Abstract
Background and Objective
Mirikizumab is a humanized anti-interleukin-23-p19 monoclonal antibody being developed for ulcerative colitis and Crohn’s disease. This analysis characterized mirikizumab pharmacokinetics using phase II and III trial data from patients with moderately to severely active ulcerative colitis.
Methods
Serum pharmacokinetic data in patients receiving mirikizumab 50–1000 mg intravenously every 4 weeks as induction treatment and mirikizumab 200 mg subcutaneously every 4 or 12 weeks as maintenance treatment across three trials (N = 1362) were analyzed using non-linear mixed-effects modeling. Covariate effects on mirikizumab exposure were evaluated using simulation-based estimations.
Results
Mirikizumab pharmacokinetics was best described by a linear two-compartment model with first-order absorption. Clearance, volume of distribution for central and peripheral compartments, and half-life were estimated at approximately 0.022 L/h (linear), 3.11 L and 1.69 L, and 9.5 days, respectively. Statistically significant effects of body weight and serum albumin levels on clearance, body weight on central and peripheral volumes of distribution, and body mass index on bioavailability were observed but effects were small relative to random inter-individual variability (% coefficient of variation: 18–64%). The subcutaneous bioavailability of mirikizumab was 48%.
Conclusions
Mirikizumab displayed pharmacokinetic characteristics typical of a monoclonal antibody where clearance increased with body weight and decreased with the albumin level, and bioavailability decreased with body mass index. These effects were small relative to random variability, indicating that a dose adjustment for patient factors is not required.
Clinical Trial Registration
ClinicalTrials.gov: NCT02589665 (28 October, 2015), NCT03518086 (8 May, 2018), NCT03524092 (14 May, 2018).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mirikizumab is a monoclonal antibody directed against the p19 subunit of interleukin-23 that has been shown to be effective and well tolerated in patients with moderately to severely active ulcerative colitis. |
Using data from one phase II (AMAC) and two phase III (LUCENT 1+2) randomized clinical trials, the population pharmacokinetics of mirikizumab is best described by a two-compartment model with first-order absorption and linear clearance. |
The mirikizumab pharmacokinetic profile was consistent with previous studies in patients with different chronic inflammatory diseases and in healthy volunteers, with a small influence of patient body weight/body mass index and a serum albumin level that is not clinically meaningful. |
1 Introduction
Ulcerative colitis (UC) is a chronic disease of unknown etiology that is characterized by inflammation of the rectum and colon resulting in symptoms such as diarrhea, rectal bleeding, urgency, and tenesmus [1, 2]. Treatment goals in UC include induction of remission (typically within a 6-week to 12-week time frame) and maintenance of remission in the longer term (assessed over 52 weeks of continuous treatment in clinical trials) [3]. Only a proportion of patients respond to current small-molecule and biologic therapies, and there is an associated increased risk of infections [4].
Interleukin-23 (IL-23) is a heterodimeric cytokine that is crucial for maintenance and amplification of T helper 17 cells and stimulation of many innate immune cells [5, 6], which are both important processes in UC and other chronic inflammatory diseases [7]. Several monoclonal antibodies that selectively target the p19 subunit of IL-23 are effective in patients with Crohn’s disease [8,9,10] and psoriasis [11,12,13,14,15,16]. Selective targeting of IL-23 is more effective than non-selective IL-23 targeting in patients with psoriasis and is a promising strategy for improved treatment of patients with moderately to severely active UC [17]. The pharmacokinetics of selective anti-IL-23 drugs for the treatment of patients with UC has not been extensively published [18].
Mirikizumab is a humanized IgG4-variant monoclonal antibody directed against the p19 subunit of IL-23 whose efficacy and safety have been assessed in adults with moderately to severely active UC in three multicenter, randomized, double-blind, placebo-controlled clinical trials: the induction and maintenance phase II AMAC trial (NCT02589665), the induction phase III LUCENT 1 trial (NCT03518086, AMAN), and the maintenance phase III LUCENT 2 trial (NCT03524092, AMBG) [19,20,21,22]. In these trials, mirikizumab was well tolerated, and clinical remission rates were higher in patients treated with mirikizumab compared with placebo during induction and maintenance therapy. Pharmacokinetic (PK) data were available from a total of 1362 patients from the three clinical studies. Data from the phase II trial were analyzed separately from the data from the two phase III trials using a non-linear mixed-effects modeling approach. The objectives of the current analyses were to characterize mirikizumab pharmacokinetics in patients with moderately to severely active UC and to evaluate the impact of the relevant patient-specific covariates on mirikizumab systemic exposures to inform dosing recommendations.
2 Methods
2.1 Patients and Study Designs
The three clinical trials were designed to evaluate the efficacy, safety, and pharmacokinetics of mirikizumab in inducing (AMAC and LUCENT 1) and maintaining (AMAC and LUCENT 2) remission in patients aged 18–75 years (AMAC) or 18–80 years (LUCENT 1+2) with moderately to severely active UC. Details of the three trials have been reported elsewhere [19,20,21,22].
The AMAC (NCT02589665) trial was conducted at 85 study sites in 14 countries between January 2016 and September 2017. LUCENT 1 (NCT03518086, AMAN) was conducted at 383 study sites in 34 countries from 18 June, 2018 to 21 January, 2021. LUCENT 2 (NCT03524092, AMBG) was conducted at 367 study sites in 34 countries from 19 October, 2018 to 3 November, 2021. These three studies were approved by the applicable ethics review boards and performed in accordance with the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, and the International Council for Harmonisation Guideline for Good Clinical Practice. All patients provided written informed consent [23].
Participating patients had a Mayo endoscopic subscore of ≥2 and a total Mayo score of 6–12 (AMAC) or a modified Mayo score 4–9 (LUCENT 1), and had not responded or were intolerant to conventional UC therapy (AMAC and LUCENT 1), biologic therapy with a target other than IL-23 (LUCENT 1), and/or Janus kinase inhibitors for UC treatment (LUCENT 1). Patients were excluded if they had previous exposure to any other biologic therapy targeting IL-23, with the LUCENT 1 trial also excluding patients who had not responded to three or more different biologic therapies. The LUCENT 2 trial accepted patients who had completed the LUCENT 1 trial.
In the AMAC trial (Fig. 1a of the Electronic Supplementary Material [ESM]), patients were randomized to placebo or mirikizumab 600-mg, 200-mg, or 50-mg induction dose regimens administered intravenously every 4 weeks (Q4W) for 12 weeks. In the mirikizumab 200-mg and 50-mg groups, doses after the first one were adjusted based on exposure. Serum concentrations of mirikizumab were assessed at weeks 2 and 6, and the dose was increased at weeks 4 and 8 according to a prespecified algorithm [21]. The dose increase ranged from 2-fold to 12-fold for patients in the 50-mg arm and from 1.5-fold to 3-fold for patients in the 200-mg arm. The 600-mg arm remained at a fixed dose during the first 12 weeks. No patient was given a dose of > 600 mg in the induction period. The dose adjustments in the 200-mg and 50-mg groups resulted in an average group dose of 250 mg and 100 mg, respectively.
Responders to induction regimens were randomized to receive 200 mg subcutaneously either Q4W or every 12 weeks as a maintenance regimen through 92 weeks, terminating with a final 16-week follow-up period. Non-responders to induction regimens had the option to discontinue from the study or enter the open-label extension period, which consisted of a 12-week induction phase with a fixed intravenous (IV) dose of mirikizumab 600 mg or 1000 mg Q4W (three doses in total), and if they responded to the induction phase, a subsequent 92-week maintenance phase with subcutaneous (SC) mirikizumab 200 mg Q4W. Patients who did not achieve a clinical response at week 12 were discontinued from the study.In the LUCENT 1 trial (Fig. 1b of the ESM), patients were randomized (3:1) to mirikizumab 300 mg or IV placebo Q4W. Once they completed the 12-week induction phase, patients could enter the LUCENT 2 trial and receive treatment based on their response in LUCENT 1.
In the LUCENT 2 trial (Fig. 1b of the ESM), mirikizumab-treated responders from LUCENT 1 (a decrease in the modified Mayo score of ≥ 2 points and a ≥ 30% decrease from baseline, and a decrease of ≥ 1 point in the rectal bleeding subscore from baseline or a rectal bleeding subscore of 0 or 1) were randomized (2:1) to mirikizumab 200 mg or SC placebo Q4W, whereas placebo-treated responders continued to receive placebo Q4W, but with SC administration. Patients who maintained clinical response continued the same regimen until week 40, whereas patients who had a loss of response were given IV mirikizumab 300 mg Q4W for three doses followed by a final assessment. LUCENT 1 trial non-responders received IV mirikizumab 300 mg Q4W in the LUCENT 2 trial. After 12 weeks, if they responded, their dose was changed to SC mirikizumab 200 mg Q4W until week 40; if they did not respond, they discontinued the trial.
Intravenous infusion of mirikizumab or placebo occurred over at least 30 minutes. Subcutaneous administration of mirikizumab or placebo was given in up to four injections (AMAC) or in two injections (LUCENT 2), with a maximum volume of 1.5 mL per injection.
2.2 Sample Collection and Analyses
For the AMAC primary and extension studies, blood samples for the PK analysis were collected every second week during the induction phase, every 4 weeks in the first part of the maintenance phase, then every 8 weeks until the end of the study, including the follow-up period. A single sample was collected prior to study drug administration (if occurring on a dosing day), and samples were taken before each IV infusion (trough) and at the end of each IV infusion (maximum plasma drug concentration [Cmax]) at weeks 0, 4, and 8 (Table 1 of the ESM).
For the LUCENT trials, a PK sample collection was performed with a total of six samples per patient in LUCENT 1 and four to six samples per patient in LUCENT 2. Blood samples were collected prior to mirikizumab dosing at weeks 0, 4, and 8 and after mirikizumab dosing at weeks 0 and 4. Post-dosing samples were collected up to 2 h after dosing, with the actual time of all dose administrations recorded in the patient’s electronic case report form. Additional samples were collected at week 12, at any unscheduled visits, at early termination visits, and 16 weeks after the last visit (Table 1 of the ESM).
Serum mirikizumab concentrations were measured in blood samples using a validated drug-tolerant quantitative enzyme immunoassay method at ICON Laboratory Services, Inc. (Whitesboro, NY, USA). The lower limit of quantification was 100 ng/mL and the upper limit of quantification was 10,000 ng/mL. Samples above the limit of quantification were diluted to yield results within the calibrated range. The inter-assay accuracy (% relative error) during validation ranged from − 9.09% to 2.30%. The inter-assay precision (% coefficient of variation [CV%]) during validation ranged from 2.18% to 6.57%. Samples were stored at − 70 °C.
2.3 Model Development
A population PK model was initially developed using the data from the phase II AMAC trial. A two-compartment model with first-order absorption for the SC maintenance doses was found to best describe the pharmacokinetics of mirikizumab. The final population PK model was developed using the combined data from LUCENT 1+2 using the same model structure as the phase II study.
Model development consisted of structural model selection, a covariate search, and a final model evaluation (Fig. 2 of the ESM). Key selection criteria were convergence of the estimation and covariance routines, reasonable parameter and variance estimates based on the known pharmacokinetics of mirikizumab, good precision of the parameter and variance estimates (relative standard error < 50%), and graphical methods to ensure the presence of reasonable characterization of data with no obvious model misspecification. For the final model development, an assessment of covariate effects was conducted. Several covariates were investigated (Table 2 of the ESM), including body weight at study entry, age, sex, race, disease severity, prior biologic therapy, concomitant use of corticosteroids or immunomodulators (tested as single and combined covariates), and an antidrug antibody (ADA) titer over time. In particular, age was evaluated as a continuous covariate, and sex, race (White, Asian, and Other [American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, multiple, and missing]), ethnicity, and prior and concomitant therapies were evaluated as categorical covariates. Baseline disease state was evaluated using serum albumin (continuous), modified Mayo score (continuous/categorical), fecal calprotectin (continuous), and C-reactive protein (continuous). Key covariate selection criteria were as per structural model selection. In addition, the criterion for a statistically significant difference in objective function value (OFV) was a ≥ 6.635-point drop in OFV (p < 0.01) for forward inclusion and a ≥ 10.828-point drop in OFV (p < 0.001) for backward elimination. A bootstrap analysis was performed to assess the precision of the final parameter estimates of the final model using the bootstrap routine in Perl-speaks-NONMEM software (Version 4.8.1, ©2018–2019 by Mats Karlsson, Rikard Nordgren, Svetlana Freiberga, Sebastian Ueckert, and Gunnar Yngman), and a visual predictive check was performed to ensure that the model maintained fidelity with the observed data using the visual predictive check algorithm in Perl-speaks-NONMEM.
Missing values of independent variables (patient characteristic data) were imputed within patients using last observation carried forward. Approximately 4% and 2% of the PK samples collected for the AMAC and LUCENT 1+2 studies, respectively, were below the quantifiable limit. Models with and without these samples were compared using a first-order conditional estimation with interaction method and a Laplacian conditional estimation with interaction method when values below the quantifiable limit were included. There were no significant changes on the estimated PK parameters, and no increased uncertainty was noted with the additional samples, so the final analysis excluded these samples.
All analyses were conducted using non-linear mixed-effects modeling [NONMEM] (Version 7.4.2; ICON Development Systems, Gaithersburg, MD, USA), R (Version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria), and Perl-speaks-NONMEM.
3 Results
3.1 Patient Disposition and Characteristics
A total of 4103 and 7578 serum mirikizumab concentration samples in 233 and 1129 patients were included in the PK analysis for the AMAC and LUCENT 1+2 trials, respectively. In the AMAC PK analysis, 59% of patients were male and 35% were naïve to biologic therapy. The mean age was 42 years (range 17–72 years) with a mean body weight of 75 kg (range 40–122 kg), a mean body mass index (BMI) of 25 kg/m2 (range 15–43 kg/m2), and a mean albumin level of 41 g/L (range 24–51 g/L). In the LUCENT 1+2 PK analysis, most patients were male (61%) and 44% were naïve to biologic therapy. The mean age was 43 years (range 18–79 years) with a mean body weight of 73 kg (range 34–152 kg), a mean BMI of 25 kg/m2 (range 14–54 kg/m2), and a mean albumin level of 43 g/L (range 21–54 g/L) (Table 1).
3.2 PK Model
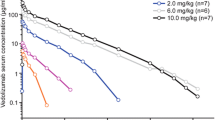
For the AMAC trial, the model-estimated apparent total body clearance (CL) of mirikizumab from plasma and the bioavailability of mirikizumab were 0.023 L/h and 42%, respectively (Table 2). For LUCENT 1+2, inter-compartmental CL was fixed to 0.00756 L/h during the model development stage based on the AMAC PK analysis results, and was estimated to be 0.0087 L/h as part of the final PK model. The final model-estimated population-typical values of CL and bioavailability were 0.022 L/h and 47.6% (geometric mean of individual post hoc estimates: 44%; geometric CV% [geoCV%]: 34%), respectively (Table 2). The typical half-life value was estimated to be 9.5 days (geometric mean: 9.33 days; geoCV%: 40%). The parameters estimated for the AMAC and LUCENT 1+2 trials were consistent with each other. A bootstrap analysis (Table 2) and visual predictive check (Fig. 1) demonstrated that the model predictions agreed with the observed data reasonably well and all parameters were estimated with adequate precision.
Visual predictive check (VPC) for the final mirikizumab pharmacokinetic model for the LUCENT 1+2 study stratified by route of administration. Blue triangles: individual observed concentration data; solid red line: median of observed concentrations; dashed red lines: 5th and 95th percentiles of observed concentrations; pink shaded area: confidence interval for the median of simulated data; blue shaded areas: confidence intervals for the 5th and 95th percentiles of simulated data. IV intravenous, SC subcutaneous
Comparison of the simulated concentration–time profile following IV mirikizumab 300 mg Q4W and SC 200 mg Q4W (Fig. 2) showed that the overall exposure with the IV regimen was approximately three-fold higher than with the SC regimen, which was consistent with the model-predicted availability of 47.6% (300 mg/(200 mg × 0.476) = 3.2). Specifically, the geometric mean (geoCV%) area under the plasma concentration–time curve during one dosing interval at steady state (AUCtau,ss) was 538 (34.4%) μg*day/mL for the IV regimen and 160 (57.6%) μg*day/mL for the SC regimen. The largest difference in the concentration profiles was at Cmax, with geometric mean steady-state (geoCV%) Cmax concentrations of 99.7 (22.7%) μg/mL for the IV regimen and 10.1 (52.1%) μg/mL for the SC regimen. The difference in trough concentrations was smaller with geometric mean steady-state (geoCV%) trough concentrations of 2.75 (101%) μg/mL for the IV regimen and 1.70 (83.3%) μg/mL for the SC regimen.
LUCENT 1+2 population pharmacokinetic model-predicted concentration–time profile. Mirikizumab model-predicted concentration–time profile following a 300-mg intravenous (IV) dose once every 4 weeks (Q4W) from weeks 0 to 12 (induction dosing period) or at steady state following a 200-mg subcutaneous (SC) dose Q4W (maintenance dosing period) from the phase III population pharmacokinetic analyses. Simulation of 1000 patients was conducted using baseline covariates in the LUCENT 1+2 dataset. The solid black and orange lines depict the median predicted concentration profile for the 300-mg IV dose Q4W from weeks 0 to 12 and the 200-mg SC dose Q4W from weeks 24 to 36, respectively, and the shaded area defines the 90% prediction interval of the simulated data
3.3 Covariate Effects
The covariate evaluation discussed here focuses on the final modeling with phase III data. In the final model based on data from the LUCENT 1+2 trial, body weight and serum albumin level were identified as statistically significant patient factors affecting mirikizumab CL. Higher CL was associated with higher body weights (Fig. 3a) and lower albumin levels (Fig. 3b). Volume of distribution increased with increasing body weight (Fig. 3c, d), whereas bioavailability decreased with increasing BMI (Fig. 3e), both in a statistically significant manner.
LUCENT 1+2 population pharmacokinetic model-estimated statistically significant covariates. LUCENT 1+2 population pharmacokinetic model-estimated a clearance versus baseline body weight, b) clearance versus albumin level, c volume of distribution of the central compartment versus baseline body weight, d volume of distribution of the peripheral compartment versus baseline body weight, and e bioavailability versus baseline body mass index (BMI). SC subcutaneous, V1 volume of distribution of the central compartment, V2 volume of distribution of the peripheral compartment
To evaluate the potential impact of body weight and albumin on mirikizumab exposure following IV and SC dose regimens, AUCtau,ss and Cmax were compared across the patient covariate quartiles. A significant overlap in the AUCtau,ss and Cmax was observed across quartile groups for weight, BMI, and albumin (Fig. 4). More specifically, with the IV dose regimen, the median AUCtau,ss in patients in the highest body weight quartile (83–152 kg) was 451 μg*day/mL versus an overall population median AUCtau,ss of 539 μg*day/mL (Fig. 4a). Similarly, the median Cmax in the highest body weight quartile was 84.4 μg/mL versus an overall population median of 99.4 μg/mL (Fig. 4b). The differences in AUCtau,ss and steady-state Cmax between the lowest body weight quartile and population median were also similar in magnitude (Fig. 4a, b). The median AUCtau,ss in patients in the lowest quartile of albumin levels (21–41 g/L) was 515 μg*day/mL versus an overall population median AUCtau,ss of 539 μg*day/mL (Fig. 4c). Similarly, the median Cmax in the lowest albumin level quartile was 102 μg/mL versus a population median of 99.4 μg/mL (Fig. 4d). With the SC dose regimen, patients with a higher baseline BMI were found to have a lower bioavailability, which would decrease both AUCtau,ss and Cmax (Fig. 4e, f). Because body weight also affected CL, and because body weight and BMI are correlated, AUC and Cmax were compared across the quartiles of body weight for the SC regimen. The median AUCtau,ss in patients in the highest body weight quartile was 124 μg*day/mL versus an overall population median AUCtau,ss of 165 μg*day/mL. Similarly, the median Cmax in the highest body weight quartile was 7.65 μg/mL versus an overall population median of 10.6 μg/mL, respectively. Overall, the differences in AUCtau,ss and Cmax for patients in the highest patient factor quartiles were small relative to the overall range in AUC and Cmax values across patients as a result of variability. The effects of body weight, BMI, and albumin levels on mirikizumab exposure were not considered clinically relevant.
LUCENT 1+2 population pharmacokinetic model-estimated effects of statistically significant covariates on the pharmacokinetics of mirikizumab. Boxplots of covariate effects on the pharmacokinetics of mirikizumab post IV (a–d) and post-SC (e, f) administration from the population pharmacokinetic analysis. The horizontal line in each box represents the median; the top and bottom sides of the box represent the 75th and 25th percentiles; the whiskers extend to the 5th and 95th percentiles; and circles represent data points outside of the 5th or 95th percentile. AUCtau,ss area under the plasma concentration–time curve during one dosing interval at steady state, Cmax,ss maximum steady-state plasma drug concentration, IV intravenous, Q quartile, SC subcutaneous
In the phase III LUCENT 1+2 studies, 1127, 678, and 195 PK samples were associated with SC injections in the abdomen, arm, and thigh, respectively. A post hoc analysis found that the injection site was not a statistically significant factor affecting bioavailability, with similar bioavailability among the three injection sites (Fig. 5a). Immunogenicity was not found to be a statistically significant factor that affected mirikizumab CL. The post hoc CL values for different ADA titer levels are presented in Fig. 5b. No significant change in CL with ADA titer levels was apparent. The majority of patients who were ADA positive had mirikizumab exposure that was consistent with exposure in patients who were ADA negative (Fig. 5c). For titers of 1:160 or higher, mirikizumab concentrations trended lower in a small number of patients, which limits definitive conclusions. None of the other covariates evaluated showed a meaningful impact on mirikizumab exposure.
LUCENT 1+2 post hoc analyses. Boxplots of LUCENT 1+2 model-estimated post hoc a bioavailability versus injection site and b clearance versus treatment-emergent antidrug antibody (TE-ADA) titers (patients were categorized by the maximum observed TE-ADA in the population pharmacokinetic analysis dataset). The horizontal line in each box represents the median; the top and bottom sides of the box represent the 75th and 25th percentiles; the whiskers extend to the 5th and 95th percentiles; and circles represent data points outside of the 5th or 95th percentile. c Observed mirikizumab concentrations in LUCENT 1 and LUCENT 2 studies following intravenous (IV) mirikizumab 300 mg every 4 weeks (Q4W) and subcutaneous (SC) 200 mg Q4W by TE-ADA titer category. Individual dots represent individual pharmacokinetic samples. The week 4 visit in LUCENT 1 included samples taken both before and after the mirikizumab infusion. The horizontal dashed line represents the lower limit of quantitation (LLOQ) of mirikizumab bioanalytical assay value (0.1 μg/mL) reported from bioanalytical results
4 Discussion
The population PK models developed in this analysis were used to characterize the PK properties of mirikizumab in patients with UC and to evaluate the impact of demographic and disease characteristic covariates on mirikizumab pharmacokinetics during induction and maintenance therapy. Data from AMAC were not pooled with data from LUCENT 1+2 as the latter were sufficient to inform the model, and the approach adopted in this analysis allowed a comparison of model parameters between the phase II and III studies. Overall, the mirikizumab pharmacokinetics in patients with UC was typical of a monoclonal antibody drug [24,25,26] and consistent with previous studies of mirikizumab in healthy volunteers (unpublished data). Clearance increased with a higher body weight and a lower serum albumin level, and bioavailability decreased with a higher BMI. However, changes in mirikizumab exposure related to changes in patient covariates were small relative to the overall variability in exposure and therefore are not considered clinically relevant.
The concentration–time data from the phase III studies were sufficient to inform the population PK model development; therefore, data from phase II and phase III studies were not combined. The population-typical CL and volume of distribution estimated by the PK model were similar between the AMAC and LUCENT 1+2 studies and to those of phase I studies of mirikizumab in patients with psoriasis and healthy volunteers (estimated CL: 0.022 L/h, NCT01947933; estimated volume of distribution: 5.74–6.91 L, NCT01947933; 4.35–5.22 L, NCT02568423; 3.98–4.43 L, NCT04137380). This indicates that the pharmacokinetics behaved consistently among different clinical trials, and there was no difference in the pharmacokinetics of mirikizumab between patient populations. In addition, the other PK parameters and inter-patient variability terms were similar between the AMAC and LUCENT 1+2 studies. Of note, the estimated bioavailability of mirikizumab resulting from the SC regimens in the LUCENT 1+2 study was slightly higher (median and geometric mean of 48% and 44%, respectively) than the bioavailability estimated in the AMAC study (median and geometric mean of 43% and 42%, respectively). This difference in bioavailability may be due to variability between the studies and to differences in the mirikizumab formulations and administration. In fact, a lyophilized formulation of mirikizumab was manually withdrawn from a vial into a syringe for administration in the AMAC trial, whereas a syringe prefilled with a mirikizumab solution formulation (planned for commercialization) was used in the LUCENT trials.
Overall, the changes in mirikizumab pharmacokinetics and exposures relative to patient factors were consistent with expectations for monoclonal antibodies. Body weight was a statistically significant covariate on CL and volume of distribution in the population PK analyses. As a result, there was an overall trend for serum concentrations to decrease as body weight increased. The effect of body weight on the pharmacokinetics of monoclonal antibodies is well established [27, 28], and simulation studies have shown that dosing by body weight is not necessarily beneficial compared with flat dosing [27, 29]. A large degree of overlap in exposures was noted when patients were stratified by body weight quartiles in the population PK analysis for mirikizumab. The result indicates that body weight does not have a clinically relevant impact on the exposure of mirikizumab, and no dose adjustment on the basis of body weight is needed.
Serum albumin level and CL of monoclonal antibodies have frequently been reported to be inversely correlated in PK studies of monoclonal antibodies [30,31,32,33]. This relationship can be explained by metabolic pathways shared by albumin and monoclonal antibodies, such as the neonatal Fc receptor rescue pathway, which plays an important role in albumin and monoclonal antibody homeostasis [34]. Lower CL with higher baseline albumin levels was observed for mirikizumab; however, the magnitude of effect was within the PK variability of mirikizumab, and therefore the effect was not considered clinically relevant.
Body mass index was identified as a statistically significant covariate affecting SC bioavailability of mirikizumab, with bioavailability decreasing as BMI increased. The bioavailability of monoclonal antibodies is thought to be influenced by several factors such as skin thickness, subcutaneous adipose tissue, and lymphatic circulation [35]. As such, the relationship between mirikizumab bioavailability and BMI is likely the result of the relationship between BMI and these physiologic factors. Body weight and BMI are highly correlated. Accounting for this correlation and the effect of both covariates on mirikizumab pharmacokinetics, i.e., the effect of weight on CL and BMI on bioavailability, a large degree of overlap in exposures was noted when patients were stratified by body weight/BMI quartiles (Fig. 4e, f). The result indicates that BMI does not have a clinically relevant impact on the exposure of mirikizumab, and no dose adjustment on the basis of BMI is needed.
Immunogenicity was not identified as a significant factor affecting mirikizumab pharmacokinetics in either the AMAC or LUCENT 1+2 studies. However, the lack of statistical significance in the models may be the result of the low number of patients who developed a high ADA titer response. Figure 5 shows boxplots of the post hoc CL values for different ADA titer levels and does not show any significant change in CL with an ADA titer. As immunogenicity and the ADA titer were not covariates in the final model, the post hoc CL value for a given patient would only increase with higher ADA titers if the patient’s ADA titer was consistently high and serum mirikizumab concentrations were consistently low throughout the study. This way, the effect of high ADA/low serum mirikizumab concentration is significant enough to influence the random-effect eta estimate for CL in those particular patients. Overall, the changes in mirikizumab pharmacokinetics and exposures relative to patient factors are consistent with expectations for a monoclonal antibody. The magnitude of the changes in exposure related to patient factors also was small relative to the overall range of exposures caused by random unexplained variability. Furthermore, there was no apparent impact on mirikizumab pharmacokinetics of other demographic factors of interest, such as age, sex, and race in the population tested.
For the modeling, the allometric exponents were not fixed to values typically reported in the literature (i.e., 0.8 and 1 for CL and volume of distribution, respectively) as this resulted in a poorer model fit (increased OFV). This may be due to the typically reported values for allometric exponents only applying to changes in body weight with a consistent body composition, without accounting for changing in body composition (i.e., fat).
The LUCENT studies had sparse PK sample collection schemes, and most PK samples were collected prior to dosing when mirikizumab concentrations are expected to be at trough. Although this is typical of phase III trials, it may not be sufficient to fully characterize the pharmacokinetics of the compound. Nevertheless, the PK parameters found in this analysis were similar to those in studies with denser sample collection schemes, such as the phase II AMAC study.
5 Conclusions
The population PK model developed with phase II and phase III data characterized the pharmacokinetics of mirikizumab well in patients with moderate-to-severe UC. The pharmacokinetics of mirikizumab is typical of a monoclonal antibody drug. None of the covariates identified as being statistically significantly correlated with mirikizumab PK parameters were predicted to be clinically relevant based on a lack of meaningful impact on mirikizumab exposures for the proposed clinical regimen for the treatment of moderate to severely active UC.
References
Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–25.
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–70.
Wetwittayakhlang P, Lontai L, Gonczi L, Golovics PA, Hahn GD, Bessissow T, et al. Treatment targets in ulcerative colitis: is it time for all in, including histology? J Clin Med. 2021;10(23):5551.
Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–70.
Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–4.
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–25.
Sewell GW, Kaser A. Interleukin-23 in the pathogenesis of inflammatory bowel disease and implications for therapeutic intervention. J Crohns Colitis. 2022;16(Suppl._2):ii3–19.
Feagan BG, Sandborn WJ, D’Haens G, Panes J, Kaser A, Ferrante M, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699–709.
Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2A study. Gastroenterology. 2017;153(1):77-86.e6.
Sands BE, Peyrin-Biroulet L, Kierkus J, Higgins PDR, Fischer M, Jairath V, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162(2):495–508.
Blauvelt A, Kimball AB, Augustin M, Okubo Y, Witte MM, Rodriguez Capriles C, et al. Efficacy and safety of mirikizumab in psoriasis: results from a 52-week, double-blinded, placebo-controlled, randomised withdrawal, phase III trial (OASIS-1). Br J Dermatol. 2022;187(6):866–77.
Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17.
Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222–6.
Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136(1):116-24.e7.
Papp K, Thaci D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–9.
Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31.
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–60.
Hanzel J, D’Haens GR. Anti-interleukin-23 agents for the treatment of ulcerative colitis. Expert Opin Biol Ther. 2020;20(4):399–406.
D’Haens G, Kobayashi T, Morris N, Lissoos T, Hoover A, Li X, et al. 884: efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-1 study. Gastroenterology. 2022;162(7):S214–314.
Dubinsky MC, Irving PM, Li X, Howaldt S, Pokrotnieks J, Krueger KA, et al. 867e: Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study. Gastroenterology. 2022;162(7):S-1393-S-4.
Sandborn WJ, Ferrante M, Bhandari BR, Berliba E, Feagan BG, Hibi T, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020;158(3):537-49.e10.
Sandborn WJ, Ferrante M, Bhandari BR, Berliba E, Hibi T, D’Haens GR, et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(1):105-15.e14.
ICH. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). http://www.ich.org. Accessed 3 Jul 2023.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51(2):119–35.
Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55(7):789–805.
Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–24.
Bajaj G, Suryawanshi S, Roy A, Gupta M. Evaluation of covariate effects on pharmacokinetics of monoclonal antibodies in oncology. Br J Clin Pharmacol. 2019;85(9):2045–58.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59.
Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(5):297–308.
Rosario M, Dirks NL, Gastonguay MR, Fasanmade AA, Wyant T, Parikh A, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42(2):188–202.
Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front Immunol. 2014;5:682.
Thomas VA, Balthasar JP. Understanding inter-individual variability in monoclonal antibody disposition. Antibodies (Basel). 2019;8(4):56.
Acknowledgements
The authors thank all study participants. Medical writing assistance was provided by Rebecca Lew, PhD, CMPP, and Loredana Spoerri, PhD, of ProScribe - Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly and Company, the manufacturer and licensee of mirikizumab. Eli Lilly and Company was involved in the study design, data collection, data analysis, and preparation of the manuscript, and paid the open access fees.
Conflicts of interest/competing interests
Laiyi Chua and Xin Cindy Zhang are employees and minor shareholders of Eli Lilly and Company. Stuart Friedrich is a former employee of Eli Lilly and Company.
Ethics approval
The AMAC, LUCENT 1, and LUCENT 2 studies were approved by the applicable ethics review boards and performed in accordance with the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, and the International Council for Harmonisation Guideline for Good Clinical Practice.
Consent to participate
All patients provided written informed consent.
Consent for publication
Not applicable.
Availability of data and material
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at http://www.vivli.org.
Code availability
Not applicable.
Authors’ contributions
All authors participated in the design and interpretation of the study results, and in the drafting, critical revision, and approval of the final version of the manuscript. SF and XCZ contributed to the study conceptualization. LC curated the data and formal analysis. Validation and visualization of the data were performed by LC and SF.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chua, L., Friedrich, S. & Zhang, X.C. Mirikizumab Pharmacokinetics in Patients with Moderately to Severely Active Ulcerative Colitis: Results from Phase III LUCENT Studies. Clin Pharmacokinet 62, 1479–1491 (2023). https://doi.org/10.1007/s40262-023-01281-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01281-z