Abstract
Background and Objectives
Topiroxostat, a selective xanthine oxidoreductase inhibitor, is used for the management of hyperuricemic patients with or without gout in Japan. Accumulating evidence has demonstrated the efficacy of topiroxostat for the treatment of hyperuricemia with or without gout. However, the safety and efficacy of topiroxostat in the clinical setting remain unclear, and there is little large-scale clinical evidence. We conducted a post-marketing observational study over 54 weeks.
Patients and Methods
Patients were centrally enrolled, and case report forms of 4491 patients were collected between April 2014 and March 2019 from 825 medical sites.
Results
Overall, 4329 patients were assessed for safety and 4253 patients for effectiveness. The overall incidence of adverse drug reactions was 6.95%, and the incidence rates of adverse drug reactions of gouty arthritis, hepatic dysfunction, and skin disorders, which are of special interest in this study, were 0.79%, 1.73%, and 0.95%, respectively. No case of serious gouty arthritis was observed. Serum urate levels decreased stably over time and showed a significant reduction rate at 54 weeks (21.19% ± 22.07%) and on the final visit (19.91% ± 23.35%) compared to the baseline. The rates for subjects who achieved serum uric acid levels ≤ 6.0 mg/dL at 18 and 54 weeks after administration were 43.80% and 48.28%, respectively.
Conclusions
This study suggests that there is no particular concern about adverse drug reactions or the efficacy of topiroxostat for hyperuricemic patients with or without gout in a post-marketing setting in Japan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The safety and efficacy of the novel non-purine selective xanthine oxidoreductase inhibitor, topiroxostat, were investigated over 54 weeks in a post-marketing study. |
There were no new findings that would raise questions about the safety of topiroxostat under actual conditions of use, and its efficacy was shown to be the same as clinical studies had reported at the time of approval. |
Topiroxostat is considered a safe and effective drug for gout and hyperuricemia in daily practice. |
1 Introduction
Hyperuricemia (defined as a serum urate level > 7.0 mg/dL in Japan) is a causative factor for urate deposition diseases such as urolithiasis and gouty arthritis [1]. Defects in single and multiple genes have been suggested as the cause of hyperuricemia. These reportedly affect nucleic acid metabolism-related enzymes that promote uric acid production or urate transporters that reduce renal excretion of uric acid [2, 3]. Hyperuricemia is broadly divided into the overproduction of uric acid, the underexcretion of it, and mixed types. Recently, the existence of a renal load type, including reduced extrarenal excretion of uric acid and the overproduction of uric acid and reduced extrarenal excretion, has also been proposed [2]. Three urate transporters, URAT1/SLC22A12, GLUT9/SLC2A9, and ABCG2/BCRP, are reported to play crucial roles in the regulation of serum urate level, and their dysfunction causes urate transport disorders (hypouricemia and/or hyperuricemia). ABCG2 variants have been shown to have stronger effects on the risk of hyperuricemia/gout than major environmental risk factors such as obesity and heavy drinking [4].
Reducing serum urate levels and maintaining it at or below 6.0 mg/dL is a major target in treating hyperuricemia to prevent gouty arthritis [5,6,7,8]. Drugs that reduce serum uric acid levels are roughly classified into two types: uric acid synthesis inhibitors that inhibit xanthine oxidoreductase (XOR) and uric acid excretion accelerators that inhibit renal uric acid reabsorption. Topiroxostat, (Topirolic® tablets and Uriadec® tablets) a non-purine selective XOR inhibitor, belongs to the group of uric acid synthesis inhibitors. It is a hybrid inhibitor that inhibits enzyme activity by covalent binding with molybdenum and by interaction with amino acid residues in the substrate-binding pocket [9, 10].
There have been several reports on the safety and efficacy of topiroxostat, mainly in development trials, and topiroxostat not only reduces serum uric acid levels [11,12,13] but also may have a possible positive effect on renal function [14,15,16,17].
We report here the results of a post-marketing study conducted to collect information on the safety, efficacy, and proper use of topiroxostat.
2 Patients and Methods
2.1 Study Design
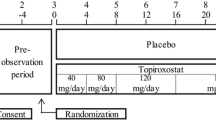
This was a prospective, observational, multicenter post-marketing study carried out in routine clinical practice, and co-sponsored by the manufacturers to investigate the safety and effectiveness of topiroxostat (Topiloric®, Fuji Yakuhin Co., Ltd., Saitama, Japan) and Uriadec® (Sanwa Kagaku Kenkyusho Co., Ltd., Aichi, Japan). The study was carried out in accordance with the Good Post-Marketing Study Practice standards specified by the Ministry of Health, Labor and Welfare in Japan.
2.2 Participants and Data Assessment
Patients were recruited from medical institutions throughout Japan and were enrolled using a central registration system from April 2014 to 31 March 2017. Each patient was followed up for 54 weeks from the date of first topiroxostat administration, using Electronic Data Capture.
This study collected patient background information, such as age, gender, BMI, reasons for using this drug, disease duration of gout or hyperuricemia, and concomitant disease.
Safety was assessed according to the incidence of adverse drug reactions (ADRs), the change in clinical laboratory tests of aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (γ-GTP), total bilirubin, and triglycerides, as well as total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, blood urea nitrogen (BUN), serum creatinine (Cr), and hemoglobin A1c (HbA1c). Urinalysis (protein and occult blood) was performed, and blood pressure, pulse, body weight, estimated glomerular filtration rate (eGFR) and urinary albumin/Cr ratio were documented. Furthermore, the incidence of cardiovascular adverse events and ADRs from renal and urinary tract disorders were also tabulated.
Efficacy endpoints were changes in serum uric acid levels, a decrease rate of serum uric acid levels at 18 weeks and 54 weeks after administration and at the final evaluation, and the achievement rate of ≤ 6 mg/dL.
Priority research factors include gouty arthritis, hepatic dysfunction, skin disorders, and safety and efficacy in special patient subgroups. These included the elderly, females, and patients with hepatic or renal dysfunction.
2.3 Statistical Analysis
The subgroup analysis of the incidence of ADRs by patient background factors and the special patient subgroups were tested using the Chi square test or Fisher’s exact test, and the analysis of changes in clinical test values was performed using the one-sample t test. A level of less than 5% (two-sided) was considered significant. Adverse events (AEs) and ADRs were categorized according to the Medical Dictionary for Regulatory Activities/Japanese edition (MedDRA/J) version 22.0. Changes in serum uric acid levels and decrease rates were analyzed using one-sample t tests, and subgroup analysis of serum uric acid decrease rates in specific patient populations was performed by analysis of variance.
2.4 Trial Registration
This PMS study was retrospectively registered on Japic-Clinical Trials Information as JapicCTI-173783 on November 22, 2017.
3 Results
3.1 Patient Disposition and Characteristics
Figure 1 shows the patient disposition in the study. In total, 4642 patients were registered at 825 medical sites across Japan. With the exception of 151 cases for which the case report form (CRF) could not be collected, 4491 CRFs (18 weeks) and 3657 CRFs (54 weeks) were collected and fixed.
Of the 4491 cases, 162 were excluded owing to the absence of visits after enrollment (n = 158), duplicate registration (n = 3), and lack of exposure to the drug (n = 1), leaving 4329 patients for the safety analyses. An additional 76 patients were excluded from the effectiveness analyses, (73—no effectiveness data available, 2—prior use of topiroxostat, 1 off-label use), leaving 4253 patients.
Table 1 summarizes the baseline characteristics of the 4329 safety analysis subjects in this study. The mean serum uric acid level was 8.11 ± 1.46 mg/dL, and the number of cases with a serum uric acid level of 7.0 mg/dL or more was 3436 (79.37%).
The details of specific patient populations (elderly, female, hepatic dysfunction, renal dysfunction) were as follows: there were 2364 (54.61%) elderly people aged ≥ 65, and 1238 (28.60%) aged > 75. There were 851 (19.66%) female patients, 434 (10.03%) hepatic dysfunction patients, 3469 (80.13%) patients with renal dysfunction.
3.2 Usage Status of this Drug
The average daily dose of the 4329 patients subject to safety analysis was 50.57 mg/day. The dose escalation was 794 cases (18.34%), and the one-step dose escalation was the highest in 638 cases (14.74%).
Of the 4329 safety analysis subjects in this study, 3203 (73.99%) completed use for one year (54 weeks), and 1126 (26.01%) discontinued or dropped out. The main reasons for discontinuation or withdrawal included no visit [365 cases (8.43%)], AEs [198 cases (4.57%)], the achievement of the treatment purpose [115 cases (2.66%)], change of hospital [104 cases (2.40%)], or failure to collect the CRF by the end of the survey due to the lack of the cooperation of a doctor [199 cases (4.60%)].
3.3 Safety Results
ADRs reported by attending physicians are summarized in Table 2. All observed ADRs are listed in the Table S2. In 4329 cases subject to safety analysis, 390 ADRs occurred in 301 cases, and the overall incidence of ADRs was 6.95%, which was lower than the 35.35% (292/826) incidence of ADRs in clinical trials up to the time of approval.
The main ADRs were abnormal hepatic function (n = 39, 0.90%), gouty arthritis (n = 34, 0.79%), pruritus and renal impairment (n = 15, 0.35% each), and liver disorders (n = 12, 0.28%).
Table 3 shows the incidence of ADRs by patient background factors. Background factors with a high incidence of ADRs included history of gouty arthritis, gout nodules, and concomitant disease (renal disease, cardiovascular disease, hypertension), with a significant difference compared to the absence of each. In addition, there was a significant difference in the incidence of ADRs in the presence or absence of gradual increased dosing, the total number of days of administration, and the total dose.
3.3.1 Changes in Clinical Test Values
Serum creatinine tended to increase after 10 weeks’ administration, and a significant difference was observed compared to the start of administration after 30 weeks’ administration, but the mean change after 54 weeks was a slight increase of 0.066, BUN did not show an increasing trend. The renal dysfunction patients (eGFR < 90 mL/min/1.73 m2: 80.13%) and the elderly (aged ≥ 65 years, 54.61%) were more likely to be affected by the natural history of these patients. Although there were also significant differences in ALT, ALP, γ-GTP, total bilirubin, triglycerides (TG), total cholesterol, HDL cholesterol, LDL cholesterol, BUN, eGFR, HbA1c, blood pressure (systolic, diastolic) and body weight, the fluctuation range was small or it was not a change for the worse.
3.3.2 Key Safety Research Items
The ADRs of gouty arthritis, hepatic dysfunction, and skin disorders were examined as research items with special interest.
In 4329 safety analysis cases, the incidence rates of ADRs of gouty arthritis, hepatic dysfunction, and skin disorders were 0.79% (34 cases), 1.73% (75 cases) and 0.95% (41 cases), respectively (Table 4). No serious gouty arthritis was observed. One case each of serious hepatic dysfunction, hepatic cirrhosis, and liver disorder occurred. There was one case each of serious skin disorder, drug eruption, and urticaria. In skin disorders, in terms of the onset period, 19 cases occurred within 42 days or fewer, and 22 cases occurred on a total dose of less than 2500 mg. The incidence was high in the early stage of administration.
3.3.3 Safety in Special Patient Populations
The incidence of ADRs is shown in elderly patients, female patients, and patients with hepatic or renal dysfunction (Table 3). Such patients have not been sufficiently studied because of the small numbers in reported clinical trials. In the stratified analysis by age, gender, and hepatic or renal function, no significant difference was found in the incidence of ADRs in any of the subgroups.
3.3.4 Other Analysis Items
Cardiovascular AEs The AE rate of cardiovascular events was 0.79% (34/4329 cases) (Table 5). There were no significant changes in the relevant laboratory test values (TG, total cholesterol, HDL cholesterol, LDL cholesterol, or in blood pressure, and pulse).
ADRs of renal and urinary tract disorders The incidence of ADRs of renal and urinary tract disorders was 0.16% (7/4329 cases), of which five were presence of blood urine and one was urinary calculus and hemorrhagic cystitis (Table 5).
3.4 Efficacy
Changes in the serum uric acid level during the administration of topiroxostat are shown in Fig. 2, and the rate of decrease of serum uric acid levels are shown in Table 6. In 4253 patients subject to efficacy analysis, the mean value of serum uric acid at the start of administration was 8.11 ± 1.46 mg/dL (4014 cases), the mean value after 18 weeks was 6.35 ± 1.47 mg/dL (2744 cases). The mean value after 54 weeks was 6.14 ± 1.31 mg/dL (2274 cases). In addition, the average value at the final evaluation, including the discontinuation of administration and the end of administration, was 6.31 ± 1.46 mg/dL (3935 cases).
The decrease rate of serum uric acid level was 19.03% ± 23.90% (2639 cases) after 18 weeks, 21.19% ± 22.07% (2191 cases) after 54 weeks, and 19.91% ± 23.35% (3706 cases) at the time of final evaluation, all showed a significant decrease compared to the start of administration.
The achievement rate of serum uric acid level of 6.0 mg/dL or less was 43.80% (1202/2744 cases) after 18 weeks, 48.28% (1098/2274 cases) after 54 weeks, and 44.55% (1753/3935 cases) at the final evaluation (Table 7). In addition, the achievement rate of 6.0 mg/dL or less in patients whose serum uric acid level exceeded 6.0 mg/dL at the start of administration was 41.87% (1004/2398 cases) after 18 weeks, and 46.05% (914/1985 cases) after 54 weeks, and 42.39% (1434/3383 cases) at the time of final evaluation (Table 7).
We examined the rate of decrease in serum uric acid levels in elderly patients, female patients, and hepatic or renal dysfunction patients. The same decrease was observed as in non-elderly patients, and patients without hepatic or renal dysfunction. Gender stratification analysis, however, showed that females had significantly higher reduction rates than males (Table 8).
4 Discussion
The safety and efficacy of topiroxostat under daily use were confirmed by this 54-week post-marketing study. In general, randomized controlled trials can provide the highest levels of clinical evidence with the least bias but cannot collect all data relevant to use in routine clinical practice. Therefore, the present study is important because it provides feedback on the use of topiroxostat in routine clinical practice.
As for the safety profile, the incidence of ADRs with topiroxostat was 6.95% in this study, indicating a lower rate compared with the aggregated results (35.35%) in the pre-approval trials. As priority items related to safety, we investigated the incidence of ADRs of gouty arthritis, hepatic dysfunction and skin disorders, and safety in the elderly, and in patients with hepatic or renal dysfunction, and in female patients. No problematic events were observed in the subgroups.
The prevalence of gout is estimated to be over 1% in men aged > 30 years and is still on the rise [18]. In addition, the occurrence of side effects of gouty arthritis associated with the treatment of hyperuricemia has become a problem. In this study, the incidence of gouty arthritis was 0.79% (34 of 4329 patients), with no serious cases, and was lower than that seen at the time of approval of 10.05% (83/826 patients). These results suggest that topiroxostat is a useful drug for patients with gout and hyperuricemia with a low incidence of gouty arthritis even when lowering serum uric acid levels.
It has been suggested that since topiroxostat is not affected by mild-to-moderate renal dysfunction, adjustment of dosage and administration is not required for these patients [14], and this study confirmed that there was no significant difference in the incidence of ADRs according to the severity of eGFR at the baseline.
As a result of examining the incidence of ADRs by patient background factors, when the total number of administration days was less than 14 and the total dose was less than 2500 mg, the incidence of ADRs was high; however, the effect of patients who discontinued the drug due to the appearance of side effects in the early stage of administration was considered.
Although there was a significant difference in the incidence rate of ADRs by some patient background factors, the tendency of the occurrence of ADRs did not differ. Further, the rate of ADRs is not remarkably high compared with the overall incidence of ADRs at 6.95% (301/4329 cases).
The incidence of cardiovascular AEs was 0.79%, and of renal and urinary tract disorders was 0.16%, indicating no particular effect on safety.
Regarding efficacy, the reduction rate of serum uric acid level at the end of treatment in clinical trials (long-term administration study at 58 weeks) was 38.44% ± 13.34% (121 patients), and the achievement rate of ≤ 6.0 mg/dL was 70.0% (77/110 subjects) after 18 weeks, and 71.9% (87/121 subjects) at the end of administration; results of this study were all lower. In clinical trials, all cases were escalated to a maintenance dose of 120 or 160 mg/day, but the average daily dose in this study under actual conditions of use was < 60 mg/day: 71.93% (3114/4329 cases), which was thought to be because the low-dose cases accounted for the majority, and the escalating cases were as low as 18.34% (794/4329 cases). Based on these results, it is considered desirable to continue increasing the dose of topiroxostat in the necessary cases.
In addition, we examined the rate of decrease in serum uric acid levels in elderly and female patients, and in patients with hepatic or renal dysfunction, where the number of cases in previous clinical trials has been too small to perform subgroup analyses. Concerning elderly patients and hepatic or renal dysfunction patients, there was no significant difference in the rate of decreases in serum uric acid levels compared to the general study population. Female patients had a higher decrease in serum uric acid levels than males.
In this study, the rate of achievement of serum uric acid level of 6.0 mg/dL or less and the decrease rate of serum uric acid level, was lower than in reported clinical trials. However, a significant decrease in serum uric acid level was observed, compared to the start of treatment, and approximately half of the patients had a serum uric acid level of ≤ 6.0 mg/dL at 54 weeks after administration. A decrease in serum uric acid levels was also observed in specific patient populations, demonstrating the efficacy of topiroxostat under actual conditions of use.
Since the urate transporter is greatly involved in the regulation of serum uric acid level, it is also interesting to observe whether XOR inhibitors affect the function of urate transporters, such as URAT1, GLUT9 and ABCG2. ABCG2 variants have been shown to have stronger effects on the risk of hyperuricemia/gout than major environmental risk factors such as obesity and heavy drinking [4]. The most common dysfunction variant rs2231142 (p.Q141K), and the prevalent variant in Japan rs72552713 (p.Q126X), as well as rare variants, increase the risk of gout and hyperuricemia, significantly influence the age of onset of gout, and are highly associated with a familial gout history. The ABCG2 dysfunction was reported as a strong independent risk for pediatric-onset hyperuricemia/gout [19]. Moreover, a significant association between rs2231142 and an increased risk of a poor response to allopurinol has been described [20]. It might be very beneficial to include these common dysfunctional ABCG2 variants in any future study about topiroxostat treatment.
5 Conclusions
As a result of the study under actual conditions of use, there were no new findings that would raise questions about the safety of topiroxostat, and the efficacy of this drug was shown to be the same as had been reported in clinical studies at the time of approval. Therefore, topiroxostat is considered to be a safe and effective drug for gout and hyperuricemia in daily practice.
References
Yamanaka H, Japanese Society of Gout and Nucleic Acid Metabolism. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucl Nucl Nucleic Acids. 2011;30:1018–29.
Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764.
Matsuo H, Tsunoda T, Ooyama K, Sakiyama M, Sogo T, Takada T, et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2. Sci Rep. 2016;6:31003.
Nakayama A, Matsuo H, Nakaoka H, Nakamura T, Nakashima H, Takada Y, et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci Rep. 2014;4:5227.
Khanna D, Fitzgerald J, Khanna P, Bae S, Singh M, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64:1431–46.
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42.
Sivera F, Andrés M, Carmona L, Kydd A, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73:328–35.
Sattui SE, Gaffo AL. Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musculoskelet Dis. 2016;8:145–59.
Okamoto K, Matsumoto K, Hille R, Eger BT, Pai EF, Nishino T. The crystal structure of xanthine oxidoreductase during catalysis: implications for reaction mechanism and enzyme inhibition. Proc Natl Acad Sci USA. 2004;101:7931–6.
Matsumoto K, Okamoto K, Ashizawa N, Nishino T. FYX-051: a novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther. 2011;336:95–103.
Hosoya T, Sasaki T, Hashimoto H, Sakamoto R, Ohashi T. Clinical efficacy and safety of topiroxostat in Japanese male hyperuricemic patients with or without gout: an exploratory, phase 2a, multicentre, randomized, double-blind, placebo-controlled study. J Clin Pharm Ther. 2016;41:298–305.
Hosoya T, Sasaki T, Ohashi T. Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: a randomized, double-blinded, controlled phase 2b study. Clin Rheumatol. 2017;36:649–56.
Hosoya T, Ogawa Y, Hashimoto H, Ohashi T, Sakamoto R. Comparison of topiroxostat and allopurinol in Japanese hyperuricemic patients with or without gout: a phase 3, multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study. J Clin Pharm Ther. 2016;41:290–7.
Hosoya T, Ohno I, Nomura S, Hisatome I, Uchida S, Fujimori S, et al. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol. 2014;18:876–84.
Hosoya T, Ishikawa T, Ogawa Y, Sakamoto R, Ohashi T. Multicenter, open-label study of long-term topiroxostat (FYX-051) administration in Japanese hyperuricemic patients with or without gout. Clin Drug Investig. 2018;38:1135–43.
Mizukoshi T, Kato S, Ando M, Sobajima H, Ohashi N, Naruse T, et al. Renoprotective effects of topiroxostat for hyperuremic patients with overt diabetic nephropathy study (ETUDE Study): a prospective, randomized, multicenter clinical trial. Nephrology. 2018;23:1023–30.
Wada T, Hosoya T, Honda D, Sakamoto R, Narita K, Sasaki T, et al. Uric acid-lowering and renoprotective effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in patients with diabetic nephropathy and hyperuricemia: a randomized, double-blind, placebo-controlled, parallel-group study (UPWARD study). Clin Exp Nephrol. 2018;22:860–70.
Hakoda M, Kasagi F. Increasing trend of asymptomatic hyperuricemia under treatment with urate-lowering drugs in Japan. Mod Rheumatol. 2019;29:880–4.
Stiburkova B, Pavelcova K, Pavlikova M, Ješina P, Pavelka K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res Ther. 2019;21:77.
Roberts RL, Wallace MC, Phipps-Green AJ, Topless R, Drake JM, Tan P, et al. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharmacogenom J. 2016;17:201–3.
Acknowledgements
The authors thank all physicians from the 825 institutions and the physicians who participated in this study, for their cooperation. This study was funded by Fuji Yakuhin Co., Ltd. and Sanwa Kagaku Kenkyusho Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YK is a medical advisor of Fuji Yakuhin Co., Ltd., and received consultant fees. TI, TH and YS are employees of Fuji Yakuhin Co., Ltd., TM, TN and KI are employees of Sanwa Kagaku Kenkyusho Co., Ltd.
Ethics approval
This study was carried out in accordance with the good post-marketing study practice standards specified by the Ministry of Health, Labor and Welfare in Japan. According to good post-marketing study practice in Japan, ethics approval was not required for this post-marketing study.
Informed consent
According to good post-marketing study practice in Japan, informed consent was not required for this post-marketing study. As such, informed consent was not obtained from the individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ishikawa, T., Maeda, T., Hashimoto, T. et al. Long-Term Safety and Effectiveness of the Xanthine Oxidoreductase Inhibitor, Topiroxostat in Japanese Hyperuricemic Patients with or Without Gout: A 54-week Open-label, Multicenter, Post-marketing Observational Study. Clin Drug Investig 40, 847–859 (2020). https://doi.org/10.1007/s40261-020-00941-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00941-3