Abstract
Background and Objectives
Vedolizumab, a humanized monoclonal antibody against the α4β7 integrin, is indicated for treatment of moderately to severely active ulcerative colitis or Crohn’s disease. In this placebo-controlled, double-blind, randomized, single ascending-dose study, the pharmacokinetics, pharmacodynamics, safety, and tolerability of vedolizumab were evaluated in healthy volunteers.
Methods
Forty-nine participants (in five cohorts) were randomly assigned in a 4:1 ratio to receive a single intravenous infusion of either vedolizumab (0.2, 0.5, 2.0, 6.0, or 10.0 mg/kg) or placebo. Blood samples were collected for measurement of vedolizumab serum concentrations and α4β7 saturation on peripheral blood lymphocytes by vedolizumab. Pharmacokinetic parameters were computed using a non-compartmental approach. Adverse events were monitored.
Results
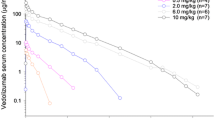
Vedolizumab maximum observed serum concentration (C max) demonstrated dose proportionality over the dose range tested. Greater than dose-proportional increases in area under the serum concentration–time curve from time 0 to infinity (AUC0–inf) and shorter terminal elimination half-life (t 1/2) were observed from 0.2 to 2.0 mg/kg, suggestive of nonlinear pharmacokinetics at lower doses. At doses higher than 2.0 mg/kg, these parameters increased dose proportionally. Saturation of α4β7 was at or near maximal levels (>90 %) at all doses and time points when vedolizumab was measurable in serum. A total of 21 of 39 (54 %) vedolizumab-treated participants were anti-drug antibody (ADA) positive, and 11 (28 %) were persistently ADA positive. Overall, no adverse event signals, including serious infections or malignancies, were apparent.
Conclusions
Vedolizumab exhibited target-mediated disposition, characterized by a rapid, saturable, nonlinear elimination process at low concentrations and a slower linear elimination process at higher concentrations. Nearly complete α4β7 saturation was observed at all doses. A single intravenous infusion of vedolizumab was well tolerated by healthy volunteers.




Similar content being viewed by others
References
Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864–75.
Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18(8):1470–9.
Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–21.
Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618–27.
Rosario M, Dirks NL, Gastonguay MR, Fasanmade AA, Wyant T, Parikh A, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42(2):188–202.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352(24):2499–507.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6(12):1370–7.
Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18(8):1470–9.
Wyant T, Estevam J, Yang L, Rosario M. Development and validation of receptor occupancy pharmacodynamic assays used in the clinical development of the monoclonal antibody vedolizumab. Cytom B Clin Cytom. 2016;90(2):168–76.
Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2016; doi:10.1136/gutjnl-2015-311079 (Epub ahead of print).
TYSABRI (natalizumab) package insert. Cambridge: Biogen Inc; 2015.
Dubinsky MC, Mahadevan U, Vermeire S, Abhyankar B, Lasch K. Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. J Crohns Colitis. 2015;9(Suppl 1):S361–2.
Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–9.
ENTYVIO (vedolizumab) package insert. Deerfield: Takeda Pharmaceuticals America, Inc; 2014.
Worobec A, Rosenberg AS. A risk-based approach to immunogenicity concerns of therapeutic protein products, part 2: Considering host-specific and product-specific factors impacting immunogenicity. BioPharm International. 2004;17(12).
Rosario M, Fox IH, Milch C, Parikh A, Feagan BG, Sandborn WJ, et al. Pharmacokinetic/pharmacodynamic relationship and immunogenicity of vedolizumab in adults with inflammatory bowel disease: additional results from GEMINI 1 and 2. Inflamm Bowel Dis. 2013;19(Suppl 1):S80.
Acknowledgments
We thank the staff at Anapharm (Montréal, Quebec, Canada) for conducting the study and collecting the study data. We are grateful to the volunteers who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This clinical study was supported by Takeda. Writing assistance was provided by Elisabeth R. Wann, PhD, Wann Medical Communications, LLC, Wilmette, Illinois, USA, and was supported by Takeda Development Center Americas, Inc., Deerfield, Illinois, USA.
Conflict of interest
Maria Rosario, Timothy Leach, Serap Sankoh, Asit Parikh, and Irving Fox are employees of Millennium Pharmaceuticals, Inc., Cambridge, Massachusetts, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company, Ltd. Asit Parikh owns stock of Takeda Pharmaceutical Company, Ltd. Catherine Scholz and Timothy Wyant were employees of Millennium Pharmaceuticals, Inc., Cambridge, Massachusetts, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company, Ltd., at the time that this study was conducted. Catherine Scholz is co-inventor of Patent 13/462,414. Brian G. Feagan has served as a consultant, advisory board member, or speaker for Abbott/AbbVie, Actogenix, Albireo Pharma, Amgen, AstraZeneca, Avaxia Biologics Inc., Avir Pharma, Axcan, Baxter Healthcare Corp., Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharmaceuticals, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GlaxoSmithKline, Ironwood Pharmaceuticals, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Hakko Kirin Co., Ltd., Lexicon, Lilly, Lucera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Novo Nordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharmaceuticals, Serono, Shire, Sigmoid Pharma, Synergy Pharmaceuticals Inc., Takeda, Teva Pharmaceutical, TiGenix, Tillotts, UCB Pharma, Vertex Pharmaceuticals, VHsquared Ltd., Warner Chilcott, Wyeth, Zealand, and Zyngenia; and has received research funding from Abbott/AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Janssen Biotech (Centocor), JnJ/Janssen, Roche/Genentech, Millennium, Pfizer, Receptos, Santarus, Sanofi, Tillotts, and UCB Pharma.
Ethical approval
This study was conducted in accordance with the ethical principles originating in or derived from the Declaration of Helsinki and its amendments, International Conference on Harmonization Good Clinical Practice Guidelines, and locally applicable laws or regulations. The protocol and informed consent form were approved by the institutional review board at the study site.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Rosario, M., Wyant, T., Leach, T. et al. Vedolizumab Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability Following Administration of a Single, Ascending, Intravenous Dose to Healthy Volunteers. Clin Drug Investig 36, 913–923 (2016). https://doi.org/10.1007/s40261-016-0437-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0437-4