Abstract
Background and Objectives
The combination of cromolyn and ibuprofen is being investigated as a treatment for early Alzheimer’s disease (AD). This study investigated the pharmacokinetics, safety, and tolerability of cromolyn and ibuprofen co-administration in healthy elderly adult volunteers.
Methods
In this open-labeled study, 26 subjects, aged 55–75 years, received co-administration of inhaled cromolyn (single dose 17.1 mg; double dose 34.2 mg total) and oral ibuprofen (single dose 10 mg; double dose 20 mg total). Blood sampling was performed for 6 h after co-administration in all subjects; cerebrospinal fluid (CSF) was collected in three to four subjects per cohort for 4 h following co-administration. Safety parameters, including adverse events (AEs), were monitored throughout the study.
Results
For cromolyn, the mean (±SD) maximum observed concentration (C max) in plasma was 46.69 ± 32.97 and 96.75 ± 46.22 ng/ml after single- and double-dose inhalation, respectively [time to C max (t max) ~22 min for each; terminal elimination half-life (t ½) ~1.8 h for each]. For ibuprofen, the plasma C max was 1090.98 ± 474.64 ng/ml and 2062.96 ± 655.13 ng/ml after single- and double-dose oral administration, respectively (t max ~1.6–1.8 h; t ½ ~1.9 h for each). For cromolyn, the CSF C max was 0.24 ± 0.08 ng/ml at 3.72 ± 0.70 h after single-dose administration and 0.34 ± 0.17 ng/ml at 3.45 ± 0.95 h after double-dose administration, and for ibuprofen, the CSF C max was 3.94 ± 1.29 ng/ml at 2.55 ± 0.96 h after single-dose administration and 8.93 ± 3.29 ng/ml at 3.15 ± 1.05 h after double-dose administration. Three (12%) subjects reported mild or moderate AEs which were unlikely to be related to study drug.
Conclusions
The combination of cromolyn and ibuprofen was safe and well tolerated. The concentrations of cromolyn and ibuprofen observed in the CSF are considered sufficient to titrate the estimated daily amyloid production and the associated inflammatory response in patients with AD.
Similar content being viewed by others
The combination of cromolyn and ibuprofen is being investigated as a treatment for early Alzheimer’s disease (AD). |
This study investigated the pharmacokinetics, safety, and tolerability of cromolyn and ibuprofen co-administration in healthy elderly adult volunteers. |
In the evaluated study population, the combination of cromolyn and ibuprofen was safe and well tolerated. |
The concentrations of cromolyn and ibuprofen observed in the cerebrospinal fluid are considered sufficient to titrate the estimated daily amyloid production and the associated inflammatory response in patients with AD. |
1 Introduction
Alzheimer’s disease (AD) is an irreversible, progressive brain disease with an average course of 8–20 years. The disease results in cognitive and functional impairment, which may affect memory, thinking skills, orientation, personality, and, in its most severe form, the ability to carry on the most basic tasks of daily life. It has been estimated that 5.4 million Americans have AD, with approximately one in nine persons over 65 years and approximately one-third of persons 85 years and older having the disease [1].
The pathogenesis of AD is believed to include two primary neuropathologies: (a) extracellular protein plaques principally composed of amyloid-beta (Aβ) peptides, also known as amyloid plaques; and (b) intracellular tangles of fibrils composed of tau protein found inside of neurons, also known as tau tangles [2,3,4]. The advent and spread of neurotoxic oligomeric aggregates of Aβ is widely regarded as the key trigger leading to neuronal damage, which then leads to the accumulation of intracellular tau tangles, and finally to neuronal cell death in AD pathogenesis. The cascade of Aβ oligomer-mediated neuronal intoxication is exacerbated by another AD trigger, namely chronic local inflammatory responses in the brain [5]. AD has a chronic neuro-inflammatory component that is characterized by the presence of abundant microglial cells associated with amyloid plaque [6, 7]. These cyclo-oxygenase (COX1/COX2)-expressing microglia, which phagocytose amyloid oligomers, become activated to secrete pro-inflammatory cytokines [5, 8, 9]. This neuro-inflammatory response, besides promoting local vascular leakage through the blood-brain barrier [10], has been implicated in driving further production of aberrant Aβ peptides 40/42 via modulation of gamma-secretase activity [11, 12] and to be detrimental to hippocampal neurogenesis in the adult brain [13]. Thus, neuro-inflammation, in combination with amyloid oligomer-mediated neuronal intoxication, creates a cycle that results in progressive neural dysfunction and neuronal cell death spreading throughout the brain in subjects with AD.
Cromolyn, which has been approved for use since the 1970s for the treatment of asthma and allergic rhinitis, has been shown to bind to and to inhibit Aβ peptide oligomerization at nanomolar concentrations in vivo [14, 15]. Inhalation of cromolyn is clinically shown to be the most effective administration route for systemic bioavailability of cromolyn [16, 17]. Studies have shown that with high inspiratory rates, the inhaled cromolyn is delivered efficiently to the human lung, with 10–15% of the inhaled drug-delivered dose absorbed into the bloodstream [16, 18].
Evidence to date suggests that long-term dosing with non-steroidal anti-inflammatory drugs (NSAIDs) can reduce AD risk in the elderly, including delayed disease onset, reduced symptomatic severity, and slowed cognitive decline [7, 19, 20]. Three mechanisms have been proposed to explain how NSAIDs inhibit the processes that contribute to AD progression: (a) inhibition of COX activity, thereby reducing or preventing microglial activation and cytokine production in the brain, (b) reduction of amyloid deposition, and (c) blockade of COX-mediated prostaglandin E2 responses in synapses [7, 11, 13, 21,22,23,24]. It has been suggested that NSAID therapy might be most efficacious in the early stages of the disease, and that NSAIDs such as indomethacin and ibuprofen, which presumably lower AB-42 peptide levels, might serve as the optimal clinical candidates [7, 13].
Given the pathogenesis of AD and the clinical evidence to date regarding cromolyn and ibuprofen, the combination of inhaled cromolyn and oral ibuprofen is currently being investigated as a treatment for early AD [25]. Thus, the primary objective of this study was to investigate the pharmacokinetics of combined cromolyn and ibuprofen administration, in plasma and cerebrospinal fluid (CSF), in healthy elderly volunteers. The secondary objective was to evaluate the safety and tolerability of cromolyn and ibuprofen, following co-administration.
2 Methods
2.1 Subjects
Men and women, age 55–75 years, with a body mass index (BMI) of 18–30 kg/m2, and in good general health, were eligible for enrollment in this study. The evaluated subject age range was selected in order to be comparable to the age range of the ongoing Phase III study [25]. Women of reproductive potential were excluded if they had a positive pregnancy test (urine or serum) or if they were pregnant or lactating. Potential subjects were excluded if they were: current smokers or ex-smokers with a remote history (>100 packs/year), symptomatic for viral infection in the last 14 days prior to dosing, had signs of active pulmonary infection or other pulmonary inflammatory conditions in the last 14 days, were currently taking medications known to be CYP2C9 inducers (i.e., carbamazepine and rifampicin), had a history of clinically significant respiratory disorders and chronic respiratory disease with impaired respiratory effort or difficulty taking inhaled drugs [e.g., chronic obstructive pulmonary disease (COPD), emphysema]. Potential subjects were also excluded if they had a forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) <60% or FEV1 <70% of predicted value or FVC <70% of predicted value in order to rule out subjects with moderate to severe respiratory obstruction. Subjects were also excluded if they were currently taking, or had taken, cromolyn within the past 30 days, if they had current NSAID use (including products containing ibuprofen while on study), and if they had allergy or hypersensitivity to cromolyn or ibuprofen. All subjects provided written informed consent prior to enrollment.
2.2 Study Design
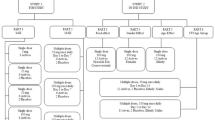
This was a Phase I, open-labeled study with a crossover design in which 26 (24 planned) healthy elderly volunteers were randomized to treatment regimen A–B or treatment regimen B–A in four cohorts [1a (A–B), 1b (B–A), 2a (B–A) and 2b (A–B); see Fig. 1]. Dose regimen A consisted of a single inhaled dose (17.1 mg) of cromolyn (Pharmaterials, Inc., Reading, UK) via a dry powder inhaler (DPI; Plastiape Spa, Osnago, Italy) plus a single oral dose (10 mg) of ibuprofen (Corealis Pharma, Laval, QC, Canada). Dose regimen B consisted of two inhaled doses of cromolyn (34.2 mg total), less than 2 min apart, plus two oral doses of ibuprofen (20 mg total). Ibuprofen tablets were administered with a total of 8 oz of water immediately following cromolyn inhalation. Dosing of group 1 (cohort 1a and 1b) was completed prior to the start of dosing of group 2 (cohort 2a and 2b).
2.3 Analytical Methods and Pharmacokinetic Assessments
Plasma concentrations of cromolyn and ibuprofen were followed for 6 h after each co-administration in all subjects; CSF was collected via lumbar puncture on a voluntary basis in three to four subjects (three planned) per cohort for up to 4 h following co-administration. Each subject came to the study site for a screening visit up to 30 days prior to the first dose (Day 1). On Day –1, eligible subjects were admitted to the clinical unit for inhaler training and safety and baseline check-ups. On Day 1 and Day 2, subjects were administered cromolyn and ibuprofen as randomized, and pharmacokinetics sampling in plasma (Day 1 and Day 2) and CSF (Day 1 only) was performed (see Fig. 1).
Following drug administration on Day 1 and Day 2, plasma samples were collected for all subjects at the following time points 0, 5, 10, 15, and 30 min, and 1, 2, 4, and 6 h. Following drug administration on Day 1, CSF samples were collected for a sub-group of 12 subjects at the following time points 0, 5, and 30 min, and 2 and 4 h. All plasma and CSF samples were to be drawn within ±10% of the scheduled time. The timing of pharmacokinetic plasma and CSF samples took priority over all other scheduled study activities except for dosing. Plasma and CSF samples were analyzed in accordance with good laboratory practice (GLP) using validated bioanalytical methods [high performance liquid chromatography tandem mass spectrometry (HPLC/MS/MS)] by KCAS Bioanalytical and Biomarker Services (Shawnee, KS, USA) (see Supplemental Table 1). The lower limit of quantification (LLOQ) for each analyte in plasma and CSF was as follows: cromolyn, plasma = 10 ng/ml, cromolyn, CSF = 0.1 ng/ml; ibuprofen, plasma = 25 ng/ml, ibuprofen, CSF = 0.3 ng/ml.
Pharmacokinetic parameters were determined with non-compartmental analysis (NCA) using Phoenix® WinNonlin® (Certara Inc.) version 6.3.0.395 by CTC Clinical Trial Consultants AB. Pharmacokinetic parameters included the area under the plasma concentration-time curve (AUC) from time 0 to infinity and from time 0 to the last measureable concentration (AUC∞ and AUClast, respectively), calculated according to the linear trapezoidal method, the maximum observed concentration (C max), the terminal elimination half-life (t ½), and the time to maximum observed concentration (t max).
2.4 Safety Assessments
Adverse events (AEs) were monitored throughout the study, from Day –1 through 30 days after the last administration of study treatment. AEs were considered to be any symptom, sign, illness, or experience that developed or worsened in severity during the course of the study. A serious adverse event (SAE) was considered to be any AE that was: fatal, life‐threatening, required or prolonged hospital stay, resulted in persistent or significant disability or incapacity, was a congenital anomaly or birth defect, or was an important medical event. AEs were coded using the Medical Dictionary for Drug Regulatory Activities (MedDRA) Version 18.0E and rated by the investigator to be of mild, moderate, or severe intensity and unrelated, possibly related, or related to study drug. Electrocardiograms (ECGs) were performed at screening and on Day –1. A physical examination was conducted and vital signs were collected each study day. Clinical laboratory parameters, including clinical chemistry, hematology, and urinalysis were evaluated at screening, Day 1, and Day 2 (see Fig. 1).
2.5 Statistical Analysis
The safety population consisted of all subjects who were assigned treatment and were administered at least once with the study drug (the all-treated population). The primary subject population for all pharmacokinetic analyses was the protocol-compliant population. This population was defined as all subjects who were randomized and received treatment with evaluable pharmacokinetic parameter data and no major protocol deviations with an impact on pharmacokinetic data. All statistical analyses were performed using SAS® (Version 9.4, SAS Institute Inc., Cary, NC, USA) by PCG Clinical Services AB. For pharmacokinetic analyses, pre-dose concentrations below the LLOQ (BLLOQ) were set to 0 at time = 0 as default by the WinNonlin program; pre-dose concentrations above LLOQ were set to 0 at time = 0 if the value was less than 5% of the C max value.
3 Results
3.1 Demographics and Baseline Characteristics
A total of 29 subjects were enrolled in the study and 26 (89.7%) were treated. Three randomized subjects did not receive study drug due to problems establishing the lumbar puncture. Two of the treated subjects (Subject 01–01 in cohort 1b and Subject 01–18 in cohort 2b) were replaced; one due to catheter clogging during CSF sampling (Subject 01–01) and one due to receiving the incorrect treatment sequence (Subject 01–18). All treated subjects, including the subjects who were replaced, completed the study. All 26 treated subjects were included in the all-treated population for safety analyses and 25 subjects were included in the protocol-compliant population for the pharmacokinetic analyses. Subject 01–01 was excluded from the protocol-compliant population based on unavailable post-dose CSF sampling due to catheter clogging; Subject 01–18 was included in the protocol-compliant population based on actual treatment given. A total of 13 subjects were included in the protocol-compliant population for CSF pharmacokinetic analyses.
The protocol-compliant population included 11 males and 14 females, with a mean age of 63.4 years (range 55–75 years) and a mean BMI of 27.0 kg/m2 (range 21.3–29.8 kg/m2). All subjects but one were White of Hispanic or Latino origin and all subjects had an FEV1/FVC ratio above 70% (range 70.7–85.1%), indicating normal lung function. Overall, the demographic characteristics were comparable between cohorts.
3.2 Pharmacokinetics
3.2.1 Cromolyn Plasma Pharmacokinetics
The mean plasma concentration-time profiles after single and double doses of cromolyn are shown in Fig. 2 and Supplemental Table 2; the pharmacokinetic parameters for cromolyn in plasma are provided in Table 1. Cromolyn was rapidly absorbed following both single- and double-dose inhalation to reach an average maximum plasma concentration of 40.53 ng/ml at 10 min after single-dose inhalation and 88.18 ng/ml at 15 min after double-dose inhalation (Fig. 2 and Supplemental Table 2).
Upon inhalation of cromolyn single dose, the cromolyn plasma concentration reached a mean (±SD) C max of 46.69 ± 32.97 ng/ml at 22.8 ± 16.6 min. The apparent t ½ of cromolyn in plasma was 1.75 ± 0.85 h indicating a relatively moderate clearance (Table 1). Upon inhalation of cromolyn double dose, cromolyn reached a C max of 96.75 ± 46.22 ng/ml at 22.2 ± 19.4 min. The apparent t ½ of cromolyn in plasma for the double dose was 1.91 ± 0.70 h, indicating similar moderate clearance from the plasma as following single-dose administration (Table 1). The AUC0–∞ increased with the dose increase from 195.71 ± 97.33 ng·h/ml following single-dose inhalation to 284.55 ± 91.29 ng·h/ml following double-dose inhalation. Similarly, the AUClast increased with the dose increase from 74.43 ± 53.89 ng·h/ml following single-dose inhalation to 198.19 ± 93.15 ng·h/ml following double-dose inhalation (Table 1).
3.2.2 Ibuprofen Plasma Pharmacokinetics
The mean plasma concentration-time profiles after single and double doses of ibuprofen are shown in Fig. 3 and Supplemental Table 3; the pharmacokinetic parameters for ibuprofen in plasma are provided in Table 2. For ibuprofen, the mean maximum plasma concentrations of 670.83 and 1442.50 ng/ml were reached 2 h after single- and double-dose administration, respectively (Fig. 3 and Supplemental Table 3).
Oral administration of the single dose of ibuprofen resulted in a plasma C max of 1,090.98 ± 474.64 ng/ml at 1.59 ± 1.43 h. The apparent t ½ in plasma was 1.93 ± 0.32 h, indicating moderate clearance from the plasma (Table 2). Oral administration of the ibuprofen double dose resulted in a C max of 2062.96 ± 655.13 ng/ml at 1.82 ± 1.27 h. The apparent t ½ in plasma was 1.88 ± 0.33 h, indicating moderate clearance from the plasma (Table 2). The AUC∞ increased with the dose increase from 3464.31 ± 443.56 ng·h/ml for a single dose to 6823.60 ± 1164.69 ng·h/ml for a double dose. The AUClast increased with the dose increase from 2737.92 ± 972.59 ng·h/ml following a single dose to 5522.21 ± 1233.99 ng·h/ml following double-dose administration (Table 2).
3.2.3 Cerebrospinal Fluid Pharmacokinetics
The pharmacokinetic parameters for cromolyn and ibuprofen in CSF following single- and double-dose administration are listed in Table 3; the mean CSF concentrations for cromolyn and ibuprofen are shown in Supplemental Tables 4 and 5, respectively. The mean cromolyn C max in the CSF during the observed time interval was 0.24 ± 0.08 ng/ml at 3.72 ± 0.70 h following single-dose inhalation and 0.34 ± 0.17 ng/ml at 3.45 ± 0.95 h following double dose inhalation (Table 3). The ibuprofen C max in CSF during the observed time interval was 3.94 ± 1.29 ng/ml at 2.55 ± 0.96 h after single-dose administration and 8.93 ± 3.29 ng/ml at 3.15 ± 1.05 h after double-dose administration. The observation period (0–4 h) for the CSF samples was too short to allow determination of the terminal half-life. For subjects with both plasma and CSF samples, the AUCCSF/AUCplasma ratios indicated that, for single-dose cromolyn, the CSF penetration was 0.15% of plasma at 2 h (n = 6) and 0.43% at 4 h (n = 5). For double-dose cromolyn, the corresponding values were 0.12% at 2 h (n = 7) and 0.33% at 4 h (n = 6). For single-dose ibuprofen, the CSF penetration was 0.20% of plasma at 2 h (n = 3) and 0.35% at 4 h (n = 3); for double-dose ibuprofen, the CSF penetration was 0.25% at 2 h (n = 7) and 0.39% at 4 h (n = 7).
3.3 Safety Assessments
Three (12%) subjects reported a total of six AEs. There were no serious AEs (SAEs), no AEs leading to withdrawal, and no AEs leading to death. The six reported AEs included nervous system disorders [n = 3 (sciatica, headache, and nerve compression)], gastrointestinal disorders [n = 2 (abdominal pain and diarrhea)] and reproductive system and breast disorder [n = 1 (testicular swelling)]. All AEs were assessed as unlikely to be related to study drug. Three events were of mild intensity and three were of moderate intensity. There were no trends or differences between treatment groups in terms of reported AEs. No clinically significant abnormal findings were identified during any physical examination and there were no clinically significant changes in mean vital sign values over time and no clinically significant abnormal vital signs. There were no apparent trends or changes in laboratory parameters over time in any treatment group and all individual out-of-range values were assessed as not clinically significant by the investigator.
4 Discussion
The co-administration of inhaled cromolyn and oral ibuprofen was safe and well tolerated in the healthy elderly population evaluated in this study. For the study, cromolyn was administered via inhalation due its relatively poor oral absorption properties [16, 17], while ibuprofen was administered via oral tablets. The study drug dosage regimens employed in this study were selected in order to match the dosage regimens currently being evaluated in a large Phase III study [25]. Consistent with prior reports, cromolyn was rapidly absorbed in plasma following inhalation, with t max values of approximately 22 min for both single- (17.1 mg) and double- (34.2 mg) dose administration. The apparent cromolyn half-life observed in the current study (1.75 h for single dose and 1.91 h for double dose) was also similar to previously reported values (1.2–4.4 h; [18]). The mean AUC∞ increased with the dose increase, from 195.71 ± 97.33 ng·h/ml following single-dose inhalation to 284.55 ± 91.29 ng·h/ml following double-dose inhalation.
To our knowledge, the pharmacokinetics of cromolyn in the CSF have not been previously reported. In the healthy elderly subjects of this study, cromolyn CSF concentrations increased over the course of the 4-h duration of the lumbar puncture for most subjects following single- and double-dose administration to reach an average C max during the observed time interval of 0.24 and 0.34 ng/ml, corresponding to cromolyn concentrations of 0.47 and 0.66 nM, respectively. Importantly, this projected level of cromolyn in the brain (0.24–0.34 ng/ml × 1500 ml brain volume = 360–510 ng of total cromolyn), is approximately 20–30 times the amount needed to titrate the estimated daily amyloid plaque production (17.7 ng) in patients with AD [12]. These data indicate that inhaled cromolyn is transported via the deep lung to the blood and then to the CSF. Analysis of the AUCCSF/AUCplasma ratios indicated that the CSF penetration of cromolyn was approximately 0.12–0.15% of plasma at 2 h and 0.33–0.43% at 4 h. While a full pharmacokinetic profile in the CSF could not be obtained due to limitations of the duration of the lumbar puncture, it is possible that the cromolyn concentrations in the CSF reached higher levels than indicated from the available data collected.
The plasma pharmacokinetics of oral ibuprofen are well characterized [26], though prior studies have employed significantly higher doses (i.e., 200–400 mg) than those used in this study (10 or 20 mg). Across a wide range of studies, the C max for oral ibuprofen has been reported to be approximately 20–40 mg/l following oral administration of 200–400 mg, with rapid absorption and peak plasma concentrations observed within 1.5–3 h post-administration [26]. In this study, the observed plasma pharmacokinetics for ibuprofen were consistent with prior studies, with mean C max values of approximately 1091 ng/ml (1 mg/l) and 2063 ng/ml (2 mg/l) following administration of 10 and 20 mg, respectively, with associated mean t max values of 1.6 and 1.8 h. Also consistent with prior studies, the apparent ibuprofen half-life in plasma was approximately 1.9 h following both single- and double-dose administration, indicating moderate plasma clearance. Similarly, the mean AUC∞ for ibuprofen reported here (3464 ng·h/ml (3.5 mg·h/l) and 6824 ng·h/ml (6.8 mg·h/l) for 10 mg and 20 mg, respectively, are consistent with prior studies, which indicate mean AUC values of approximately 100 mg·h/l following 400-mg oral administration [26].
Relatively few studies have reported on the pharmacokinetics of ibuprofen in the CSF [27,28,29]. Following the oral administration of 800 mg of racemic ibuprofen, Bannwarth et al. reported CSF C max values of 168 and 315 µg/l for the (R)-enantiomer and the (S)-enantiomer, respectively, for a total ibuprofen C max of 483 µg/L [27]. In a pediatric population, Kokki et al. reported a CSF C max of 541 µg/L at 30 min after the intravenous administration of 10 mg/kg ibuprofen [28]. In the current study, oral administration of 10 mg or 20 mg of ibuprofen resulted in CSF C max levels during the observed time interval of approximately 4 and 9 µg/L (corresponding to 19 nM and 43 nM), respectively. In the Bannwarth study, ibuprofen was undetectable in the CSF 30 min after oral administration [27], and in the current study, most subjects did not have quantifiable levels of ibuprofen in the CSF until the 2-h analysis time point. Analysis of the AUCCSF/AUCplasma ratio for subjects with available data indicated that the CSF penetration of ibuprofen was approximately 0.20–0.25% of plasma at 2 h and 0.35–0.39% of plasma at 4 h. While differences in study design, ibuprofen dosing, and pharmacokinetic sample timing make direct comparisons across studies difficult, Bannwarth et al. reported AUCCSF/AUCplasma ratios of 0.9–1.5%, while Har-Even et al. reported a CCSF/Cplasma ratio of 1.1–0.7% in pediatric patients following oral administration of 10 mg/kg ibuprofen [29]. In the current study, the concentration of ibuprofen in the CSF increased up to the 4 h duration of the lumbar puncture. As with cromolyn, a full pharmacokinetic profile in the CSF could not be obtained due to limitations of the duration of the lumbar puncture.
4.1 Limitations
As noted above, the protocol-specified 4-h duration of the lumbar puncture was insufficient to obtain a full pharmacokinetic profile for either cromolyn or ibuprofen in the CSF. For the plasma pharmacokinetics analyses, the addition of more sampling times during both the absorption and elimination phases may have allowed for a more complete characterization of the plasma concentration-time profiles for both cromolyn and ibuprofen. The inter-subject variability was perhaps larger than expected for both cromolyn and ibuprofen. While all subjects received the same inhaler training and performed the same standardized cromolyn inhalation procedure, it is possible that slight differences in inhalation technique or unidentified differences in lung function across subjects affected the delivery of cromolyn to the lung. For ibuprofen, the observed inter-subject variability was largely driven by a single subject whose pharmacokinetic parameters for single-dose ibuprofen, especially C max and T max, were substantially reduced as compared to other subjects. No dosing irregularities were reported for this subject, and the reason(s) behind these aberrant values are unknown. In addition, the study used a relatively low ibuprofen dose (10 or 20 mg), which has not been previously evaluated, thus limiting comparison to prior studies with respect to inter-subject variability. Changes to the study population to include/exclude subjects with differing degrees of respiratory obstruction might be expected to affect the observed pharmacokinetics of inhaled cromolyn.
5 Conclusions
The observed plasma concentrations and half-life of cromolyn and ibuprofen following single- and double-dose administration were consistent with values reported previously. The levels of cromolyn and ibuprofen observed in the CSF are estimated to be sufficient to titrate the estimated daily 17.7 ng of amyloid production and the associated inflammatory response. The co-administration of cromolyn and ibuprofen was well tolerated by the healthy elderly subjects in the study.
References
Alzheimer’s A. Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509.
Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3(1):75–80.
Gotz J, Schild A, Hoerndli F, Pennanen L. Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: insight from transgenic mouse and tissue-culture models. Int J Dev Neurosci. 2004;22(7):453–65.
Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol. 2010;119(5):523–41.
Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation. 2012;9:151. doi:10.1186/1742-2094-9-151.
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22.
Imbimbo BP, Solfrizzi V, Panza F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front Aging Neurosci. 2010;2:19.
Griffin WS. Alzheimer’s—looking beyond plaques. F1000 Med Rep. 2011;3:24.
Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Soothing the inflamed brain: effect of non-steroidal anti-inflammatory drugs on Alzheimer’s disease pathology. CNS Neurol Disord Drug Targets. 2011;10(1):57–67.
Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38.
Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23(20):7504–9.
Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10(9):698–712.
Gasparini L, Ongini E, Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J Neurochem. 2004;91(3):521–36.
Hori Y, Takeda S, Cho H, Wegmann S, Shoup TM, Takahashi K, et al. A food and drug administration-approved asthma therapeutic agent impacts amyloid beta in the brain in a transgenic model of Alzheimer disease. J Biol Chem. 2015;290(4):1966–78.
Elmaleh DR, Shoup TM, Fischman AJ, Takahashi K, Larvie M, Vogan E. Evaluation of F-18 radiolabeled cromolyn as a potential Aβ polymerization inhibitor and PET tracer. HAI (Human Amyloid Imaging), Miami Florida. 2014. p. 63.
Richards R, Dickson CR, Renwick AG, Lewis RA, Holgate ST. Absorption and disposition kinetics of cromolyn sodium and the influence of inhalation technique. J Pharmacol Exp Ther. 1987;241(3):1028–32.
Aswania OA, Corlett SA, Chrystyn H. Relative bioavailability of sodium cromoglycate to the lung following inhalation, using urinary excretion. Br J Clin Pharmacol. 1999;47(6):613–8.
Keller M, Schierholz J. Have inadequate delivery systems hampered the clinical success of inhaled disodium cromoglycate? Time for reconsideration. Expert Opin Drug Deliv. 2011;8(1):1–17.
In’t Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–21.
Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327(7407):128. doi:10.1136/bmj.327.7407.128.
Mackenzie IR, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50(4):986–90.
Alafuzoff I, Overmyer M, Helisalmi S, Soininen H. Lower counts of astroglia and activated microglia in patients with Alzheimer’s disease with regular use of non-steroidal anti-inflammatory drugs. J Alzheimers Dis. 2000;2(1):37–46.
Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–6.
Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, et al. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131(Pt 3):651–64.
https://clinicaltrials.gov/ct2/show/NCT02547818. Accessed June 2017.
Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34(2):101–54. doi:10.2165/00003088-199834020-00002.
Bannwarth B, Lapicque F, Pehourcq F, Gillet P, Schaeverbeke T, Laborde C, et al. Stereoselective disposition of ibuprofen enantiomers in human cerebrospinal fluid. Br J Clin Pharmacol. 1995;40(3):266–9.
Kokki H, Kumpulainen E, Lehtonen M, Laisalmi M, Heikkinen M, Savolainen J, et al. Cerebrospinal fluid distribution of ibuprofen after intravenous administration in children. Pediatrics. 2007;120(4):e1002–8.
Har-Even R, Stepensky D, Britzi M, Soback S, Chaim AB, Brandriss N, et al. Plasma and cerebrospinal fluid concentrations of ibuprofen in pediatric patients and antipyretic effect: Pharmacokinetic-pharmacodynamic modeling analysis. J Clin Pharmacol. 2014;54(9):1023–30.
Acknowledgements
The authors acknowledge the clinical research staff at Panax Clinical Research for the conduct of the study, Bengt Dahlström, PhD of CTC Clinical Trial Consultants for the pharmacokinetic analyses, PCG Clinical Services for clinical data management, biostatistics, and medical writing support, and Brooke Harrison, PhD for technical assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AZ Therapies, Inc.
Conflicts of Interest
David Brazier, Jim Keane, Katie Barrett, and David R. Elmaleh are employees of the study sponsor, AZ Therapies, Inc. Robert Perry is an employee of Panax Clinical Research, the site of the clinical study.
Ethics Approval
The study was approved in writing on 16-APR-2015 by the Liberty Institutional Review Board (IRB) in DeLand, FL, USA. All study procedures were in accordance with the 1964 Helsinki declaration (and its amendments).
Informed Consent
The subject informed consent (IC) was reviewed and approved by the IRB for the study. The formal consent of a subject, using the IRB-approved consent form, was obtained before the subject underwent any study procedure. The IC was signed by the subject and the investigator/designated research professional obtaining the consent. Subjects willing to allow lumbar puncture for CSF collection were also consented using a procedure-specific IC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brazier, D., Perry, R., Keane, J. et al. Pharmacokinetics of Cromolyn and Ibuprofen in Healthy Elderly Volunteers. Clin Drug Investig 37, 1025–1034 (2017). https://doi.org/10.1007/s40261-017-0549-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-017-0549-5