Abstract
Background and Objectives
Suvorexant (MK-4305) is an orexin receptor antagonist approved for the treatment of insomnia in the USA and other regions. This randomized, double-blind, placebo-controlled, sequential-panel, Phase 1 trial assessed the safety, tolerability, and pharmacokinetic data following single and multiple dosing of suvorexant in healthy men (aged 18–45 years).
Methods
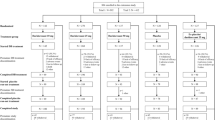
Within allocated panels, subjects (n = 8) were randomized to receive nightly doses of suvorexant (10, 20, 40, 80, and 100 mg) administered orally for 14 days, or placebo. Safety assessments included daily adverse event (AE) monitoring; pharmacokinetic data were obtained through periodic sampling.
Results
Of 40 subjects randomized, 39 completed the trial. The incidence of any AEs in the 10 and 20 mg groups was 67 and 83%, respectively, while 100% of subjects reported AEs in the dose groups of 40, 80, and 100 mg and the placebo group. The most frequently reported AEs were somnolence (n = 19 subjects), fatigue (n = 17), and headache (n = 15). Following single and multiple dosing, median time to reach maximum observed concentration ranged from 1.5 to 4.0 h and the apparent terminal half-life ranged from 7.7 to 14.5 h. Across the investigated doses, accumulation ratios for the area under the concentration–time curve and the maximum observed concentration were independent of dose and ranged from 1.21 to 1.60 and 1.00 to 1.46, respectively.
Conclusions
Suvorexant was generally well tolerated after single and multiple dosing for 14 days. The findings support the once-nightly dosing regimen.


Similar content being viewed by others
References
Coleman P, Gotter A, Herring WJ, Winrow C, Renger J. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509–33.
Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64(3):389–420.
Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22(13):5282–6.
Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14(7):1075–81.
Roecker AJ, Coleman PJ. Orexin receptor antagonists: medicinal chemistry and therapeutic potential. Curr Top Med Chem. 2008;8(11):977–87.
Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66.
Buysse D. Clinical pharmacology of other drugs used as hypnotics. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed., Chapter 43. St. Louis, MO: Elsevier Saunders; 2011. p. 492–509.
Roth T, Roehrs TA. Issues in the use of benzodiazepine therapy. J Clin Psychiatry. 1992;53(Suppl):14–8.
Roth T, Roehrs T. Pharmacotherapy for insomnia. Sleep Med Clin. 2010;5(4):529–39.
Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ. Orexin receptors as therapeutic drug targets. Prog Brain Res. 2012;198:163–88.
Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–74.
Michelson D, Snyder E, Paradis E, Chengan-Liu M, Snavely DB, Hutzelmann J, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–71.
Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, et al. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61.
Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283–93.
Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136–48.
Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12(9):1215–25.
Cui D, Cabalu T, Yee KL, Small J, Li X, Liu B, et al. In vitro and in vivo characterisation of the metabolism and disposition of suvorexant in humans. Xenobiotica. 2016;46:882–95.
Gotter AL, Winrow CJ, Brunner J, Garson SL, Fox SV, Binns J, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90–106.
Vermeeren A, Sun H, Vuurman EF, Jongen S, Van Leeuwen CJ, van Oers AC, et al. On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. Sleep. 2015;38(11):1803–13.
Merck Sharp & Dohme Corp. Belsomra (suvorexant) [package insert]. 2014. https://www.merck.com/product/usa/pi_circulars/b/belsomra/belsomra_pi.pdf. Accessed 28 Apr 2016.
US Department of Health and Human Services. 204569Orig1s000 pharmacology review. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204569Orig1s000PharmR.pdf. Accessed 13 Jul 2017.
Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in elderly patients with insomnia: pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25(7):791–802.
Acknowledgments
Christopher Lines, PhD, of Merck & Co., Inc., Kenilworth, NJ, USA provided comments and edits on a draft of the manuscript. The authors would like to thank Hong Sun and Dexter Kennedy for serving as clinical monitors for this trial, Xiaodong Li for his contributions to the statistical analyses, Gail Murphy for her contributions to the approval of dose-escalation decisions, and Bo Liu for data collection. Medical writing support, under the direction of the authors, was provided by Hicham Naimy, PhD, and Adele Blair, PhD, of CMC AFFINITY, a division of Complete Medical Communications Ltd., Glasgow, UK in accordance with Good Publication Practice (GPP3) guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This trial and medical writing assistance was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of interest
KLY, JM, DP, WL, NL, TC, and REW are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options. At the time of the trial, SR was an employee of SGS Life Sciences Services, a Contract Research Organization contracted by Merck & Co., Inc., Kenilworth, NJ, USA, to conduct the trial.
Ethical approval
All procedures performed in trials involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual subjects included in the trial.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yee, K.L., McCrea, J., Panebianco, D. et al. Safety, Tolerability, and Pharmacokinetics of Suvorexant: A Randomized Rising-Dose Trial in Healthy Men. Clin Drug Investig 38, 631–638 (2018). https://doi.org/10.1007/s40261-018-0650-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0650-4