anti-rheumatic agents. Drug Saf. 2002;25:173–97.
Bieber J, Kavanaugh A. Consideration of the risk and treatment of tuberculosis in patients who have rheumatoid arthritis and receive biologic treatments. Rheum Dis Clin N Am. 2004;30:257–70.
Wolfe F, Michaud K, Anderson J, Ubansky K. Tuberculosis infection in patients with rheumatoid arthritis and effect of infliximab therapy. Arthritis Rheum. 2004;50:372.
Lee JH, Silfman NR, Gershon SK, Edwards ET, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor α antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–70.
Cañete JD, Hernández MV, Sanmartí R. Safety profile of biological therapies for treating rheumatoid arthritis. Expert Opin Biol Ther. 2017;17(9):1089–103.
Hernández MV, Sanmartí R, Cañete JD. The safety of tumor necrosis factor-alpha inhibitors in the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2016;15(5):613–24.
Kay J, Fleischmann R, Keystone E, et al. Five-year safety data from 5 clinical trials of subcutaneous golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheum. 2016;43:2120–30.
Frampton JE. Golimumab: a review in inflammatory arthritis. BioDrugs. 2017;31(3):263–74.
Rossini M, Viapiana O, Orsolini G, et al. Why golimumab in the treatment of psoriatic arthritis, ankylosing spondylitis and rheumatoid arthritis? Reumatismo. 2015;66(4):285–303.
Papagoras C, Voulgari PV, Drosos AA. Golimumab, the newest TNF-α blocker, comes of age. Clin Exp Rheumatol. 2015;33:570–7.
Eng PM, Seeger JD, Loughlin J, et al. Supplementary data collection with case-cohort analysis to address potential confounding in a cohort study of thromboembolism in oral contraceptive initiators matched on claims-based propensity scores. Pharmacoepidemiol Drug Saf. 2008;17:297–305.
Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. In: Paper 214-26 in proceedings of the twenty-sixth annual SAS users group international conference. Long Beach: SAS Institute, Inc; 2001. http://www2.sas.com/proceedings/sugi26/p214-26.pdf. Accessed 24 Oct 2019.
Parsons LS. Performing a 1:N case-control match on propensity score. In: Paper 165-29 in proceedings of the twenty-ninth annual SAS users group international conference. Montréal: SAS Institute, Inc; 2004. http://www2.sas.com/proceedings/sugi29/165-29.pdf. Accessed 24 Oct 2019.
Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiologic data. Berlin: Springer; 2009.
Carnahan RM. Mini-Sentinel’s systematic reviews of validated methods for identifying health outcomes using administrative data: summary of findings and suggestions for future research. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):90–9.
Brandenburg NA, Phillips S, Wells KE, Woodcroft KJ, Amend KL, Enger C, et al. Validating an algorithm for multiple myeloma based on administrative data using a SEER tumor registry and medical record review. Pharmacoepidemiol Drug Saf. 2019;28(2):256–63.
Funch D, Ross D, Gardstein BM, et al. Performance of claims-based algorithms for identifying incident thyroid cancer in commercial health plan enrollees receiving antidiabetic drug therapies. BMC Health Serv Res. 2017;17(1):330.
Funch D, Holick C, Velentgas P, Clifford R, Wahl PM, McMahill-Walraven C, et al. Algorithms for identification of Guillain-Barre Syndrome among adolescents in claims databases. Vaccine. 2013;31(16):2075–9.
Sands BE, Duh MS, Cali C, Ajene A, Bohn RL, Miller D, et al. Algorithms to identify colonic ischemia, complications of constipation and irritable bowel syndrome in medical claims data: development and validation. Pharmacoepidemiol Drug Saf. 2006;15(1):47–56.
Townsend L, Walkup JT, Crystal S, Olfson M. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):163–73.
Feinstein AR. Clinical epidemiology: the architecture of clinical research. Philadelphia: WB Saunders; 1985.
Tamim H, Monfared AAT, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf. 2007;16:250–8.
Hider SL, Tanveer W, Brownfield A, et al. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology. 2009;48:1152–4.
Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumatol. 2011;6:617–23.
Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology. 2013;52:2136–48.
Deb A, Dwibedi N, LeMasters T, Hornsby JA, Wei W, Sambamoorthi U. Burden of depression among working-age adults with rheumatoid arthritis. Arthritis. 2018;2018:8463632.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–14.
Matcham F, Norton S, Scott DL, et al. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology. 2016;55:268–78.
Rathbun AM, Harrold LR, Reed GW. Temporal effect of depressive symptoms on the longitudinal evolution of rheumatoid arthritis disease activity. Arthritis Care Res. 2015;67(6):765–75.
Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. 2019;6(2):164–73.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35.
Uguz F, Akman C, Kucuksarac S, Tufekci O. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorder in patients with rheumatoid arthritis. Psychiatry Clin Neurosci. 2009;63(1):50–5.
Guloksuz S, Wichers M, Kenis G, et al. Depressive symptoms in Crohn’s disease: relationship with immune activation and tryptophan availability. PLoS One. 2013;8(3):e60435.
Horst S, Chao A, Rosen M, et al. Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig Dis Sci. 2015;60(2):465–70.
Acknowledgements
The authors wish to acknowledge the contributions of the following colleagues from Optum Epidemiology: Xiangmei Gu and Li Zhou for their assistance with the study analysis, Nicole Brooks for her assistance with study operations, and Veena Hoffman.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported through a research contract between Optum Epidemiology and Janssen Biologics B.V., who is the Marketing Authorization Holder of golimumab.
Conflict of Interest
NJZ and JDS are employed by Optum and hold stock/stock options in the parent company of Optum (United HealthGroup, Inc.). AG, WN, MO-L, and SE are or were employees of Janssen Biologics B.V. at the time of this study while SDC and YW are employees of Janssen Scientific Affairs, LLC. AG, WN, MO-L, SE, SDC, and YW hold stock/stock options of Johnson & Johnson.
Ethics Approval
Medical record abstraction and the NDI search were performed only after receiving approval from the New England Institutional Review Board and a waiver of authorization from the Privacy Board.
Informed Consent
Informed consent was not required as the HIPAA Privacy Rule (45 CFR 164.512(i)(2)) permits protected health information (PHI) to be used or disclosed for research, without patient authorization. This study was an observational study to provide surveillance of prespecified safety outcomes among patients with rheumatic disease and given the size of the insurance population from which study subjects were drawn (approximately 14 million health insurance participants), individual-level consent was deemed impractical.
Consent to Participate
Not applicable.
Consent for Publication
All co-authors have consented for publication of this study.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ziyadeh, N.J., Geldhof, A., Noël, W. et al. Post-approval Safety Surveillance Study of Golimumab in the Treatment of Rheumatic Disease Using a United States Healthcare Claims Database. Clin Drug Investig 40, 1021–1040 (2020). https://doi.org/10.1007/s40261-020-00959-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00959-7