Abstract
Background and Objectives
Oxycodone tamper resistant (OTR) is a new extended-release abuse-deterrent formulation providing improvements in the tamper resistant characteristics. This study aimed to investigate the pharmacokinetic properties of the new OTR tablets and evaluate the bioequivalence of oxycodone from OTR and the original extended release (ER) formulation tablets administered with an opioid antagonist in patients with chronic pain.
Methods
In this open-label, randomized, cross-over study, the enrolled patients were randomised to receive a single dose of 40 mg OTR or 40 mg OXYCONTIN® (OXY) tablet administered with naltrexone blockade under fasting conditions. Serial blood samples for pharmacokinetic analysis were collected. Plasma oxycodone was quantified by a high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) method. Tolerability was evaluated by monitoring adverse events, physical examinations, 12-lead ECG and laboratory tests.
Results
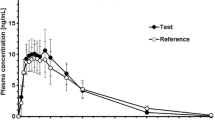
A total of 38 patients were enrolled and 33 subjects completed the study. After a single dose of 40 mg tablets, pharmacokinetic results of the new OTR tablet were found to be similar to those of original extended-release oxycodone tablet. OTR 40 mg was bioequivalent to OXY 40 mg and was well tolerated in patients with chronic pain.
Conclusions
The new OTR formulation could provide a new choice in the treatment of chronic pain and reduce the potential for oxycodone abuse.
Chictr.org identifier: ChiCTR1800017253.


Similar content being viewed by others
References
Riley J, Eisenberg E, Muller-Schwefe G, et al. Oxycodone: a review of its use in the management of pain. Curr Med Res Opin. 2008;24(1):175–92.
Lugo RA, Kern SE. The pharmacokinetics of oxycodone. J Pain Palliat Care Pharmacother. 2005;18(4):17–30.
The Medical Letter Inc. Abuse-deterrent opioids. Med Lett Drugs Ther. 2017;59(1522):95–6.
Pergolizzi JV Jr, Raffa RB, Taylor R Jr, et al. Abuse-deterrent opioids: an update on current approaches and considerations. Curr Med Res Opin. 2018;34(4):711–23.
Keast SL, Owora A, Nesser N, et al. Evaluation of abuse-deterrent or tamper-resistant opioid formulations on overall health care expenditures in a state medicaid program. J Manag Care Spec Pharm. 2016;22(4):347–56.
Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704.
Nguyen V, Raffa RB, Taylor R, et al. The role of abuse-deterrent formulations in countering opioid misuse and abuse. J Clin Pharm Ther. 2015;40(6):629–34.
Investigator’s Brochure of Oxycodone Tamper Resistant (Reformulated Oxycontin), Purdue Pharma, released date Jun/2016, edition number 16.1.
Shen Y, Luo Z, Yu Q, et al. Pharmacokinetics of dimemorfan phosphate tablets in healthy Chinese volunteers. Eur J Clin Pharmacol. 2017;73(6):709–15.
Bioavailability and bioequivalence studies submitted in NDAs or INDs-general considerations. US FDA. 2014. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioavailability-and-bioequivalence-studies-submitted-ndas-or-inds-general-considerations. Accessed 16 Oct 2019.
Pain and Policy Studies Group. Opioid consumption data. https://ppsg-chart.medicine.wisc.edu/. Accessed 1 Jan 2019.
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Abusedeterrent opioids—evaluation and labeling: guidance for industry. Silver Spring: US DoH; 2015.
OxyContin (oxycodone hydrochloride) extended-release tablets [package insert]. Stamford (CT): Purdue Pharma L.P.; 2016.
Poyhia R, Seppala T, Olkkola KT, et al. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol. 1992;33(6):617–21.
Mandema JW, Kaiko RF, Oshlack B, Reder RF, Stanski DR. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42(6):747–56.
Kokki M, Välitalo P, Rasanen I, Aaltomaa S, Ojanperä I, Eskelinen M, et al. Absorption of different oral dosage forms of oxycodone in the elderly: a cross-over clinical trial in patients undergoing cystoscopy. Eur J Clin Pharmacol. 2012;68(10):1357–63.
Kinnunen M, Piirainen P, Kokki H, et al. Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet. 2019;58(6):705–25.
Pöyhiä R, Vainio A, Kalso E. A review of oxycodone’s clinical pharmacokinetics and pharmacodynamics. J Pain Symptom Manag. 1993;8(2):63–7.
Chen ZR, Irvine RJ, Somogyi AA, et al. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48(22):2165–71.
Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–25.
Kokki H, Kokki M. Central nervous system penetration of the opioid oxycodone. In: Preedy VR, editor. Neuropathology of drug addictions and substance misuse, vol. 3. 1st ed. New York: Academic; 2016 (ISBN 9780128006344).
Bostrom E, Hammarlund-Udenaes M, Simonsson US. Blood–brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology. 2008;108(3):495–505.
Bostrom E, Simonsson US, Hammarlund-Udenaes M. In vivo blood–brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab Dispos. 2006;34(9):1624–31.
Lemberg KK, Siiskonen AO, Kontinen VK, et al. Pharmacological characterization of noroxymorphone as a new opioid for spinal analgesia. Anesth Analg. 2008;106(2):463–70 (table of contents).
Kokki M, Valitalo P, Kuusisto M, et al. Central nervous system penetration of oxycodone after intravenous and epidural administration. Br J Anaesth. 2014;112(1):133–40.
Sadiq MW, Bostrom E, Keizer R, et al. Oxymorphone active uptake at the blood–brain barrier and population modeling of its pharmacokinetic–pharmacodynamic relationship. J Pharm Sci. 2013;102(9):3320–31.
Vadivelu N, Chang D, Helander EM, et al. Ketorolac, oxymorphone, tapentadol, and tramadol: a comprehensive review. Anesthesiol Clin. 2017;35(2):e1–20.
Klimas R, Witticke D, El Fallah S, et al. Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin Drug Metab Toxicol. 2013;9(5):517–28.
Lalovic B, Kharasch E, Hoffer C, et al. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79(5):461–79.
Samer CF, Daali Y, Wagner M, et al. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol. 2010;160(4):907–18.
Samer CF, Daali Y, Wagner M, et al. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol. 2010;160(4):919–30.
Zwisler ST, Enggaard TP, Noehr-Jensen L, et al. The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic Clin Pharmacol Toxicol. 2009;104(4):335–44.
Zwisler ST, Enggaard TP, Mikkelsen S, et al. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol Scand. 2010;54(2):232–40.
Stamer UM, Zhang L, Book M, et al. CYP2D6 genotype dependent oxycodone metabolism in postoperative patients. PLoS One. 2013;8(3):e60239.
Gudin J, Levy-Cooperman N, Kopecky EA, et al. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties. Pain Med. 2015;16(11):2142–51.
Webster LR, Bath B, Medve RA, et al. Randomized, double-blind, placebo-controlled study of the abuse potential of different formulations of oral oxycodone. Pain Med. 2012;13(6):790–801.
Franke RM, Morton T, Devarakonda K. Pooled post hoc analysis of population pharmacokinetics of oxycodone and acetaminophen following a single oral dose of biphasic immediate-release/extended-release oxycodone/acetaminophen tablets. Drug Des Dev Ther. 2015;9:4587–97.
Liukas A, Kuusniemi K, Aantaa R, et al. Elimination of intravenous oxycodone in the elderly: a pharmacokinetic study in postoperative orthopaedic patients of different age groups. Drugs Aging. 2011;28(1):41–50.
Morton T, Franke R, Devarakonda K. Pooled post hoc analysis of population pharmacokinetics of oxycodone and acetaminophen following multiple oral doses of biphasic immediate-release/extended-release oxycodone/acetaminophen tablets. Pain Pract. 2016;16(6):730–6.
Andreassen TN, Klepstad P, Davies A, et al. Influences on the pharmacokinetics of oxycodone: a multicentre cross-sectional study in 439 adult cancer patients. Eur J Clin Pharmacol. 2011;67(5):493–506.
Elder NM, Atayee RS, Best BM, et al. Observations of urinary oxycodone and metabolite distributions in pain patients. J Anal Toxicol. 2014;38(3):129–34.
Davis MP. Pharmacokinetic and pharmacodynamic evaluation of oxycodone and naltrexone for the treatment of chronic lower back pain. Expert Opin Drug Metab Toxicol. 2016;12(7):823–31.
Pergolizzi JV Jr, Taylor R Jr, LeQuang JA, et al. Managing severe pain and abuse potential: the potential impact of a new abuse-deterrent formulation oxycodone/naltrexone extended-release product. J Pain Res. 2018;11:301–11.
Chindalore VL, Craven RA, Yu KP, et al. Adding ultralow-dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of Oxytrex. J Pain. 2005;6(6):392–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was sponsored and funded by Mundipharma (China) Pharmaceutical Co. Ltd. (Beijing, People’s Republic of China).
Conflict of interest
The authors have declared that no financial relationships with any organizations that might have an interest in the submitted work; no other relationships or activities that could influence the submitted work.
Ethical approval
The study protocol was approved by the Independent Ethics Committee of West China Hospital, Sichuan University (Chengdu, China). All procedures in this study were carried out in accordance with the Helsinki declaration.
Informed consent
Written informed consent was obtained from each subject before screening procedures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, Z., Miao, J., Shu, S. et al. Pharmacokinetics and Bioequivalence Evaluation of a New Oxycodone Tamper-Resistant Tablet Administered with an Opioid Antagonist in Patients with Chronic Pain. Clin Drug Investig 40, 139–148 (2020). https://doi.org/10.1007/s40261-019-00870-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00870-w